Abstract
Matrix metalloproteinases (MMPs) modulate development, inflammation, and repair in lungs. Tissue inhibitors of MMPs (TIMPs) interact with MMPs, controlling the intensity and nature of the response to injury. Absence of MMP-9, -2, and -8 activities is associated with altered lung inflammation during allergic sensitization. To test the hypothesis that the absence of TIMP-1 enhances allergic lung inflammation, airway hyperreactivity (AHR), and lung remodeling in asthma, we studied TIMP-1 null (TIMP-1 KO) mice and their WT controls using an ovalbumin (OVA) asthma model. TIMP-1 KO mice, compared to WT controls, developed an asthma phenotype characterized by AHR, pronounced cellular lung infiltrates, greater reduction in lung compliance, enhanced Th2 cytokine mRNA and protein expression, and altered collagen lung content associated with enhanced MMP-9 activity. Our findings support the hypothesis that TIMP-1 plays a protective role by preventing AHR and modulating inflammation, remodeling, and cytokine expression in an animal model of asthma.
Keywords: Asthma, Bronchial hyperreactivity, TIMP-1, MMPs, Airway remodeling, Intercellular matrix
Introduction
Airway inflammation, reactivity, and remodeling in asthma depend upon many interrelated processes. There is increasing recognition that the lung matrix plays an active role in the inflammatory response and resultant remodeling in asthma. It has been shown that within the lung intercellular matrix, matrix metalloproteinases (MMPs; specifically MMP-2, MMP-8, MMP-9, and MMP-12) and tissue inhibitors of metalloproteinases (TIMPs; specifically TIMP-1 and TIMP-2) modulate the nature and outcome of inflammation, namely healing versus pathologic remodeling [1,2].
Thus far, attention has focused primarily on the role of MMPs in asthma with less attention given to their intrinsic inhibitors, TIMPs. However, due to the complexity of MMP/TIMP interactions, the precise roles of TIMPs cannot be fully understood by studying their targets alone (MMPs). Additionally, TIMP-1 is recognized as having biologic effects independent of MMPs, for example producing alterations in cellular apoptosis and sex hormone steroidogenesis [3,4].
Studies of MMPs have partially elucidated a role for MMP-9 in airway inflammation and hyperreactivity in animal models of ovalbumin (OVA)-induced asthma. Reports suggest that inhibition [5] or genetic knockout of MMP-9 [6] results in an asthma phenotype. However this has not been universally observed [7,8].
Additionally, MMP-2 appears to play a more defined role in lung inflammation through cell trafficking. Corry et al. observed that in MMP-2 KO mice sensitized with OVA and Aspergillus develop intense parenchymal injury with impaired cell trafficking into the airspace [9], suggesting that TIMP-1 targeted MMPs serve important and interrelated functions [7].
Furthermore, examination of ratios of MMPs to TIMPs (TIMP-1 or TIMP-2) [1,10,11–13] has provided insight into the importance of interrelationships between MMPs and TIMPs. Specifically, in individuals with asthma, the MMP-9/TIMP-1 ratio has been shown to be elevated when compared with non-asthmatics [14].
Studies of TIMP-1, which specifically modulates the activities of MMP-2, MMP-9 and MMP-12, have demonstrated an increase in TIMP-1 protein in human asthma [15], in human and animal models of idiopathic pulmonary fibrosis [16,17], and in lung injury associated with adult respiratory distress syndrome (ARDS) [18]. Finally, TIMP-1 polymorphisms have recently been associated, through linkage analyses, with airway hyperreactivity and asthma in Australian women [19]. Taken together, these observations suggest an important role for TIMP-1 in allergic lung inflammation.
In order to better define the role of TIMP-1 in asthma, we utilized TIMP-1 KO mice in a murine asthma model. We hypothesized that TIMP-1 deficiency would result in an asthmatic phenotype by creating a ‘permissive’ environment wherein target MMP activity would be enhanced. Our results support this hypothesis by demonstrating that TIMP-1 deficient mice developed altered lung mechanics, particularly increased airway reactivity, increased lung inflammation (cellular infiltration), altered cytokine gene expression, and enhanced Th2 cytokine expression in response to allergic (OVA) sensitization, compared to isogenic WT littermate controls. Some of the results of these studies have been previously reported in abstract form [20,21].
Methods
Experimental animals and genotyping
Homozygous C57/BL6 TIMP-1 null mice (TIMP-1 KO) [22–24] and wild-type (WT) backcross littermates of both sexes were utilized. Genotype was confirmed by PCR as previously described [22,25]. Animal protocols complied with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by Institutional Animal Care and Use Committees of the University at Buffalo and Veterans Administration Health Care System of Western New York.
Experimental protocol
On days 0 and 14, TIMP-1 KO and WT mice (ages 6–8 weeks) were sensitized by 200 µl intraperitoneal (i.p.) injection with 10 µg chicken OVA (Grade III, Sigma, St. Louis, MO) and 1 mg alum adjuvant (AlK[SO]4[H2O]12) emulsified in sterile phosphate buffered saline (PBS) (OVA groups). SHAM mice received OVA-free injections. On day 21, mice were challenged with 30 ml of aerosolized 1% (wt/vol) OVA or PBS (SHAM group) for 30 min on 7 consecutive days using an ultrasonic nebulizer [26]. On day 29 mice were anesthetized with intraperitoneal pentobarbital, and lung mechanics measured during methacholine (MCh) challenge. Following euthanasia, lungs were harvested for histopathology, protein, and gene expression analyses. On days 0 and 28, blood was drawn for IgE analysis to confirm OVA sensitization. Serum OVA-specific IgE was measured by ELISA. Lung tissue for collagen, zymography and lung cytokines was obtained from mice anesthetized with halothane and exsanguinated via the inferior vena cava on day 28 or 29.
