Summary
Fulminant hepatic failure (FHF) is a devastating disease. Liver transplantation is the definitive treatment. However, a third of these patients die due to brain edema before a donor becomes available. Cerebrospinal fluid (CSF) drainage and decompressive craniectomy have been used to treat brain edema in brain trauma and hemispheric stroke. However, their role in brain edema associated with FHF has not been examined. In this study we evaluated the potential effects of CSF drainage and decompressive craniectomy on survival in FHF using an experimental model in rats. In CSF drainage experiments all animals had ventriculostomy placed. Five days later FHF was induced with d-galactosamine. Those FHF rats that progressed into comatose stages either received CSF aspiration or did not. In separate experiments the study rats had either a decompressive craniectomy or a sham procedure. FHF was induced 5 days later. We found that both CSF drainage and decompressive craniectomy significantly increased survival of FHF rats compared with the controls: 53.2 ± 1.1 vs. 48.7 ± 1.5 h (P = 0.031), and 69.4 ± 3.9 vs. 53.7 ± 3.2 h (P = 0.009), respectively. In conclusion, these findings suggest that CSF drainage and decompressive craniectomy may increase the window of opportunity for liver transplantation.
Keywords: brain edema, craniectomy, cerebrospinal fluid, liver failure
Introduction
Since 1970 brain edema has been recognized as a unique complication of fulminant hepatic failure (FHF) [1]. When the disease progresses into the comatose phases, brain edema occurs [1-3], changing FHF from being a severe disease to a lethal condition. Brain edema remains a major determinant of outcome in FHF.
Brain edema in FHF results from the increased permeability across the blood–brain barrier (BBB) [4,5]. Uncontrolled edema leads to brain herniation and death [6,7]. The clinical management has included head elevation, mechanical hyperventilation, administration of mannitol, use of ultrafiltration, the induction of thiopental coma, and recently hypothermia [8,9]. Each of these therapies has limited effects, and today liver transplantation is the definitive treatment for FHF [10-13]. However, the window of opportunity for a liver replacement is quite limited; those who died awaiting a donor liver died at a median waiting time of 5 days vs. those who underwent transplantation received their graft on median day 3 following the study admission. As 30% of FHF deaths are due to brain edema [14], it is critical to examine potential therapeutic options that effectively control the development and progression of brain edema to prolong the waiting period for a liver transplant.
Within the rigid skull, the total intracranial volume (ICV) is fixed. When the compliance or elastance of the brain parenchyma reaches its maximal state, a small change in ICV will result in an exponential change in intracranial pressure (ICP) [6,8]. In 1993, Thuomas et al. [15] examined the progression of brain herniation and correlated it with physiologic changes followed by dynamic magnetic resonance (MR) imaging. Compression and displacement of the ipsilateral ventricle was observed at the balloon volume of 2–3% of ICV. At 4–5%, transtentorial herniation occurred. When the balloon reached 8–10% of ICV, respiratory arrest occurred because of foramen magnum herniation [15]. Thus, a compensatory mechanism to protect against the development of brain herniation must provide at least 10% reduction or accommodation in ICV. In normal humans cranial cerebrospinal fluid (CSF) volume constitutes 11% of the total ICV [16]. Cranial CSF is composed of ventricular and extraventricular components. In the absence of anatomical anomaly or obstruction, CSF flows freely between the two cranial ventricular and extraventricular compartments. A successful cranial CSF removal may provide up to 11% of the total ICV to accommodate the swollen brain.
Ventricular CSF drainage remains an effective physiologic method to lower ICP. It is a key component in the standard management of brain edema associated with traumatic brain injury [17,18]. With aggressive CSF drainage to control ICP, the mortality is reduced and neurologic outcomes are improved [14,19,20]. Recently decompressive craniectomy has been used to control brain edema because of traumatic and ischemic brain injuries [21-25]. This method provides larger space volume than ventriculostomy. A craniectomy in a FHF patient would seem dangerous because of the underlying coagulopathy. However, the therapeutic potential of cranial CSF drainage via either ventriculostomy or decompressive craniectomy has not been previously evaluated in the settings of FHF. In this study a protocol was designed to examine the potential effects of CSF drainage with ventriculostomy and decompressive craniectomy on the survival outcomes in a rat model of experimental FHF. Our findings show that CSF drainage either by ventriculostomy or craniectomy prolongs survival in FHF rats.
