Abstract
The aim of the present study was to examine whether endocannabinoids cause PPARγ-mediated vascular actions. Functional vascular studies were carried out in rat aortae. Anandamide and N-arachidonoyl-dopamine (NADA), but not palmitoylethanolamide, caused significant vasorelaxation over time (2 hours). Vasorelaxation to NADA, but not anandamide, was inhibited by CB1 receptor antagonism (AM251, 1 μM), and vasorelaxation to both anandamide and NADA was inhibited by PPARγ antagonism (GW9662, 1 μM). Pharmacological inhibition of de novo protein synthesis, nitric oxide synthase, and super oxide dismutase abolished the responses to anandamide and NADA. Removal of the endothelium partly inhibited the vasorelaxant responses to anandamide and NADA. Inhibition of fatty acid amide hydrolase (URB597, 1 μM) inhibited the vasorelaxant response to NADA, but not anandamide. These data indicate that endocannabinoids cause time-dependent, PPARγ-mediated vasorelaxation. Activation of PPARγ in the vasculature may represent a novel mechanism by which endocannabinoids are involved in vascular regulation.
1. Introduction
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors which control the transcription of many families of genes. They have a large ligand binding pocket and are pharmacologically promiscuous, being activated by a number of structurally diverse natural and synthetic ligands including some angiotensin II receptor antagonists [1], statins [2], retinoic receptor antagonists [3], flavinoids [4], and citrus fruit compounds [5]. An increasing body of evidence now also suggests that cannabinoids activate PPARs, and this may mediate some of the biological effects of cannabinoids [6], in addition to activation of two well-established 7-transmembrane cannabinoid receptors (CB1 and CB2).
The first evidence of cannabinoid interactions with PPAR came in 2002 in a study by Kozak and colleagues who showed that lipoxygenase metabolism of the endocannabinoid, 2-arachidonoylglycerol (2-AG), produced a metabolite that increases the transcriptional activity of PPARα [7]. Fu et al. (2003) then showed that the appetite-suppressing and weight-reducing effects of another endocannabinoid-related agent, oleoylethanolamide (OEA), were absent in PPARα knock-out mice [8]. Guzmán et al. (2004) also showed that the stimulatory effect of OEA on lipolysis in vivo was absent in PPARα knock-out mice [9]. Palmitoylethanolamide (PEA), which is structurally related to OEA, similarly activates PPARα transcriptional activity, causing anti-inflammatory actions that were absent in PPARα knock-out mice [10]. Other endocannabinoids that have been shown to activate PPARα include noladin ether and virodhamine [11].
As well as activating PPARα, it was shown in 2003 [12] that the synthetic cannabinoid, ajulemic acid (an analogue of a tetrahydrocannabinol metabolite) binds to and increases the transcriptional activity of PPARγ. We have since shown that the principal active ingredient of Cannabis sativa, Δ9-tetrahydrocannabinol (THC), activates the transcriptional activity of PPARγ and stimulates adipogenesis, a PPARγ property [13]. The endocannabinoids anandamide and 2-AG have anti-inflammatory effects which are sensitive to PPARγ antagonism [14, 15], although it was not clear whether these effects were through activation of PPARγ directly, or via metabolites of the endocannabinoids. Subsequent research has shown that anandamide directly binds to PPARγ [16, 17], activates PPARγ transcriptional activity, and stimulates the differentiation of fibroblasts to adipocytes [16]. Other cannabinoids that activate the transcriptional activity of PPARγ include the endocannabinoid/endovanilloid, N-arachidonoyl-dopamine (NADA), the synthetic cannabinoids WIN55212-2 and CP55940, and the phytocannabinoid, cannabidiol [18].
We have shown that THC causes time-dependent, endothelium-dependent, PPARγ-mediated vasorelaxation of the rat isolated aorta [13]. This response was dependent on nitric oxide (NO) and superoxide dismutase (SOD) activity [13]. Furthermore, subsequent studies showed that 2-hour incubation with THC (10 μM) in vitro blunts subsequent contractile responses and enhances vasodilator responses in isolated arteries, which was also inhibited by a PPARγ antagonist [19]. These experiments similarly indicated a role for increased SOD activity stimulated by THC. Together, these studies suggest that THC, through activation of PPARγ, leads to increased synthesis of SOD, promoting vasorelaxation by preventing NO being scavenged by endogenous superoxides. This is in agreement with research showing that, in addition to direct effects on NO production, PPARγ ligands enhance NO bioavailability in blood vessels through induction of SOD [20].