Measurement of lung mechanics
Lung mechanics were measured by plethysmography (Buxco Technologies, Troy, NY) to characterize the physiologic response to OVA sensitization between groups. Mice were anesthetized and instrumented for mechanical ventilation for administration of a methacholine challenge. Respiratory system resistance (Rrs) and dynamic lung compliance (Cdyn) were recorded and dose–response curves were constructed.
Histological analysis of lung tissue
Inflammatory and remodeling responses to OVA sensitization were evaluated by lung histopathologic analyses. Lung orientation for cutting and paraffin embedding was adapted from Ellis [27] with modification to optimize visualization of relatively uniform sizes of cross cut bronchi for quantitative analysis. Sections were processed and stained with H and E or Masson Trichrome for light microscopic examination of cellular infiltrates and collagen respectively. A pathologist (YVF), blinded to experimental group, determined inflammation scores for 10 bronchi (100–150 µm diameter) per mouse for 20 mice in each experimental group. Inflammation was graded 0 to V as described by Tourney [28] with modifications as follows: Grade 0 — no inflammation; Grade I — occasional bronchial cuffing with inflammatory cells; Grade II —>50% of bronchi surrounded by a thin layer (1–4) of mononuclear or eosinophilic cells; Grade III — >50% of bronchi surrounded by a thick layer (5–8) of mononuclear or eosinophilic cells; Grade IV — >50% of bronchi surrounded by a layer of >9 cells; Grade V–Grade IV with prominent lymphoid aggregates and interstitial infiltration.
Differential and total cell counts were determined for SHAM- and OVA-treated groups. At least 20 high power fields (HPF) per experimental group were sampled, generating a differential cell count of ≥1000 cells (OVA-treated groups), and ≥200 cells (SHAM-treated groups). Eosinophils (Eos), lymphocytes (Lymphs), histiocytes, polymorphonuclear cells (PMNs), and plasma cells were identified on H and E stains.
Measurement of OVA-specific IgE Antibody by ELISA
On days 0 and 28, blood was drawn for IgE analysis to confirm OVA sensitization. Serum levels of OVA-specific murine IgE were measured by ELISA using a semi-quantitative sandwich ELISA. Briefly, 100 µl/well solution of 1 mg/ml OVA (Sigma Grade III) stock was coated on to a 96-well plate and incubated overnight at 4 °C. Standard curves ranging from 0–30,000 ng/ml were generated using murine purified commercial IgE (BD Pharmingen, San Diego CA; cat # 557079). The biological controls and samples were appropriately diluted and used in a range of dilutions (1:10, 1:50 and 1:100). All samples and standards were added to the OVA coated plates and incubated overnight. Plates were washed, incubated for an additional 1 h at room temperature with a 100 µl/well solution of 1:250 biotinylated anti-mouse antibody (BD Pharmingen, San Diego CA; cat # 553419) in blocking buffer (0.5% BSA). A 1:1000 dilution of avidin–horseradish peroxidase (BD Pharmingen, San Diego CA; Cat # 554058) in blocking buffer was subsequently added, that binds to the biotinylated anti-mouse antibody. This was followed by color development using the ABTS substrate (Sigma Chemical Co; St Louis, MO) and spectrophotometric quantitation at 405 nm. To minimize inter- and intra-assay variability pooled samples were used as biological controls. The OVA-specific IgE titers of the samples were expressed as ELISA units (EU)/ml.
Hydroxyproline assay
Quantitative hydroxyproline assay of lungs (n=10 per group) was performed on protocol day 29 as an indicator of collagen content. A modification of previously described methods was utilized [29].
RNA extraction and quantitative real-time polymerase chain reaction (QPCR)
Lung gene expression was examined to determine differential immunologic responses to OVA sensitization between genotypes. Lung tissue (entire lung) was placed in RNAlater (Ambion, Austin, TX) and stored at −20 °C. Cytoplasmic RNA was extracted using an acid guanidinium-thiocyanate–phenol–chloroform method [30]. RNA was reverse transcribed to cDNA and relative abundance of each mRNA species assessed using QPCR. All data were controlled by performing measurements on the endogenous reference gene β-actin [31].
Lung cytokine analyses
Lungs were harvested on protocol days 28 or 29 (24 and 48 h) after the seventh day of lung aerosol delivery of either saline (sham groups) or OVA (sensitized groups). Lung homogenates from 109 mice were assayed by ELISA for cytokine concentration (IL-1β, TNF-α, IL-2, IL-12, MCP-1, IFN-γ, MIP-1α, MIP-2, IL-4, IL-5, IL-6, IL-10, IL-13, RANTES, and eotaxin) as previously described in this laboratory. [32,33] Cytokine concentrations in lung homogenates were determined by ELISA using matched antibody pairs and protein standards (R & D Systems, Inc., Minneapolis, MN). Briefly, 96-well plates were coated with the capture antibody and incubated overnight at 4 °C. After washing, the plates were coated with the appropriate blocker (Pierce Biotechnology, Inc., Rockford, IL) and incubated for 1 h at room temperature. Blocker was removed by washing, and dilutions of standards and samples were added. Plates were incubated at room temperature for 2 h, and then washed. The detection antibody was added to the wells and incubated for 2 h. The plates were washed, and Streptavidin Perioxidase (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was added to the wells. After 30 min incubation, the plates were washed, and a solution of tetramethylbenzidine (™B, Sigma, St. Louis, MO) was added. After an additional 30 min incubation in the dark, the color reaction was stopped by addition of 2 N H2SO4. Absorbance was read at 465 nm (with a 590 nm correction). Samples were run simultaneously to avoid inter-assay variability.