Materials and methods
Animals
Male Sprague–Dawley rats, 200–300 g, were purchased from Harlan (Indianapolis, IN, USA). The rats were housed in a conventional rat room in 12-h light/dark cycles and were allowed free access to food and water. The use of animals in this investigation was institutionally approved according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Placement of ventriculostomy cannula for ICP measurement and CSF drainage
The upper skull was shaved and prepped in standard sterile fashion [26]. The head was positioned on a stereotactic guide instrument. The midline incision was made. The bregma was identified as the intersecting point of the midline and coronal suture lines. The cannulation hole, 0.8 mm caudal and 1.5 mm lateral to the bregma, was made with a dental drill. The ventriculostomy cannula (Single guide, Gauge 22, 4.8 mm tubing length below pedestal; Plastics One, Inc., Roanoke, VA, USA, Category No. C313G/Spc) was inserted vertically deep into the brain parenchyma. CSF was visualized egressing through the cannula, indicating a successful intraventricular cannulation. The catheter was cemented and secured and capped. The animal was allowed to recover and was kept in an individual cage for 5 days prior to the FHF study.
Decompressive craniectomy in rats
Decompressive craniectomy was performed as described previously [21]. The animals were anesthetized with Nembutal 60 mg/kg and maintained with inhalational 2% isoflurane. A bone flap (10 × 5 mm) was created in the bilateral temporal bone with a dental drill, and the bone was removed using a microscissor. The meningeal layer was incised but not removed. The cortical tissue was not violated. The temporalis muscle and skin were closed with continuous absorbable sutures. The sham controls received the same general anesthesia and skin flap exposure without a craniectomy. The animals were allowed to recover for 5 days prior to FHF study.
FHF induction in rats using d-galactosamine
This model resembles FHF in humans in all major aspects [27]. The overall mortality is 80% within 50 h from induction of d-galactosamine. This drug is specifically taken up by the liver, and by depleting the pool of uridine nucleotide it results in hepatocellular necrosis. There is no known direct effect of this agent on central nervous system (CNS) or BBB. d(+)-galactosamine has been most commonly used in rodents and dogs with reproducible clinical, biochemical, and histologic patterns. FHF was induced by intraperitoneally injecting a single dose of d(+)-galactosamine hydrochloride (CalBiochem, San Diego, CA, USA) of 2.5 g/kg of body weight [27,28]. Rats were kept separately with free access to food and sugar water (8 g table sugar/200 ml water). Each rat received 10 ml of D10% 1/4 NS subcutaneously every 6–8 h [29].
Clinically, FHF rats progressed through the four stages of encephalopathy [29] starting with stage I when the rats are less active than normal rats; stage II, the rats are lethargic and show ataxic movement; stage III, the rats are sleeping most of the time but maintain the reflexes of the extremities to tactile stimulus; stage IV, the rats show loss of extremity reflexes but maintain the corneal reflexes to soft cotton tip. The loss of corneal reflexes indicates brain herniation. Death of the animal inevitably ensued shortly following the loss of corneal reflexes [30]. Stages I and II are referred to as precoma, and stages III and IV as comatose.
ICP monitoring, blood pressure measurement, and CSF drainage
At the inception of stage III, ICP readings were monitored using BIOPAC Systems MP100 workstation. Tail mean arterial pressure (MAP; Model 20-NW Cuff Pump and Model 179 Blood Pressure Analyzer, Goleta, CA, USA) was measured. Body temperature was measured hourly using a rectal probe (digital dual channel thermometer, VWR M107766). Heat lamp and thermal pad were used to keep body temperature close to 37 °C. CSF was aspirated every 2 h using a 1-ml syringe, which has been shown effective for CSF removal.
BBB permeability assessed by Evans blue extravasation
Evans blue (EB) has been widely used to assess the increased BBB permeability [31]. Four ml/kg of a 2% (w/v) solution of EB were injected intravenously. The dye was allowed to circulate 60 min. The animals were killed with Nembutal overdose (100 mg/kg) followed by 1 ml of saturated KCl intravenously. The brains were removed and liver biopsies were performed.
Brain water determination using specific gravity method
This method has been widely used for determinations of brain water, i.e. brain edema [32,33]. The cerebral cortex and cerebellum were removed after killing and stored on an anhydrous tray at 4 °C to be processed within 30 min. The gray matter of the cerebral cortex and the cerebellum were cut into 2 mm slices. Then 1-mm3 punch biopsy specimens were obtained. These pieces were carefully placed in a bromobenze-kerosene density-gradient column to measure specific gravity, and the equilibrium positions were recorded after 2 min as previously reported [32,33]. The column was calibrated in advance with six potassium sulfate solutions of different concentrations with known specific gravities. All the gradients used were linear with correlation coefficients of ≥0.998. The conversions from specific gravity to brain water were performed as previously described [34]. Eight measurements were made per cerebral cortex and four measurements per cerebellum.
Statistical analysis
Results were expressed as mean ± SEM. Statistical comparisons were performed using the anova followed by t-test with a Bonferroni adjustment. The Kaplan–Meier cumulative survival analysis was performed using log-rank (Mantel-Cox) analysis. A P-value of <0.05 was considered statistically significant.