There has been much interest surrounding the vascular actions of endocannabinoids. The mechanisms underpinning the acute vasorelaxant response to endocannabinoids include activation of sensory nerves [21–23], activation of the CB1 receptor, and activation of a novel endothelial cannabinoid receptor [23–25]. In light of the growing evidence that endocannabinoids activate PPARγ [14–18], the aim of the present study was to investigate whether similar time-dependent, PPARγ-mediated vasorelaxation to endocannabinoids occurs in the rat aorta as observed for THC, and to investigate the underlying mechanisms.
2. Material and Methods
2.1. In Vitro Vascular Studies
Male Wistar rats (250–350 g) were stunned by a blow to the back of the head and killed by cervical dislocation. The aortae were removed rapidly and placed into cold modified Krebs-Henseleit buffer (composition, mM: NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, CaCl2 2, and D-glucose 10). The aortae were dissected free of adherent connective and adipose tissue and cut into rings 3-4 mm long, and mounted on fixed segment support pins using the Multimyograph system (Model 610M, Danish Myo Technology, Denmark) as previously described [13, 19, 23]. Once mounted, all vessels were kept at 37°C in modified Krebs-Henseleit buffer and gassed with 5% CO2 in O2. The aortae were stretched to an optimal passive tension of 9.8 mN tension. Vessels were allowed to equilibrate and the contractile integrity of each was tested by its ability to contract to 60 mM KCl by at least 4.9 mN. Vessels were contracted with a combination of U46619 (10–100 nM, a thromboxane prostanoid receptor agonist), and the α-adrenoceptor agonist methoxamine (1–5 μM) to increase tension.
When stable contraction was maintained, the vasorelaxant effect of a single concentration of endocannabinoid or vehicle control (0.1% ethanol) on induced tone was assessed as the reduction in tone over time. The endocannabinoids chosen were anandamide (5 μM) and NADA (10 μM), both previously demonstrated to be PPARγ ligands [14, 16–18], and PEA (10 μM), which activates PPARα but not PPARγ [10]. For every experimental protocol, vehicle-treated and endocannabinoid-treated experiments were performed in adjacent segments of the same artery.
To assess any possible contribution of vasorelaxation mediated through cannabinoid receptors, some experiments were performed in the presence of the cannabinoid CB1 receptor antagonist AM251 (1 μM), or the CB2 receptor antagonist AM630 (1 μM), both added 10 minutes before contracting the vessels.
To assess the contribution of PPARγ activation, some experiments were performed in the presence of the PPARγ antagonist GW9662 (1 μM) added 10 minutes prior to precontraction. To establish whether the time-dependent vasorelaxant effects of endocannabinoids were dependent upon de novo protein synthesis, some experiments were performed in the presence of the protein synthesis inhibitor cycloheximide (10 μM).
To investigate the role of endothelium-derived relaxing factors in the time-dependent vasorelaxation to endocannabinoids, some vessels were denuded of their endothelium by abrasion with a human hair. The role of endothelium-derived nitric oxide (NO) was investigated using the NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 300 μM, present throughout). To establish whether endocannabinoids cause increased expression of superoxide dismutase (SOD) activity, some experiments were performed in the presence of the SOD inhibitor diethyldithiocarbamate (DETCA, 3 mM), added 30 minutes prior to precontraction of arteries.
To assess whether the actions of endocannabinoids are due to their breakdown to other biologically active compounds that may act at PPARγ, some vessels were treated with the FAAH inhibitor, URB597 (1 μM, added 10 minutes prior to precontraction).
2.2. Statistical Analysis
In each protocol, the number of animals in each group is represented by n, and values are expressed as mean ± SEM. The difference between endocannabinoid-treated and vehicle-treated vessels (adjacent segments from the same aorta) under each experimental protocol were analysed by paired Student's t-test.
2.3. Drugs
All drugs were supplied by Sigma Chemical Co. (UK) except where stated. Anandamide, NADA, PEA, AM251, AM630, and GW9662 were obtained from Tocris (UK). L-NAME, DETCA, and cycloheximide were dissolved in the Krebs-Henseleit solution. Anandamide, NADA, PEA, and URB597 were dissolved in ethanol at 10 mM with further dilutions made in distilled water. AM251, AM630, and GW9662 were dissolved in DMSO to 10 mM, with further dilutions in distilled water.