Zymography
Lung tissue was frozen at −80 °C. Tissue from each mouse was crushed and suspended for protein extraction in a solution containing 2 M urea, 1 M NaCl, and 50 mM Tris (pH 7.5) as described by Gueders et al. [2]. WT and TIMP-1 KO sensitized mice were compared for MMP-9 enzymatic activity on zymogram gels (Invitrogen Inc., Carlsbad, CA). Protein extracts were mixed with equal amounts of sample buffer and electrophoresis was conducted on the aforementioned SDS gel. Samples were incubated in 2×Triton X-100 buffer at 37 °C overnight, then washed and stained for 30 min in Coomassie Blue. Gelatinase MMP-9 activity was detected as a clear lysis band against a blue background. Band intensity was quantified using a scanning gel documentation center (Syngene; Frederick, MD) using Genetool Software version 2.11a. On each gel we utilized recombinant mouse MMP-9 (R & D Systems Inc., Minneapolis, MN) as an internal standard and for band identification (78.4 kDa).
Statistical analysis of data
Data are expressed as means±SEM. Two-way repeated measures ANOVA was used to determine differences between groups for OVA-specific IgE, and airway reactivity to MCh challenge (Rrs and Cdyn). Differences between groups for lung hydroxyproline content, cytokine, differential and absolute lung cell counts, and baseline lung mechanics were determined using a one-way ANOVA. Inflammation scores were analyzed using a Mann–Whitney-U test for non-parametric data, as were zymograms (non-normal distributions) and expressed as medians. Gene expression differences between genotypes, in response to sensitization, were analyzed using a two-tailed T-test. Significant F-ratios were followed with appropriate post-hoc testing. A P value of <0.05 was considered statistically significant. Analyses were performed 188 M.F. Sands et al. using STATISTICA (SoftStat, Tulsa, OK) or Prism (GraphPad Software, Inc., San Diego, CA) software.
Results
Serum levels of OVA-specific IgE are increased with OVA sensitization
To confirm that OVA antigen presentation resulted in immunologic sensitization, serum OVA-specific IgE levels were compared before and following sensitization (n=220).
Results are reported in relative ELISA units (EU). There were no differences in IgE between the four groups prior to sensitization (IgE was 0 EU/ml for all groups pre-treatment). No difference was noted between WT-SHAM (post=318±156 EU/ml) and KO-SHAM (post=53±25 EU/ml). Following sensitization, compared to genotype SHAM controls, serum IgE levels were increased in the WT-OVA (1554±401 EU/ml) (p <0.001) and TIMP-1 KO-OVA (4687 ± 1437 EU/ml) (p<0.001) groups but not in the sham-treated animals. The serum levels of IgE were not different between TIMP-1 KO-OVA and WT-OVA groups.
Airway reactivity following SHAM or OVA sensitization in TIMP-1 deficient and WT mice
Baseline measurements
For these experiments, 131 mice were studied. At baseline, there were no differences between the four groups in weight, gender distribution, or Rrs (Table 1). In sham-treated animals, baseline Cdyn was significantly higher in the TIMP-1 KO-SHAM group relative to the WT-SHAM (p<0.0001). OVA sensitization was associated with lower baseline Cdyn in both WT-OVA (p=0.028) and TIMP-KO-OVA (p<0.0001) groups relative to their respective sham groups. The difference in baseline Cdyn between the TIMP-SHAM and TIMP-KO-OVA groups was twice as great as that observed between the WT-SHAM and WT-OVA groups (Table 1) (p<0.0001). TIMP-1 deficient mice, there-fore, developed a greater reduction in lung compliance when sensitized as compared to the WT mice.
Table 1.
Baseline pulmonary measurements
| WT-SHAM (n=32) | WT-OVA (n=33) | TIMP-1 KO-SHAM (n=33) | TIMP-1 KO-OVA (n=33) | |
|---|---|---|---|---|
| Rrs (cmH2O)/L/s) | 1.74±0.02 | 1.79±0.05 | 1.71±0.03 | 1.81±0.06 |
| Cdyn (ml/cmH2O) | 0.0237±0.0007 | 0.0211±0.0008 a | 0.0263±0.0011 a | 0.0213±0.0005 b |
No significant differences in gender or weight were noted between genotypes.
Data are expressed as means±SEM. Rrs — respiratory system resistance; Cdyn — dynamic compliance of the respiratory system.
Different from WT-SHAM.
Different from TIMP-1 KO-SHAM.