Results
FHF induction in rats
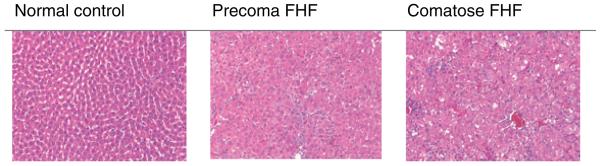
To reproduce the model of FHF in our laboratory, 27 rats were injected with d-galactosamine. Of these 27 rats, 23 d-galactosamine-treated rats progressed through the stages of encephalopathy. FHF-induced rats developed stage I of encephalopathy at 22.6 ± 5.2 h, stage II at 36.4 ± 6.7 h, stage III at 46.4 ± 4.5 h, and stage IV at 50.3 ± 6.6 h (Table 1). Four rats (15%) developed stages I and II, then they spontaneously recovered. Those rats that entered stage III, i.e. comatose stage, ultimately died. Thus, once the d-galactosamine-treated rats entered comatose stages, they had a mortality of 100%. Serum aminotransferase (ALT) levels increased with increasing severity of FHF (Table 1). Histologic sections of livers from the d-galactosamine-treated rats showed hepatocellular injury in the precoma FHF rats. The comatose FHF rats had significant necrosis and apoptosis of hepatocytes with parenchymal hemorrhage (Fig. 1). The saline-injected rats served as normal control and their liver histology was normal.
Table 1.
Experimental FHF in rats.
| Stage of encephalopathy | Time of onset (h) | Clinical manifestations | ALT (U/l) |
|---|---|---|---|
| 0 | 0 | Normal | 31 ± 0.3 |
| I | 22.6 ± 5.2 | Ataxic | 2334 ± 219 |
| II | 36.4 ± 6.7 | Lethargic | 3177 ± 134 |
| III | 46.4 ± 4.5 | Comatose, arousable, extremity reflexes intact | 3479 ± 200 |
| IV | 50.3 ± 6.6 | Comatose, loss of extremity reflexes, corneal reflexes intact | 3837 ±118 |
| Brain herniation | – | Loss of corneal reflexes | NA |
Following a single intraperitoneal injection with D-galactosamine, FHF rats progressed through the clinical stages of encephalopathy from I to IV and eventual death. ALT increased with increasing stages of FHF. The clinical stages and ALT levels correlated with the hepatocellular necrosis in liver histology.
FHF, fulminant hepatic failure; ALT, alanine aminotransferase; NA, not applicable.
Figure 1.
Liver histology of study rats. The liver section from the saline control rats was normal. Fulminant hepatic failure (FHF) rats from stages I to IV of encephalopathy showed increasing hepatocellular necrosis, parenchymal disruption, and hemorrhage. The extent of hepatocellular necrosis correlated with the serum transaminases and encephalopathy. Precoma indicates stages I and II; comatose FHF indicates stages III and IV of encephalopathy.
Increased brain EB extravasation, brain water, and ICP in FHF rats
To demonstrate the presence of increased BBB permeability, EB dye was used. Brains of comatose FHF rats had marked blue staining (Fig. 2). Control rats did not have any EB staining. The precoma FHF rats had minimal staining (not shown). The marked staining in the comatose FHF brains confirmed the increased BBB permeability to small circulating molecules, such as the EB and water. These results agreed with previous reports [27,35]. Accordingly, the FHF rats showed increases of 3.0% and 4.4% of brain water in the cerebrum and cerebellum, respectively, compared with the control rats (Table 2). These increases are consistent with previous report [4]. Consequently, the comatose FHF rats had significantly higher ICP above the precoma FHF rats (9.3 ± 2.0 vs. 3.6 ± 0.1 mmHg, P = 0.007; N = 5).
Figure 2.
Increased brain extravasation in fulminant hepatic failure (FHF). Evans blue (EB) was used to illustrate the increased blood–brain barrier (BBB) permeability, i.e. increased brain extravasation, in FHF rats. The brain from a control rat had trace EB staining. In contrast, the comatose FHF rat brain showed marked blue staining, indicating increased extravasation of small molecules including EB dye, water, and ammonia. The precoma FHF brain had minimal staining (not shown).
Table 2.
Increased brain water in FHF rats.
| Brain water (%) | Control rats | FHF rats | Net water increase (%) |
|---|---|---|---|
| Cortex | 77.7 ± 0.2 | 80.7 ± 0.2 | 3.0 ± 0.2** |
| Cerebellum | 78.8 ± 0.1 | 83.2 ± 0.1 | 4.4 ± 0.1** |
| Number of animals | 5 | 6 |
Consistent with increased BBB permeability, FHF rats had significant increase in brain water in the cortex and cerebellum.