3. Results
3.1. Time-Dependent Vasorelaxant Effects of Endocannabinoids
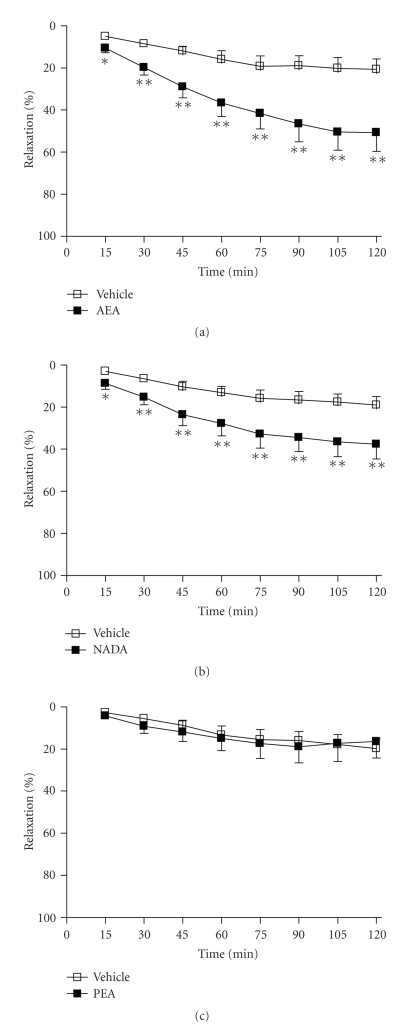
Anandamide (5 μM) caused significant time-dependent relaxation of the rat aorta compared to vehicle-treated arteries at all time-points over the course of 2 hours (2 hours, vehicle 21 ± 5% versus AEA 51 ± 8% relaxation, n = 12, P < .01, see Figure 1(a)). NADA (10 μM) also caused significant time-dependent relaxation of the rat aorta compared to vehicle control at all time-points studied over the course of 2 hours (2 hours, vehicle 19 ± 4% versus NADA 38 ± 7% relaxation, n = 12, P < .01, see Figure 1(b)). By contrast, PEA (10 μM) did not have any significant effect on the rat aorta compared to vehicle (2 hours, vehicle 20 ± 5% versus PEA 17 ± 9% relaxation, n = 12, Figure 1(c)).
Figure 1.
The mean vasorelaxant response to (a) AEA, (b) NADA, and (c) PEA versus vehicle (0.1% EtOH) over 2 hours in preconstricted aortae. Data are given as means with error bars representing SEM. (*P < .05, **P < .01, Student's t-test, n = 12).
3.2. Receptor Sites of Action
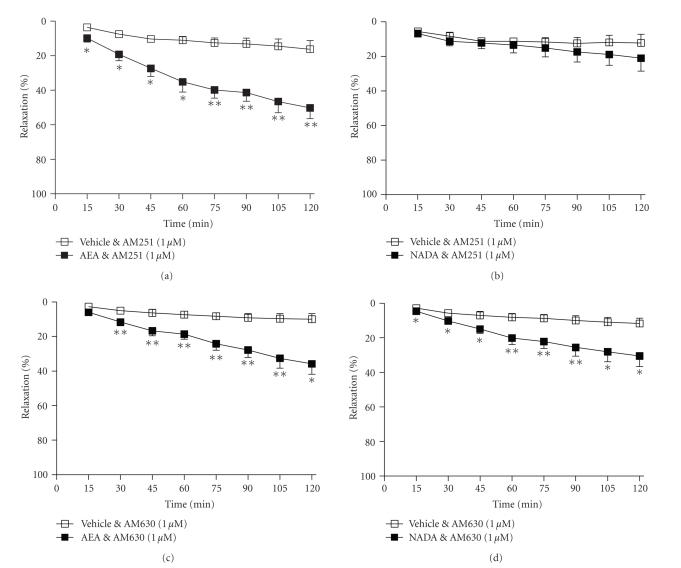
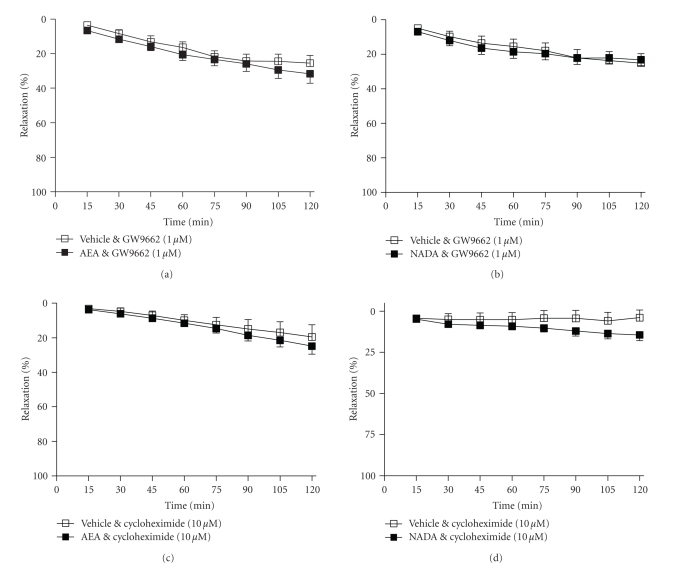
In the presence of the cannabinoid CB1 receptor antagonist, AM251 (1 μM), the vasorelaxant response to anandamide was not affected (2 hours, vehicle 16 ± 4% versus AEA 50 ± 5% relaxation, n = 9, P < .01, Figure 2(a)). By contrast, in the presence of AM251, the vasorelaxant response to NADA was abolished (2 hours, vehicle 12 ± 4% versus NADA 21 ± 6% relaxation, n = 9, nonsignificant, Figure 2(b)). The CB2 receptor antagonist AM630 (1 μM) did not affect the vasorelaxant response to either anandamide (2 hours, vehicle 10 ± 3% versus AEA 36 ± 5% relaxation, n = 9, P < .05, Figure 2(c)) or NADA (2 hours, vehicle 12 ± 2% versus NADA 31 ± 5% relaxation, n = 8, P < .05, Figure 2(d)). In the presence of the PPARγ receptor antagonist GW9662 (1 μM), the vasorelaxant effects of both anandamide (2 hours, vehicle 26 ± 4% versus AEA 32 ± 5% relaxation, n = 12, nonsignificant, Figure 3(a)) and NADA (2 hours, vehicle 25 ± 4% versus NADA 23 ± 3% relaxation, n = 9, nonsignificant, Figure 3(b)) were abolished.