Response to MCh Challenge
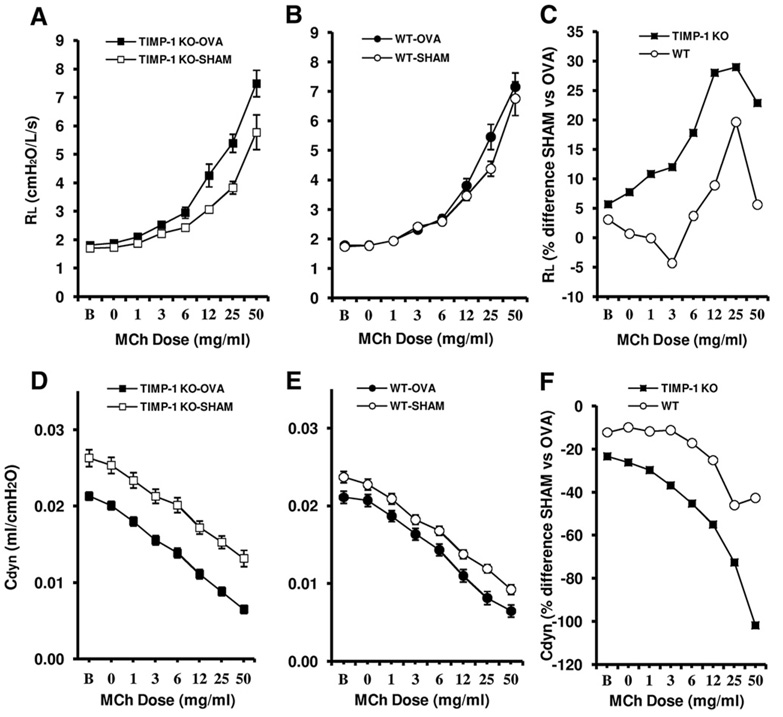
Airway hyperreactivity (AHR) is a characteristic feature of the pulmonary allergic response and has been associated with recruitment of inflammatory cells to the lung. To determine whether deficiency in TIMP-1 had a direct effect on the development of airway reactivity, AHR was measured 48 h following the final day of OVA nebulization in TIMP-1 KO and WT mice. MCh challenge revealed similar airway reactivity in the WT-SHAM, WT-OVA, and TIMP-1 KO-SHAM groups (Figs. 1A and B). The TIMP-1 KO-OVA group demonstrated an enhanced MCh responsiveness compared to the TIMP-KO-SHAM group (p=0.031), whereas the WT-OVA-sensitized mice did not develop enhanced MCh responsiveness relative to WT-SHAM mice (Figs. 1A and B). Dynamic compliance during MCh challenge was lower in the TIMP-1 KO-OVA (p<0.001) and WT-OVA (p<0.01) relative to their respective SHAM groups (Figs. 1D and E). MCh challenge was associated with similar reductions in Cdyn in all four groups with the two fold difference in baseline Cdyn associated with OVA sensitization in the TIMP-1 KO-OVA group being sustained throughout the challenge (Fig. 2D). The slope of the compliance curves during the MCh challenge was similar for all groups. The TIMP-1 KO group demonstrated greater mean increases in airway resistance and decreases in dynamic compliance as compared to the WT group in response to OVA sensitization (Figs. 1C and F). Furthermore, the TIMP-1 KO mice demonstrated increases in MCh-induced lung resistance (vs SHAM) at lower doses than WT (the dose–response curves are shifted to the left), also indicating greater MCh sensitivity.(Fig. 1C). The reduction in percent change at MCh dose 50 may reflect that the mice were approaching the maximal physiologic response to MCh in the sensitized groups.
Figure 1.
OVA-induced changes in respiratory system resistance (Rrs) and dynamic compliance (Cdyn) during methacholine (MCh) challenge. MCh challenge was associated with increases in Rrs and reduction in Cdyn. TIMP-1 KO-OVA (panel A) showed enhanced airway reactivity (Rrs) relative to TIMP-1 KO-SHAM (p<0.05) whereas responses in WT-OVA and WT-SHAM were similar (panel B). Panel C demonstrates that TIMP-1 KO mice undergo a greater percent increase in MCh-induced lung resistance (RL) than do WT mice when comparing SHAM and OVA-sensitized groups. Cdyn was greater in TIMP-1 KO-SHAM relative to WT-SHAM (panels D and E) (p=0.002). Reductions in Cdyn were observed in all groups in response to MCh challenge. Panel F shows the greater percentage drop in lung compliance (Cdyn) in TIMP-1 KO mice (100%) vs WT mice (40%) in SHAM vs OVA-treated states. Data represented as mean±SEM.
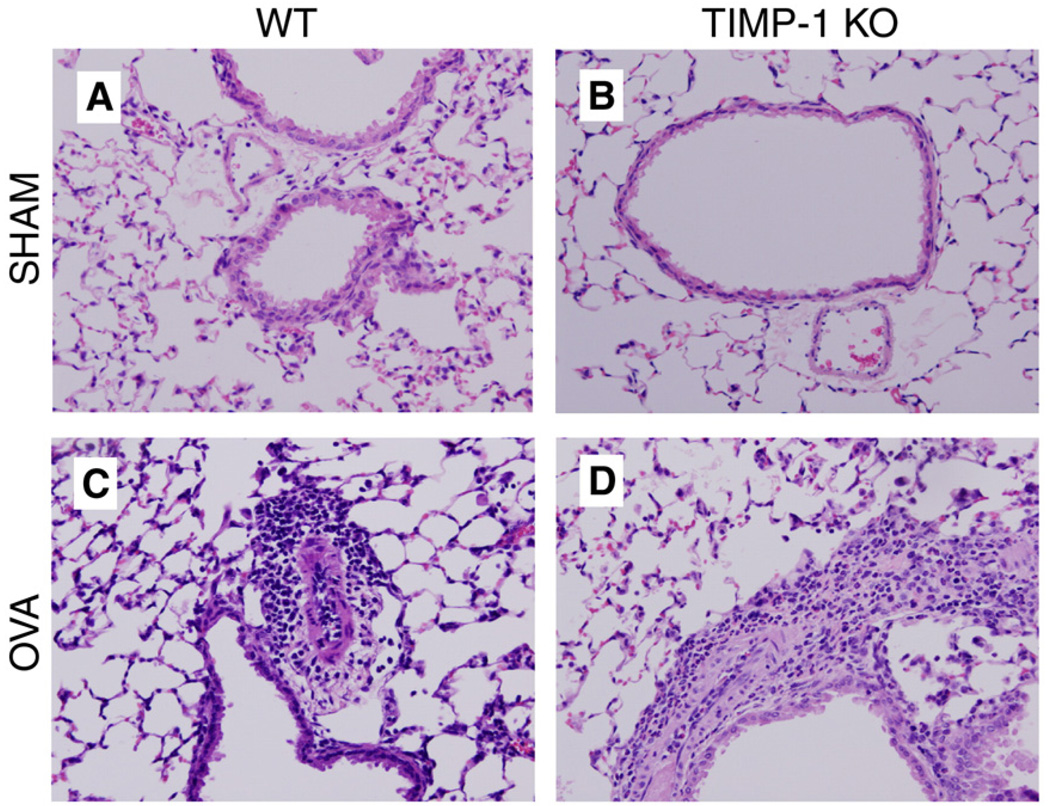
Figure 2.