FHF, fulminant hepatic failure; BBB, blood–brain barrier.
P < 0.001.
CSF drainage prolonged the survival in FHF-induced rats
To determine the effect of CSF drainage, the study rats had a ventriculostomy placed for CSF aspiration. Five days later FHF was induced following the injection of d-galactosamine. A total of 33 FHF-induced rats progressed into the comatose stages (Table 3). Thirteen received CSF drainage by syringe aspiration; 19 did not. The volumes of CSF removed varied from 80 to 1410 μl (mean: 351 ± 119 μl). Figure 3 illustrates gradual reductions of ICP following successive CSF aspirations in a FHF rat. In a rat of about 300 g of body weight, the cranial volume of CSF is about 250 μl [36]. Thus, overall the CSF aspirations achieved in this study were from a one-third to five times the cranial CSF volume. However, there was no correlation between the survival and the volume of aspirated CSF (not shown). There was no difference in MAP and blood glucose between the two study groups. The FHF rats that received CSF drainage had higher ICP (9.95 ± 2.15 vs. 6.28 ± 0.8 mmHg, P = 0.053). Yet, the FHF rats that were treated with CSF drainage had a significant prolongation of survival time compared with those FHF rats that had no CSF drainage (Fig. 4; P = 0.019). The FHF rats that received CSF drainage had an increase in the mean survival of 4 h (53.2 ± 1.1 vs. 48.7 ± 1.5, P = 0.031).
Table 3.
Physiologic parameters of the study rats.
| FHF without CSF drainage (n = 19) |
FHF with CSF drainage (n = 14) |
|
|---|---|---|
| MAP (mmHg) | 122 ± 5 (4) | 118 ± 5 (4) |
| Serum glucose (mg/dl) | 157 ± 30 (10) | 128 ± 26 (10) |
| CSF volume (μl) | None | 351 ± 119 (12) |
| ICP (mmHg) | 6.2 ± 0.8 (18) | 9.95 ± 2.15 (6)** |
Ventriculostomy was placed 5 days prior to FHF induction. Among the 33 FHF rats that progressed into comatose stages, 19 received CSF drainage via syringe aspiration, and 14 did not. There was no difference in MAP and serum glucose between the two study groups. The initial ICP readings showed a trend of higher readings in the CSF drainage group (P = 0.053). The numbers in parentheses indicate the number of animals in which the measurement was made.
FHF, fulminant hepatic failure; CSF, cerebrospinal fluid; MAP, mean arterial pressure; ICP, intracranial pressure.
Figure 3.
Serial cerebrospinal fluid (CSF) aspirations resulted in reduction of intracranial pressure (ICP) in a fulminant hepatic failure (FHF) rat.
Figure 4.
Kaplan–Meier cumulative survival in fulminant hepatic failure (FHF) rats with cerebrospinal fluid (CSF) drainage. Both groups of the study animals had ventriculostomy placement 5 days prior to FHF induction with d-galactosamine. CSF drainage was carried out with 1-ml syringe starting at the inception of comatose stages. Those FHF rats that received CSF aspiration showed a prolonged survival (P = 0.019).
Decompressive craniectomy increased FHF rat survival
To evaluate the effect of decompressive craniectomy on survival of FHF rats, nine FHF rats had craniectomy and eight had sham procedure. Five days later FHF was induced. The animals were clinically monitored from the time of FHF induction to the time of death. As illustrated in Fig. 5, the craniectomized FHF rats had a significantly increased survival when compared with the FHF sham controls (P = 0.014). The mean survival among the craniectomized FHF rats was 69.4 ± 3.9 h when compared with 53.7 ± 3.2 h among sham FHF animals (P = 0.009). The liver histology showed the same degree of hepatocellular necrosis in both groups of the study animals.
Figure 5.
Kaplan–Meier cumulative survival of craniectomized fulminant hepatic failure (FHF) rats. The rats had craniectomy or sham procedure 5 days prior to the induction of FHF with d-galactosamine. The survival time from the induction to the time of death was recorded. FHF rats with craniectomy had a significant increase in survival when compared with the sham controls (P = 0.014).
Discussion
Brain swelling in FHF occurs in the comatose stages and is a major cause of death among FHF patients [4]. In this study we have demonstrated that providing additional space volume for the swollen brain could prolong the survival of FHF animals. This concept was developed by Harvey Cushing [37] in the early 1900s. Dr Cushing [37] demonstrated that a decompressive craniectomy was effective to relieve the ICP due to intracranial masses. Recently decompressive craniectomy has been employed in the clinical management of patients who experience a large hemispheric stroke, which carries mortality as much as 80% [38] in rats [39] and humans [40,41]. These results underscore the principle that if adequate additional ICV is timely provided to compensate for the swelling because of brain edema, the lethal complications of brain compression and herniation may be ameliorated.