Figure 2.
The effects of the CB1 receptor antagonist AM251 (1 μM, (a), and (b)) and the CB2 receptor antagonist AM630 (1 μM, (c), and (d)) on vasorelaxation to anandamide and NADA. Data are given as means with error bars representing SEM. (*P < .05, **P < .01, Student's t-test.)
Figure 3.
The effects of the PPARγ antagonist GW9662 (1 μM, (a), and (b)) and the protein synthesis inhibitor, cycloheximide (10 μM, (c), and (d)) on vasorelaxation to anandamide and NADA. Data are given as means with error bars representing SEM.
3.3. Mechanisms of Action
In the presence of the protein synthesis inhibitor, cycloheximide (10 μM), the vasorelaxant effects of both anandamide (2 hours, vehicle 20 ± 6% versus AEA 25 ± 4% relaxation, n = 8, nonsignificant, Figure 3(c)) and NADA (2 hours, vehicle 4 ± 4% versus NADA 14 ± 3% relaxation, n = 9, nonsignificant, Figure 3(b)) were abolished.
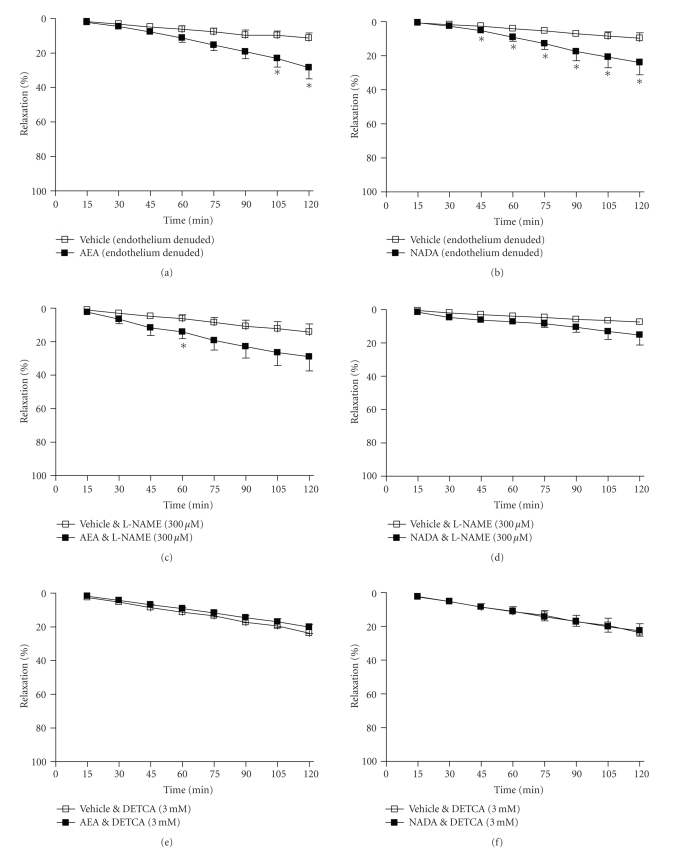
Removal of the endothelium limited the vasorelaxant effects of anandamide such that arteries treated with anandamide were significantly different from vehicle-treated arteries only at 105 and 120 minutes (2 hours, vehicle 11 ± 3% versus AEA 29 ± 6% relaxation, n = 11, P < .05, see Figure 4(a)). Similarly, removal of the endothelium limited the vasorelaxant response to NADA (2 hours, vehicle 10 ± 3% versus AEA 24 ± 6% relaxation, n = 9, P < .05, see Figure 4(b)). The NOS inhibitor, L-NAME (300 μM), inhibited the vasorelaxant response to anandamide (2 hours, vehicle 16 ± 5% versus AEA 31 ± 8% relaxation, n = 11, nonsignificant, Figure 4(c)) and NADA (2 hours, vehicle 6 ± 1% versus NADA 15 ± 5% relaxation, n = 8, nonsignificant, Figure 4(c)). Similarly, the SOD inhibitor, DETCA (3 mM) abolished the vasorelaxant response to both anandamide (2 hours, vehicle 20 ± 4% versus AEA 20 ± 8% relaxation, n = 8, nonsignificant, Figure 4(e)) and NADA (2 hours, vehicle 24 ± 4% versus NADA 22 ± 3% relaxation, n = 8, nonsignificant, Figure 4(f)).