Hematoxylin and Eosin stains of mouse lungs showing broncho-vascular bundles (magnification ×200). (Panel A) WT-SHAM, (Panel B) TIMP-1 KO-SHAM. Neither shows inflammation. (Panel C) Grade III inflammation in WT-OVA group. Panel D: TIMP-1 KO-OVA mouse with Grade V infiltrates of eosinophils and mononuclear cells>9 cells deep (lymphoid aggregates not in field).
Lung histological changes are greater in sensitized TIMP-1 KO Mice
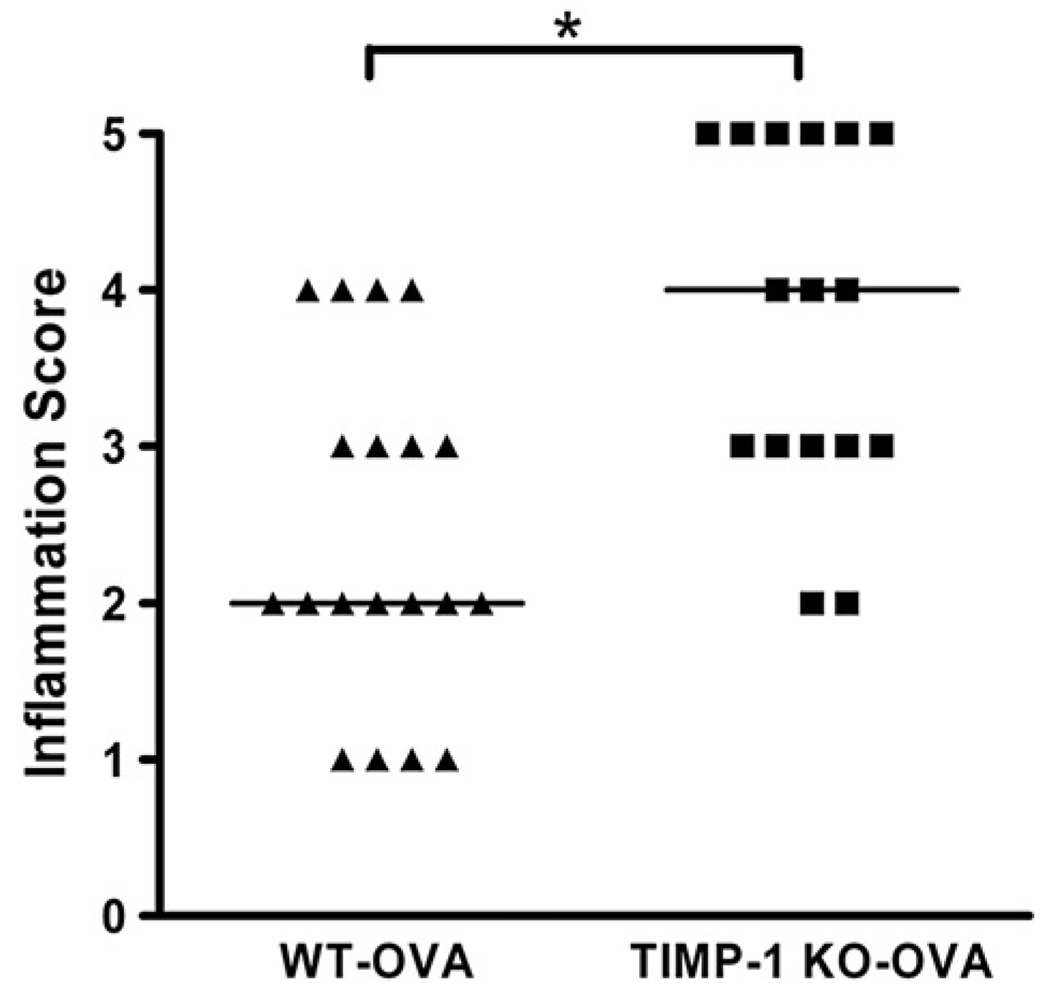
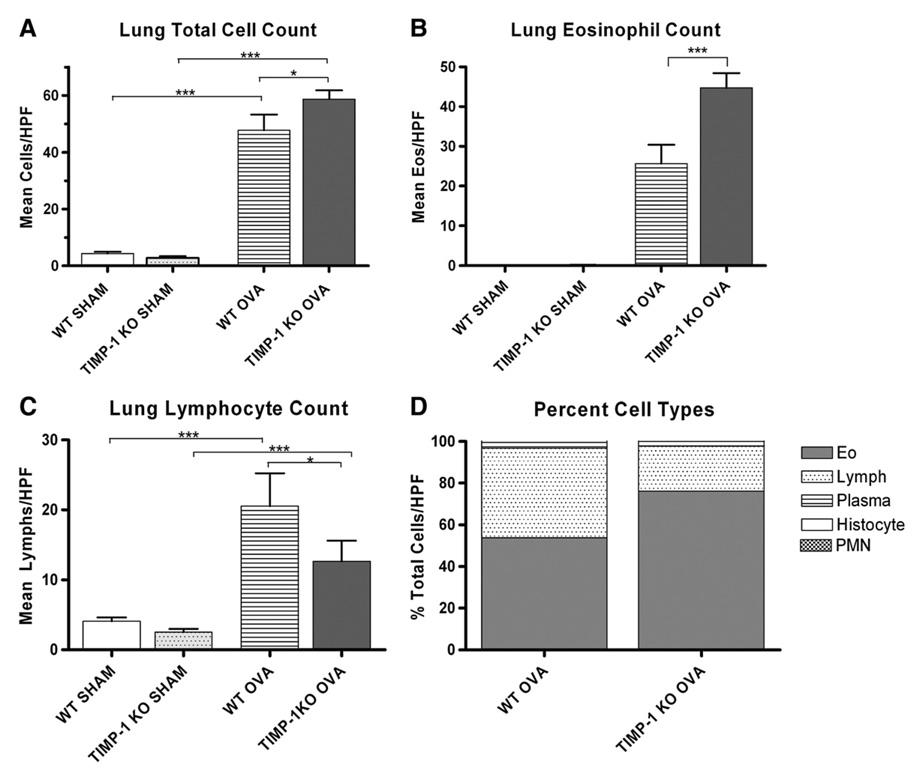
To examine the role of TIMP-1 in modulating the inflammatory response, we examined the magnitude of peribronchial cellular infiltration in response to sensitization. No inflammation was observed in either SHAM-sensitized group (Fig. 3). Furthermore, no emphysematous changes were identified in any of the four groups. Mild to moderate inflammation (Grades II–IV) was observed in the WT-OVA group whereas sensitization in the TIMP-1 KO-OVA group was associated with predominantly moderate to severe inflammation (Grades III–V) (Fig. 3 and Fig.4) (p=0.002). It was striking that only TIMP-1 KO-OVA mice developed Grade V inflammation, which included interstitial infiltrates and large lymphoid aggregates. The inflammation in both sensitized groups consisted predominantly of lymphocytic and eosinophilic peribronchial and perivascular infiltrates (Fig. 3). To further characterize the nature and intensity of the lung cellular infiltrates, the broncho-vascular bundles were subjected to differential cell counts (see Methods) and summarized in Fig. 4. Mean total cell counts (Fig. 4A) per (1000×) high power field (HPF) were: WT-SHAM (4.35±0.59) vs. TIMP-1 KO-SHAM (2.79±0.60) (p>0.05); WT-OVA (47.74±5.54) vs TIMP-1 KO-OVA (58.72±3.10) (p<0.05). WT-SHAM vs WT-OVA (p<0.001); KO-SHAM vs KO-OVA (p<0.001).