Fulminant hepatic failure is a complex medical condition. Many FHF patients have multiorgan failure [42]. Thus, general anesthesia needed for a surgical procedure in these comatose subjects can be lethal, particularly in animals. The insertion of an ICP catheter or decompressive craniectomy during the advanced stages of FHF can result in high mortality and morbidity. The surgical interventions would result in intracranial hemorrhage that is potentially difficult to control due to underlying coagulopathy. For these reasons, the insertion of an ICP catheter and the decompressive craniectomy were performed 5 days prior to our experimental study to avoid the potential confounding factors.
Craniectomy provides a larger space volume for the brain and effectively reduces ICP [43]. As it is clinically known that ICP is significantly reduced by decompressive craniectomy [23,44], we did not measure the ICP in the craniectomized animals. Nevertheless, the mean survival in craniectomized FHF rats was 15 h beyond that of the sham FHF animals. This is probably the longest improvement in survival observed in experimental FHF. Early works with an inhibitor of glutamate synthetase prolonged the survival in FHF animals for 3.7 h [45]. Hypothermia delayed the development of comatose stages for 5 h, as it reduced brain edema formation [46]. However, the survival was not reported [46].
Previous studies have demonstrated that edema in the brain resolves by clearing the excess fluid into CSF [47,48]. The CSF component was shown to contribute one-third of the ICP increase in brain edema in severe head injury [49]. Using the model of acute dilutional hyponatremia in rats, Melton and Nattie showed that it is the CSF compartment that provides the required space volume to accommodate the swollen brain [36]. In addition, ventricular CSF drainage remains the most effective and rapid physiologic method to lower ICP. Thus, CSF drainage may provide a mechanism to control the progression of cerebral edema. Our results showed that CSF drainage in FHF rats prolonged the median survival for 4 h. However, as the skull is intact, the effectiveness of CSF drainage must be limited. As reflected in our data, once the CSF limit is reached, the death rate in the treated FHF rats is the same as those FHF rats without CSF drainage.
Liver transplantation has evolved, becoming the definitive treatment of this disease. However, the window of opportunity is very limited. In the face of organ shortage, many of these patients die before availability of a donor. In the large series reviewed by Dr William Lee, who leads the Acute Liver Failure Study Group in the USA, the median difference between those FHF patients who receive a liver transplant versus those who die without one is only 48 h [50]. The same window of time has been observed in United Kingdom [51]. Expanding this window by appropriate interventions will increase the number of patients with FHF able to receive the definitive liver transplant. Yet, the gain in survival in FHF rats that had CSF drainage and decompressive craniectomy is modest, 4 and 15 h respectively. It remains, however, to determine the exact translation of a survival advantage in small animals to humans. It should be noted that there has been evidence that an increase in survival measured in days in mice may result into years of survival in humans. Therefore, we believe that the small increase in survival in our animal study deserves further investigations for this lethal disease with few therapeutic options.
There are several additional limitations in our study. First, the pre-existing ventriculostomy and craniectomy limit the interpretations of the results in correlation with clinical management of FHF. We did not see a correlation of the CSF volume to the advantage in the survival rate. This is probably a direct result of the ventriculostomy being carried out prior to the experimental induction of FHF. Although it would be ideal to carry out the ventriculostomy during the comatose stages of the FHF animals, the associated mortality would confound the outcomes of the current study objective. Secondly, in our experimental condition, the complexity in the management of FHF is not addressed [42]. Sepsis is a major complication and challenge in patients with FHF. However, it is clear from our clinical experience with FHF patients at our medical center that infectious complications associated with the placement of ventriculostomy have been minimal without a death [52]. Therefore, we do not believe that infection would have played a major role in the experimental results reported in this study. Thirdly, although not seen in this study, the possibility of reverse herniation associated with ventriculostomy could not be ruled out.
Although the use of decompressive craniectomy is dangerous in FHF due to the severe coagulopathy, there is a report of decompressive craniectomy in a FHF patient with Reye's syndrome in 1976 [53]. In this report the coagulopathy was constantly normalized with exchange transfusion, and decompressive craniectomy was performed, allowing a successful outcome of the patient. A more recent report demonstrates a case in which a bifrontal decompressive craniectomy is performed on a patient with posttraumatic acute liver dysfunction [54]. The avoidance of the lethal complication of brain edema allows the patient to recover neurologically as the liver recovers [54]. These reports support a potential role of brain decompression in the clinical management of brain edema in FHF.