Figure 4.
The effects of removing the endothelium ((a), (b)), inhibiting nitric oxide synthase (L-NAME, 300 μM, (c), and (d)), and inhibiting superoxide dismutase (DETCA, 3 mM, (e), and (f)) on vasorelaxation to anandamide and NADA. Data are given as means with error bars representing SEM. (*P < .05, Student's t-test).
3.4. Endocannabinoid Metabolism
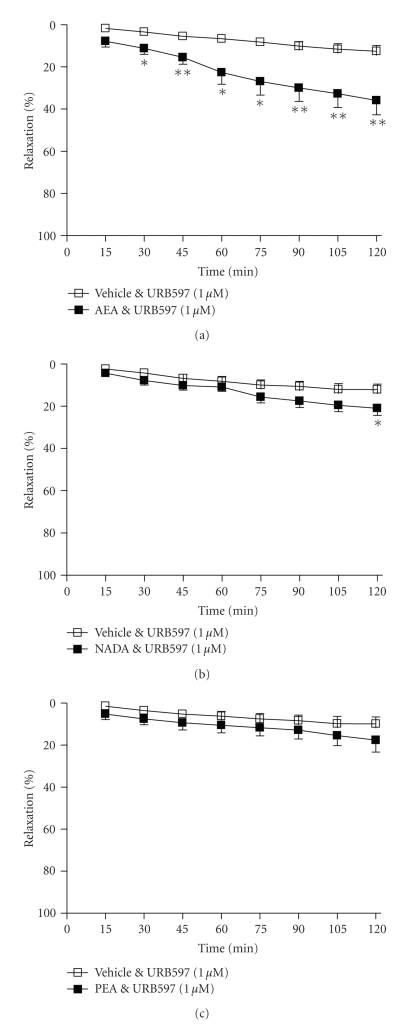
The presence of the FAAH inhibitor, URB597 (1 μM) did not affect the vasorelaxant effect of anandamide (2 hours, vehicle 13 ± 2% versus AEA 36 ± 6% relaxation, n = 10, P < .01, Figure 5(a)), and did not alter the vascular response to PEA (2 hours, vehicle 10 ± 2% versus PEA 18 ± 4% relaxation, n = 7, nonsignificant, Figure 5(c)). URB597 did inhibit the vasorelaxant response to NADA such that NADA-treated and vehicle-treated arteries were significantly different at 2 hours only (2 hours, vehicle 12 ± 2% versus NADA 21 ± 3% relaxation, n = 9, P < .05, Figure 5(b)).
Figure 5.
The effects of the FAAH inhibitor, URB597 (1 μM) on vasorelaxation to (a) anandamide, (b) NADA, and (c) PEA. Data are given as means with error bars representing SEM. (*P < .05, **P < .01, Student's t-test).
4. Discussion
In the present study, we have examined whether endocannabinoids cause time-dependent, PPARγ-mediated vascular effects as previously shown for the phytocannabinoid, THC [13, 19]. In these studies, we demonstrate for the first time that the endocannabinoids anandamide and NADA cause PPARγ-mediated, time-dependent vasorelaxation of rat aortae, which is dependent on de novo protein synthesis, nitric oxide production and superoxide dismutase activity. These are similar mechanisms to those found to underlie the vasorelaxant effects of the PPARγ agonists, rosiglitazone [26], and THC [13].
On the basis that PPARγ agonists cause time-dependent vasorelaxation of isolated aortae [13, 26], and that endocannabinoids activate PPARγ [14–18], we investigated whether endocannabinoids produce time-dependent vasorelaxation. The endocannabinoids chosen were anandamide and NADA, both previously demonstrated to activate PPARγ [14, 16–18], and PEA, which activates PPARα but not PPARγ [10]. We found that, like rosiglitazone and THC, anandamide and NADA produced a slowly developing decrease in tone of precontracted aortae that was significantly greater than that seen in vehicle-treated segments of the same artery. The vascular response to anandamide and NADA was inhibited by the PPARγ antagonist, GW9662, and by inhibition of de novo protein synthesis. In contrast, PEA did not cause vasorelaxation of the rat aorta. This is in agreement with our previous finding that the PPARα ligand, bezafibrate, does not cause time-dependent vasorelaxation of isolated aortae [13]. These results demonstrate that PPARγ-, but not PPARα-active endocannabinoids cause time-dependent vascular effects.