Figure 3.
Inflammation scores for OVA-sensitized WT and TIMP-1 KO groups. Median inflammation score was greater in TIMP-1 KO-OVA relative to WT-OVA (p=0.002). The most severe inflammation (Grade V) was observed only in the TIMP-1 KO-OVA group.
Figure 4.
Differential cell counts of lung H and E stained sections. Total cell counts (A); WT and TIMP-1 KO-SHAM groups were not different. Following sensitization both genotypes demonstrated significant increases in cellular infiltrates in broncho-vascular bundles (***p<0.001). Total cell counts were greater in sensitized TIMP-1 than WT mice (*p<0.05). Lung Eosinophil Counts (B); eosinophilia was seen only in sensitized groups. TIMP-1 KO sensitized mice developed greater eosinophil counts than WT sensitized mice (***p<.001). Both genotypes developed lymphocytic infiltration (C) following sensitization (***p<0.001), but WT sensitized mice had greater absolute lymphocyte counts than TIMP-1 mice (*p<0.05). Percentage of cell types (D); TIMP-1 KO-OVA mice showed a greater percentage of eosinophils and less lymphocytes in their infiltrates than WT-OVA mice. Plasma cells and histiocytes were rare.
Lung eosinophil counts/HPF (Fig. 4B) were: 0 for WT and TIMP-1 KO-SHAM groups; WT-OVA (25.63±4.76) vs TIMP-1 KO-OVA (44.72±3.67) (p<0.001). SHAM vs OVA for each genotype (p<0.001).
Lung lymphocyte counts (Fig. 4C) were: WT-SHAM (4.05±0.57) vs TIMP-1 KO-SHAM (2.50±0.48) (p>0.05); WT-OVA (20.53 ±4.70) vs TIMP-1 KO-OVA (12.64 ±2.93) (p<0.05); SHAM vs OVA for each group (p<0.001).
Fig. 4C depicts differential cell counts for WT and TIMP-1 OVA groups, revealing greater eosinophil counts in TIMP-1 (76.05%) vs WT(53.69%), and less lymphocytes in TIMP-1 (21.50%) vs WT(42.99%). Plasma, histiocytes, and polymorphonuclear cells accounted for <1%).