In conclusion, our results provide evidence that both ventriculostomy and decompressive craniectomy can serve as a bridge to transplantation. Although the craniectomy provides a longer survival advantage (15 vs. 4 h) over the former method, its clinical application is limited by the underlying severe coagulopathy. With recent advances in transfusion medicine, decompressive craniectomy may deserve further consideration and investigation. Expanding the window of opportunity will allow a FHF patient to timely receive a life-saving liver transplant or allow enough time for the native liver to recover.
Acknowledgments
The authors wish to thank Dr James Meschia for his critical review of the manuscript and Ms Kathleen Norton for editorial assistance.
Part of this work was supported by a grant from National Institute of Health DK064361 (JHN) and the Deason Foundation.
References
- 1.Ware AJ, D'Agostino AN, Combes B. Cerebral edema: a major complication of massive hepatic necrosis. Gastroenterology. 1971;61:877. [PubMed] [Google Scholar]
- 2.Silk DB, Trewby PN, Chase RA, et al. Treatment of fulminant hepatic failure by polyacrylonitrile-membrane haemodialysis. Lancet. 1977;2:1. doi: 10.1016/s0140-6736(77)90001-0. [DOI] [PubMed] [Google Scholar]
- 3.Canalese J, Gimson AE, Davis C, Mellon PJ, Davis M, Williams R. Controlled trial of dexamethasone and mannitol for the cerebral oedema of fulminant hepatic failure. Gut. 1982;23:625. doi: 10.1136/gut.23.7.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blei AT. Pathogenesis of brain edema in fulminant hepatic failure. Prog Liver Dis. 1995;13:311. [PubMed] [Google Scholar]
- 5.Blei AT, Larsen FS. Pathophysiology of cerebral edema in fulminant hepatic failure. J Hepatol. 1999;31:771. doi: 10.1016/s0168-8278(99)80361-4. [DOI] [PubMed] [Google Scholar]
- 6.Cordoba J, Blei AT. Cerebral edema and intracranial pressure monitoring. Liver Transpl Surg. 1995;1:187. doi: 10.1002/lt.500010310. [DOI] [PubMed] [Google Scholar]
- 7.Alper G, Jarjour IT, Reyes JD, Towbin RB, Hirsch WL, Bergman I. Outcome of children with cerebral edema caused by fulminant hepatic failure. Pediatr Neurol. 1998;18:299. doi: 10.1016/s0887-8994(97)00218-x. [DOI] [PubMed] [Google Scholar]
- 8.Bingaman WE, Frank JI. Malignant cerebral edema and intracranial hypertension. Neurol Clin. 1995;13:479. [PubMed] [Google Scholar]
- 9.Bernal W, Wendon J. Acute liver failure; clinical features and management. Eur J Gastroenterol Hepatol. 1999;11:977. doi: 10.1097/00042737-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Ascher NL, Lake JR, Emond JC, Roberts JP. Liver transplantation for fulminant hepatic failure. Arch Surg. 1993;128:677. doi: 10.1001/archsurg.1993.01420180079015. [DOI] [PubMed] [Google Scholar]
- 11.Hoofnagle JH, Carithers RL, Jr, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21:240. [PubMed] [Google Scholar]
- 12.Bismuth H, Samuel D, Castaing D, et al. Orthotopic liver transplantation in fulminant and subfulminant hepatitis. The Paul Brousse experience. Ann Surg. 1995;222:109. doi: 10.1097/00000658-199508000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goss JA, Shackleton CR, Maggard M, et al. Liver transplantation for fulminant hepatic failure in the pediatric patient. Arch Surg. 1998;133:839. doi: 10.1001/archsurg.133.8.839. [DOI] [PubMed] [Google Scholar]
- 14.Greenwald BM, Ghajar J, Notterman DA. Critical care of children with acute brain injury. Adv Pediatr. 1995;42:47. [PubMed] [Google Scholar]
- 15.Thuomas KA, Vlajkovic S, Ganz JC, et al. Progressive brain compression. Changes in vital physiological variables, correlated with brain tissue water content and brain tissue displacement. Experimental MR imaging in dogs. Acta Radiol. 1993;34:289. [PubMed] [Google Scholar]
- 16.Matsumae M, Kikinis R, Morocz IA, et al. Age-related changes in intracranial compartment volumes in normal adults assessed by magnetic resonance imaging. J Neurosurg. 1996;84:982. doi: 10.3171/jns.1996.84.6.0982. [DOI] [PubMed] [Google Scholar]