Some of the vasorelaxant effects of cannabinoids are due to activation of other target sites such as the CB1 or CB2 receptor [27], and we explored whether the vasorelaxant response to endocannabinoids might be partially mediated by any of these. We found that neither the CB1 nor CB2 receptor antagonists had any significant effect on vasorelaxation to anandamide. However, vasorelaxation to NADA was inhibited by the CB1 receptor antagonist, AM251. It is possible, therefore, that NADA may activate cannabinoid receptors at the cell surface, initiating intracellular signalling that may lead to PPARγ activation. For example, it has been shown that statins activate PPARs through activation of extracellular signal-regulated kinase (ERK)1/2 and p38 mitogen-activated protein kinase (MAPK) [28]. Both of these pathways can be activated by cannabinoid receptor activation [29, 30].
Further analysis of the time-dependent vasorelaxant effects of anandamide and NADA showed that these responses are partially endothelium-dependent and NO-dependent, as previously demonstrated for rosiglitazone and THC [13, 26]. We have also previously demonstrated that the PPARγ-mediated vascular effects of cannabinoids are due to increases in SOD activity [13, 19]. Similarly, in the present study, the time-dependent effects of anandamide and NADA were abolished in the presence of a SOD inhibitor, DETCA, suggesting the vasorelaxant effects of anandamide and NADA are mediated by upregulation of SOD, preventing NO of being scavenged by endogenous superoxides. This is in agreement with other work showing PPARγ ligands cause the induction of Cu/Zn-SOD [20], and with numerous studies that have shown that PPARγ ligands increase NO production and bioavailability in vitro and in vivo [31–34].
There are several potential mechanisms by which cannabinoids can activate PPARγ including direct binding, metabolism to other compounds that activate PPARs, or via intracellular signalling cascades. To establish whether endocannabinoids are metabolised into PPARγ-active compounds, we performed some experiments in the presence of the FAAH inhibitor, URB597. The vasorelaxant effects of anandamide were not affected by URB597, which is consistent with previous studies showing that anandamide directly binds to PPARγ [16, 17]. It also suggests that prolonging the effects of anandamide by preventing its breakdown does not enhance the PPARγ-mediated vasorelaxant response. By contrast, the vasorelaxant effects of NADA were inhibited by URB597, suggesting that it is the conversion of this compound to PPARγ-active metabolites that mediate the effects of NADA. There are no data presently available demonstrating a direct interaction between NADA and the PPARγ ligand binding domain.
In summary, these data provide evidence for the first time that the endocannabinoids anandamide and NADA, but not the related acylethanolamide PEA, activate PPARγ in the vasculature, leading to NO-dependent vasorelaxation. PPARγ agonists have a number of positive cardiovascular effects, which include increased availability of NO, in vivo reductions in blood pressure and attenuation of atherosclerosis [35–37]. Similarly, endocannabinoids have a number of beneficial effects on the cardiovascular system such as cardiac protection [38–40], benefits in hypertension [41, 42], and potential benefits in atherosclerosis [43]. PPARγ activation by some endocannabinoids may represent a novel mechanism by which they are involved in the regulation of the cardiovascular system.
Acknowledgments
The first author was previously supported by a Leverhulme Trust Early Career Fellowship. The authors would like to thank Dr. Richard Roberts for the use of a myograph.