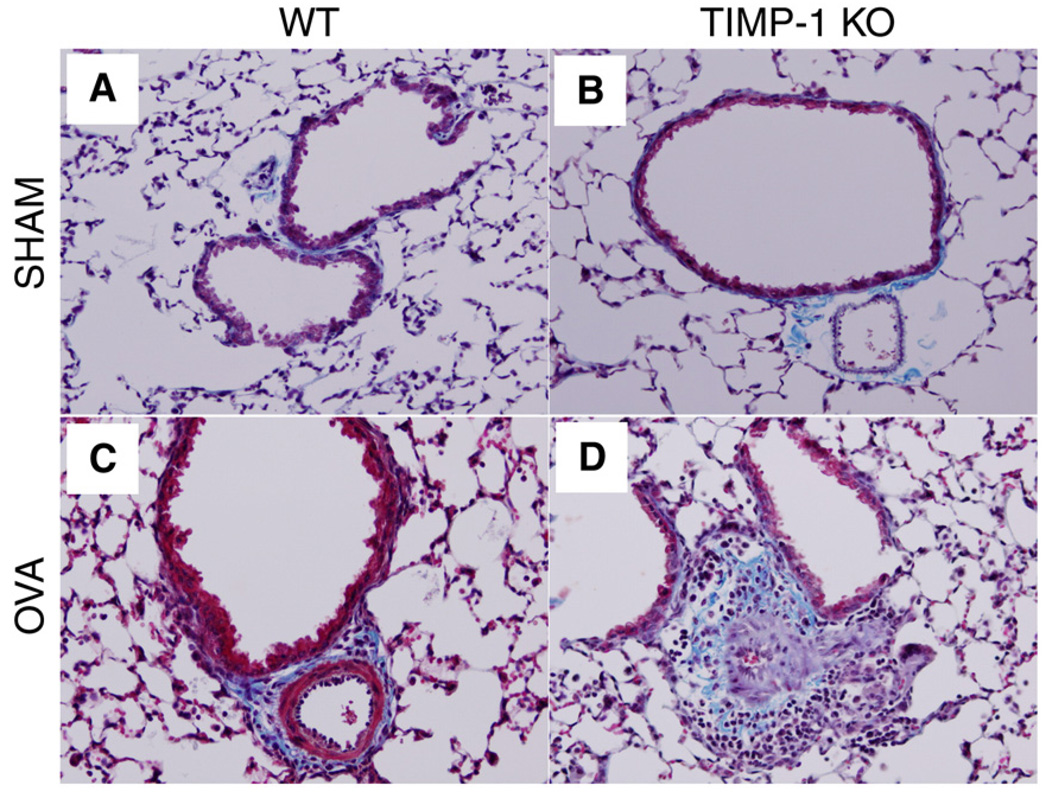
Immunostaining (CD68+) for monocyte/macrophages revealed significant increases (compared to SHAM) in sensitized WT and TIMP-1 KO mice, but no effect for genotype (data not shown). Infiltrates were most intense at areas deep to bronchial bifurcations. Trichrome staining for collagen revealed deposition of collagen within the inflammatory infiltrates (Fig. 5). Some epithelial desquamation was also noticed in sensitized animals. Morphometric analyses revealed increases in peribronchial collagen in both genotypes when comparing sensitized to SHAM groups, but no difference between genotypes could be appreciated by this methodology.
Figure 5.
Masson Trichrome stain of broncho-vascular bundles (magnification × 200). Collagen stains blue. Note normal architecture in panel A (WT-SHAM) and panel B (TIMP-1 KO-SHAM). (Panel C) OVA-sensitized WT mouse evidences mild cellular infiltration and collagen deposition between the vascular lumen and the larger bronchial lumen. (Panel D) Severe peribronchial/perivascular cellular infiltration of eosinophils and mononuclear cells, with interwoven collagen (blue fibrils) deposits.
Sensitization results in increased matrix hydroxyproline deposition
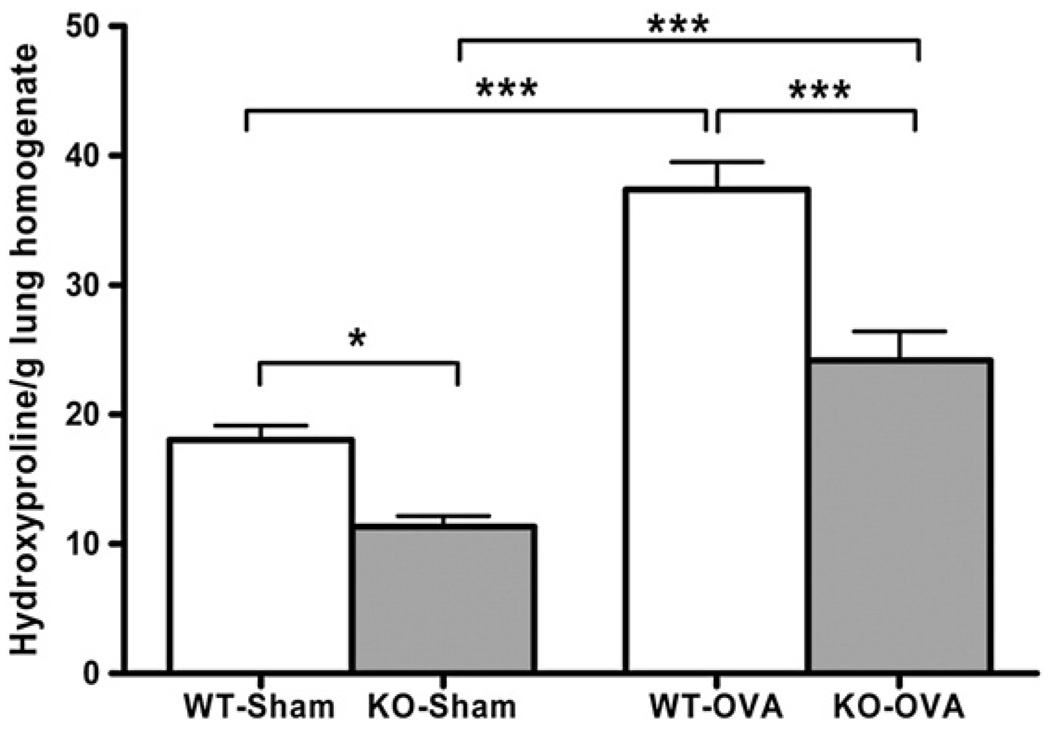
Hydroxyproline content in lung homogenates from 40 mice was utilized as an index of matrix collagen content (Fig. 6). Sensitization-induced remodeling was associated with significant increases in hydroxyproline in both WT (p<0.001) and TIMP-1 KO (p<0.001) mice whether hydroxyproline was normalized against lung albumin or per gram lung tissue. Furthermore, hydroxyproline content, normalized for lung mass (Fig. 6), revealed that WT-SHAM mice had significantly greater hydroxyproline content (18.04±1.09 ng/g) than TIMP-I KO-SHAM mice (11.33±0.84), and this was also true in sensitized WT mice (37.38±2.13) vs TIMP-1 KO mice (24.16±2.27). These data are consistent with the lung mechanics data, wherein the lung compliance was greater in TIMP-1 KO than WT-SHAM mice, suggesting that less collagen may account for greater compliance. Additionally, less collagen, if responsible for greater lung compliance, might contribute to greater changes in airway caliber during bronchoconstriction, and thus enhanced AHR in the sensitized TIMP-1 mice compared to sensitized WT mice.