- 17.Chestnut RM. Evaluation and management of severe closed head injury. In: Tindall GT, Cooper RR, Barrow DL, editors. The Practice of Neurosurgery. 1st edn. Williams & Wilkins Electronic; Baltimore, MD, USA: 1996. pp. 1401–1424. [Google Scholar]
- 18.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care Intracranial pressure treatment threshold. J Neurotrauma. 2000;17:493. doi: 10.1089/neu.2000.17.493. [DOI] [PubMed] [Google Scholar]
- 19.Rosner MJ, Rosner SD, Johnson AH. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg. 1995;83:949. doi: 10.3171/jns.1995.83.6.0949. [DOI] [PubMed] [Google Scholar]
- 20.Lane PL, Skoretz TG, Doig G, Girotti MJ. Intracranial pressure monitoring and outcomes after traumatic brain injury. Can J Surg. 2000;43:442. [PMC free article] [PubMed] [Google Scholar]
- 21.Doerfler A, Forsting M, Reith W, et al. Decompressive craniectomy in a rat model of ‘alignant’ cerebral hemispheric stroke: experimental support for an aggressive therapeutic approach. J Neurosurg. 1996;85:853. doi: 10.3171/jns.1996.85.5.0853. [DOI] [PubMed] [Google Scholar]
- 22.Steiner T, Ringleb P, Hacke W. Treatment options for large hemispheric stroke. Neurology. 2001;57(5 Suppl 2):S61. doi: 10.1212/wnl.57.suppl_2.s61. [DOI] [PubMed] [Google Scholar]
- 23.Schneider GH, Bardt T, Lanksch WR, Unterberg A. Decompressive craniectomy following traumatic brain injury: ICP, CPP and neurological outcome. Acta Neurochir Suppl. 2002;81:77. doi: 10.1007/978-3-7091-6738-0_20. [DOI] [PubMed] [Google Scholar]
- 24.Figaji AA, Fieggen AG, Peter JC. Early decompressive craniotomy in children with severe traumatic brain injury. Childs Nerv Syst. 2003;19:666. doi: 10.1007/s00381-003-0804-3. [DOI] [PubMed] [Google Scholar]
- 25.Albanese J, Leone M, Alliez JR, et al. Decompressive craniectomy for severe traumatic brain injury: evaluation of the effects at one year. Crit Care Med. 2003;31:2535. doi: 10.1097/01.CCM.0000089927.67396.F3. [DOI] [PubMed] [Google Scholar]
- 26.Brakkee JH, Wiegant VM, Gispen WH. A simple technique for rapid implantation of a permanent cannula into the rat brain ventricular system. Lab Anim Sci. 1979;29:78. [PubMed] [Google Scholar]
- 27.Dixit V, Chang TM. Brain edema and the blood brain barrier in galactosamine-induced fulminant hepatic failure rats. An animal model for evaluation of liver support systems. ASAIO Trans. 1990;36:21. [PubMed] [Google Scholar]
- 28.Gove CD, Hughes RD, Ede RJ, Williams R. Regional cerebral edema and chloride space in galactosamine-induced liver failure in rats. Hepatology. 1997;25:295. doi: 10.1002/hep.510250207. [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann C, Ferenci P, Pifl C, et al. Hepatic encephalopathy in thioacetamide-induced acute liver failure in rats: characterization of an improved model and study of amino acid-ergic neurotransmission. Hepatology. 1989;9:594. doi: 10.1002/hep.1840090414. [DOI] [PubMed] [Google Scholar]
- 30.Webster S, Gottstein J, Levy R, Blei AT. Intracranial pressure waves and intracranial hypertension in rats with ischemic fulminant hepatic failure. Hepatology. 1991;14(4 Pt 1):715. doi: 10.1016/0270-9139(91)90063-2. [DOI] [PubMed] [Google Scholar]
- 31.Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 1988;8:282. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- 32.Marmarou A, Poll W, Shulman K, Bhagavan H. A simple gravimetric technique for measurement of cerebral edema. J Neurosurg. 1978;49:530. doi: 10.3171/jns.1978.49.4.0530. [DOI] [PubMed] [Google Scholar]
- 33.Larsen FS, Gottstein J, Blei AT. Cerebral hyperemia and nitric oxide synthase in rats with ammonia-induced brain edema. J Hepatol. 2001;34:548. doi: 10.1016/s0168-8278(00)00069-6. [DOI] [PubMed] [Google Scholar]
- 34.Traber PG, Ganger DR, Blei AT. Brain edema in rabbits with galactosamine-induced fulminant hepatitis. Regional differences and effects on intracranial pressure. Gastroenterology. 1986;91:1347. doi: 10.1016/0016-5085(86)90186-1. [DOI] [PubMed] [Google Scholar]
- 35.Livingstone AS, Potvin M, Goresky CA, Finlayson MH, Hinchey EJ. Changes in the blood-brain barrier in hepatic coma after hepatectomy in the rat. Gastroenterology. 1977;73(4 Pt 1):697. [PubMed] [Google Scholar]