References
- 1.Janke J, Schupp M, Engeli S, et al. Angiotensin type 1 receptor antagonists induce human in-vitro adipogenesis through peroxisome proliferator-activated receptor-γ activation. Journal of Hypertension. 2006;24(9):1809–1816. doi: 10.1097/01.hjh.0000242405.68461.84. [DOI] [PubMed] [Google Scholar]
- 2.Jasińska M, Owczarek J, Orszulak-Michalak D. Statins: a new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacological Reports. 2007;59(5):483–499. [PubMed] [Google Scholar]
- 3.Schupp M, Curtin JC, Kim RJ, Billin AN, Lazar MA. A widely used retinoic acid receptor antagonist induces peroxisome proliferator-activated receptor-γ activity. Molecular Pharmacology. 2007;71(5):1251–1257. doi: 10.1124/mol.106.033662. [DOI] [PubMed] [Google Scholar]
- 4.Salam NK, Huang TH-W, Kota BP, Kim MS, Li Y, Hibbs DE. Novel PPAR-gamma agonists identified from a natural product library: a virtual screening, induced-fit docking and biological assay study. Chemical Biology & Drug Design. 2008;71(1):57–70. doi: 10.1111/j.1747-0285.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuroyanagi K, Kang M-S, Goto T, et al. Citrus auraptene acts as an agonist for PPARs and enhances adiponectin production and MCP-1 reduction in 3T3-L1 adipocytes. Biochemical and Biophysical Research Communications. 2008;366(1):219–225. doi: 10.1016/j.bbrc.2007.11.119. [DOI] [PubMed] [Google Scholar]
- 6.O'Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. British Journal of Pharmacology. 2007;152(5):576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozak KR, Gupta RA, Moody JS, et al. 15-lipoxygenase metabolism of 2-arachidonylglycerol: generation of a peroxisome proliferator-activated receptor α agonist. The Journal of Biological Chemistry. 2002;277(26):23278–23286. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- 8.Fu J, Gaetani S, Oveisi F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α . Nature. 2003;425(6953):90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 9.Guzmán M, Lo Verme J, Fu J, Oveisi F, Blázquez C, Piomelli D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α) The Journal of Biological Chemistry. 2004;279(27):27849–27854. doi: 10.1074/jbc.M404087200. [DOI] [PubMed] [Google Scholar]
- 10.Lo Verme J, Fu J, Astarita G, et al. The nuclear receptor peroxisome proliferator-activated receptor-α mediates the anti-inflammatory actions of palmitoylethanolamide. Molecular Pharmacology. 2005;67(1):15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Alexander SPH, Kendall DA, Bennett AJ. Cannabinoids and PPARα signalling. Biochemical Society Transactions. 2006;34(6):1095–1097. doi: 10.1042/BST0341095. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Li H, Burstein SH, Zurier RB, Chen JD. Activation and binding of peroxisome proliferator-activated receptor γ by synthetic cannabinoid ajulemic acid. Molecular Pharmacology. 2003;63(5):983–992. doi: 10.1124/mol.63.5.983. [DOI] [PubMed] [Google Scholar]
- 13.O'Sullivan SE, Tarling EJ, Bennett AJ, Kendall DA, Randall MD. Novel time-dependent vascular actions of Δ9- tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor gamma. Biochemical and Biophysical Research Communications. 2005;337(3):824–831. doi: 10.1016/j.bbrc.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 14.Rockwell CE, Kaminski NE. A cyclooxygenase metabolite of anandamide causes inhibition of interleukin-2 secretion in murine splenocytes. Journal of Pharmacology and Experimental Therapeutics. 2004;311(2):683–690. doi: 10.1124/jpet.104.065524. [DOI] [PubMed] [Google Scholar]
- 15.Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor γ independently of cannabinoid receptors 1 and 2. Molecular Pharmacology. 2006;70(1):101–111. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- 16.Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARγ transcriptional activation and 3T3-L1 preadipocyte differentiation. European Journal of Pharmacology. 2005;517(3):174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Gasperi V, Fezza F, Pasquariello N, et al. Endocannabinoids in adipocytes during differentiation and their role in glucose uptake. Cellular and Molecular Life Sciences. 2007;64(2):219–229. doi: 10.1007/s00018-006-6445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Sullivan SE, Bennett AJ, Kendall DA, Randall MD. Cannabinoids and peroxisome proliferator-activated receptor gamma (PPARγ). In: Proceedings of the International Cannabinoid Research Society (ICRS '06); June 2006; Tihany, Hungary. p. 59. [Google Scholar]
- 19.O'Sullivan SE, Kendall DA, Randall MD. Further characterization of the time-dependent vascular effects of Δ9-tetrahydrocannabinol. Journal of Pharmacology and Experimental Therapeutics. 2006;317(1):428–438. doi: 10.1124/jpet.105.095828. [DOI] [PubMed] [Google Scholar]
- 20.Hwang J, Kleinhenz DJ, Lassègue B, Griendling KK, Dikalov S, Hart CM. Peroxisome proliferator-activated receptor-γ ligands regulate endothelial membrane superoxide production. American Journal of Physiology. 2005;288(4):C899–C905. doi: 10.1152/ajpcell.00474.2004. [DOI] [PubMed] [Google Scholar]
- 21.Zygmunt PM, Petersson J, Andersson DA, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400(6743):452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 22.Harris D, McCulloch AI, Kendall DA, Randall MD. Characterization of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. Journal of Physiology. 2002;539(3):893–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Sullivan SE, Kendall DA, Randall MD. Vascular effects of Δ9-tetrahydrocannabinol (THC), anandamide and N-arachidonoyldopamine (NADA) in the rat isolated aorta. European Journal of Pharmacology. 2005;507(1–3):211–221. doi: 10.1016/j.ejphar.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 24.Offertáler L, Mo F-M, Bátkai S, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Molecular Pharmacology. 2003;63(3):699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- 25.Hoi PM, Hiley CR. Vasorelaxant effects of oleamide in rat small mesenteric artery indicate action at a novel cannabinoid receptor. British Journal of Pharmacology. 2006;147(5):560–568. doi: 10.1038/sj.bjp.0706643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunnane SE, Chan YY, Randall MD. Rosiglitazone-induced vasorelaxation in the rat aorta. Proceedings of the British Pharmacological Society. 2004;2(2, abstract 096P) [Google Scholar]
- 27.Randall MD, Kendall DA, O'Sullivan SE. The complexities of the cardiovascular actions of cannabinoids. British Journal of Pharmacology. 2004;142(1):20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yano M, Matsumura T, Senokuchi T, et al. Statins activate peroxisome proliferator-activated receptor γ through extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent cyclooxygenase-2 expression in macrophages. Circulation Research. 2007;100(10):1442–1451. doi: 10.1161/01.RES.0000268411.49545.9c. [DOI] [PubMed] [Google Scholar]
- 29.Upham BL, Rummel AM, Carbone JM, et al. Cannabinoids inhibit gap junctional intercellular communication and activate ERK in a rat liver epithelial cell line. International Journal of Cancer. 2003;104(1):12–18. doi: 10.1002/ijc.10899. [DOI] [PubMed] [Google Scholar]
- 30.Demuth DG, Molleman A. Cannabinoid signalling. Life Sciences. 2006;78(6):549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 31.Bagi Z, Koller A, Kaley G. PPARγ activation, by reducing oxidative stress, increases NO bioavailability in coronary arterioles of mice with Type 2 diabetes. American Journal of Physiology. 2004;286(2):H742–H748. doi: 10.1152/ajpheart.00718.2003. [DOI] [PubMed] [Google Scholar]
- 32.Cho D-H, Choi YJ, Jo SA, Jo I. Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) γ-dependent and PPARγ-independent signaling pathways. The Journal of Biological Chemistry. 2004;279(4):2499–2506. doi: 10.1074/jbc.M309451200. [DOI] [PubMed] [Google Scholar]
- 33.Ling HY, Feng SD, Zhou SH, Wang BX, Liu XQ, Hu B. Effects of rosiglitazone on aortic function in rats with insulin resistant-hypertension. Sheng Li Xue Bao. 2005;57(2):125–131. [PubMed] [Google Scholar]
- 34.Majithiya JB, Paramar AN, Balaraman R. Pioglitazone, a PPARγ agonist, restores endothelial function in aorta of streptozotocin-induced diabetic rats. Cardiovascular Research. 2005;66(1):150–161. doi: 10.1016/j.cardiores.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 35.Bishop-Bailey D. Peroxisome proliferator-activated receptors in the cardiovascular system. British Journal of Pharmacology. 2000;129(5):823–834. doi: 10.1038/sj.bjp.0703149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsueh WA, Bruemmer D. Peroxisome proliferator-activated receptor γ: implications for cardiovascular disease. Hypertension. 2004;43(2):297–305. doi: 10.1161/01.HYP.0000113626.76571.5b. [DOI] [PubMed] [Google Scholar]
- 37.Duan SZ, Usher MG, Mortensen RM. Peroxisome proliferator-activated receptor-γ-mediated effects in the vasculature. Circulation Research. 2008;102(3):283–294. doi: 10.1161/CIRCRESAHA.107.164384. [DOI] [PubMed] [Google Scholar]
- 38.Underdown NJ, Hiley CR, Ford WR. Anandamide reduces infarct size in rat isolated hearts subjected to ischaemia-reperfusion by a novel cannabinoid mechanism. British Journal of Pharmacology. 2005;146(6):809–816. doi: 10.1038/sj.bjp.0706391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamontagne D, Lépicier P, Lagneux C, Bouchard JF. The endogenous cardiac cannabinoid system: a new protective mechanism against myocardial ischemia. Archives des Maladies du Coeur et des Vaisseaux. 2006;99(3):242–246. [PubMed] [Google Scholar]
- 40.Pacher P, Haskó G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. British Journal of Pharmacology. 2008;153(2):252–262. doi: 10.1038/sj.bjp.0707582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bátkai S, Pacher P, Osei-Hyiaman D, et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110(14):1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarzani R. Endocannabinoids, blood pressure and the human heart. Journal of Neuroendocrinology. 2008;20(supplement 1):58–62. doi: 10.1111/j.1365-2826.2008.01677.x. [DOI] [PubMed] [Google Scholar]
- 43.Mach F, Steffens S. The role of the endocannabinoid system in atherosclerosis. Journal of Neuroendocrinology. 2008;20(supplement 1):53–57. doi: 10.1111/j.1365-2826.2008.01685.x. [DOI] [PubMed] [Google Scholar]