- 36.Melton JE, Nattie EE. Intracranial volume adjustments and cerebrospinal fluid pressure in the osmotically swollen rat brain. Am J Physiol. 1984;246(4 Pt 2):R533. doi: 10.1152/ajpregu.1984.246.4.R533. [DOI] [PubMed] [Google Scholar]
- 37.Cushing H. The establishment of cerebral hernia as a decompressive measure for inaccessible brain tumors; with the description of intermuscular methods of making bone defect in temporal and occipital regions. Surg Gynecol Obstet. 1905;1:297. [Google Scholar]
- 38.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 39.Forsting M, Reith W, Schabitz WR, et al. Decompressive craniectomy for cerebral infarction. An experimental study in rats. Stroke. 1995;26:259. doi: 10.1161/01.str.26.2.259. [DOI] [PubMed] [Google Scholar]
- 40.Rieke K, Schwab S, Krieger D, et al. Decompressive surgery in space-occupying hemispheric infarction: results of an open, prospective trial. Crit Care Med. 1995;23:1576. doi: 10.1097/00003246-199509000-00019. [DOI] [PubMed] [Google Scholar]
- 41.Schwab S, Steiner T, Aschoff A, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998;29:1888. doi: 10.1161/01.str.29.9.1888. [DOI] [PubMed] [Google Scholar]
- 42.McGuire BM. The critically ill liver patient: fulminant hepatic failure. Semin Gastrointest Dis. 2003;14:39. [PubMed] [Google Scholar]
- 43.Jaeger M, Soehle M, Meixensberger J. Effects of decompressive craniectomy on brain tissue oxygen in patients with intracranial hypertension. J Neurol Neurosurg Psychiatry. 2003;74:513. doi: 10.1136/jnnp.74.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo DS, Kim DS, Cho KS, Huh PW, Park CK, Kang JK. Ventricular pressure monitoring during bilateral decompression with dural expansion. J Neurosurg. 1999;91:953. doi: 10.3171/jns.1999.91.6.0953. [DOI] [PubMed] [Google Scholar]
- 45.Rose C, Michalak A, Rao KV, Quack G, Kircheis G, Butterworth RF. L-ornithine-L-aspartate lowers plasma and cerebrospinal fluid ammonia and prevents brain edema in rats with acute liver failure. Hepatology. 1999;30:636. doi: 10.1002/hep.510300311. [DOI] [PubMed] [Google Scholar]
- 46.Rose C, Michalak A, Pannunzio M, Chatauret N, Rambaldi A, Butterworth RF. Mild hypothermia delays the onset of coma and prevents brain edema and extracellular brain glutamate accumulation in rats with acute liver failure. Hepatology. 2000;31:872. doi: 10.1053/he.2000.5923. [DOI] [PubMed] [Google Scholar]
- 47.Reulen HJ, Tsuyumu M, Tack A, Fenske AR, Prioleau GR. Clearance of edema fluid into cerebrospinal fluid. A mechanism for resolution of vasogenic brain edema. J Neurosurg. 1978;48:754. doi: 10.3171/jns.1978.48.5.0754. [DOI] [PubMed] [Google Scholar]
- 48.Marmarou A, Hochwald G, Nakamura T, Tanaka K, Weaver J, Dunbar J. Brain edema resolution by CSF pathways and brain vasculature in cats. Am J Physiol. 1994;267(2 Pt 2):H514. doi: 10.1152/ajpheart.1994.267.2.H514. [DOI] [PubMed] [Google Scholar]
- 49.Marmarou A, Maset AL, Ward JD, et al. Contribution of CSF and vascular factors to elevation of ICP in severely head-injured patients. J Neurosurg. 1987;66:883. doi: 10.3171/jns.1987.66.6.0883. [DOI] [PubMed] [Google Scholar]
- 50.Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 51.Riordan SM, Williams R. Cause and prognosis in acute liver failure. Liver Transpl Surg. 1999;5:86. doi: 10.1002/lt.500050107. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen JH, Wharen R, Kramer D, et al. Intracranial ventriculostomy is safe in the management of cerebral edema in patients with acute liver failure. Liver Transpl. 2002;8:C-52. Abstract 207. [Google Scholar]
- 53.Ausman JI, Rogers C, Sharp HL. Decompressive craniectomy for the encephalopathy of Reye's syndrome. Surg Neurol. 1976;6:97. [PubMed] [Google Scholar]
- 54.Mussack T, Huber SM, Ladurner R, Hummel T, Mutschler W. Bilateral decompressive craniectomy due to intracranial hypertension during acute posttraumatic liver dysfunction. J Trauma. 2005;58:1061. doi: 10.1097/01.ta.0000171989.63817.8c. [DOI] [PubMed] [Google Scholar]