Figure 6.
Lung hydroxyproline content in SHAM- and OVA-sensitized groups. OVA sensitization resulted in increased hydroxyproline content in lung homogenates of both TIMP-1 KO groups. Between genotypes, hydroxyproline content was greater in both the SHAM- (p<0.05) and OVA-sensitized (p<0.001) conditions when WT was compared to TIMP-1 KO mice. Data represented as mean±SEM.
Gene (mRNA) expression
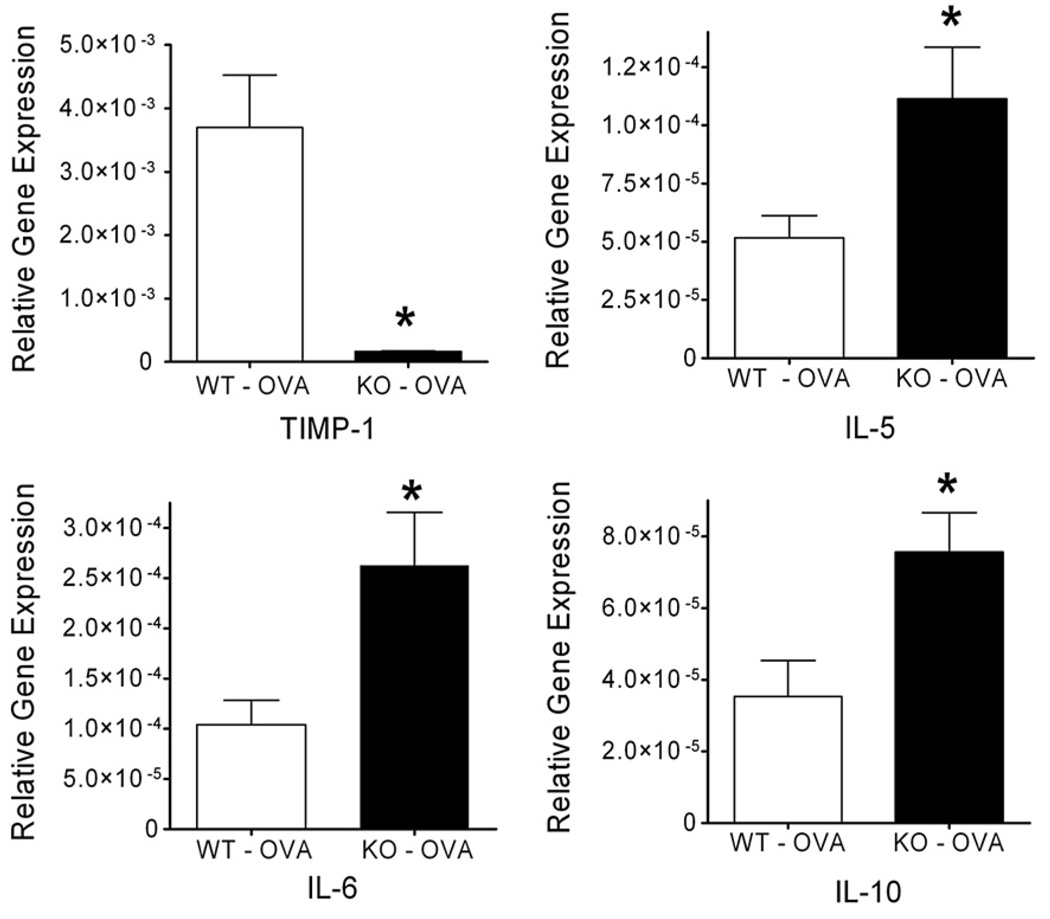
Extracts of mRNA were obtained from lung homogenates of mice that underwent pulmonary function analysis. After reverse transcription, cDNA was amplified via QPCR to analyze relative gene expression for cytokines, collagens and collagen precursors, MMPs and TIMP-1 and TIMP-2. WT and TIMP-1 KO mice after OVA sensitization (per above protocol) were compared (N=9 each group). Sensitized TIMP-1 KO mice demonstrated greater relative gene expression than WT mice (normalized against beta-actin) for the following genes; IL-5 (p=0.036), IL-6 (p=0.023), IL-10 (p=0.018) and collagen 1α2 (p=0.035) (Fig. 7). IL-4 trended toward greater relative expression in sensitized TIMP-1 KO than in WT mice (p=0.083). No significant differences between WT and TIMP-1 KO sensitized mice were observed for MMP-9, -2, -12 or in TIMP-2. TIMP-1 gene expression was, as anticipated, profoundly impaired in the KO animals vs WT (p<0.001). Additional gene expression analyses were performed, but no differences were observed between genotypes (see Supplement Table 1).
Figure 7.
Gene expression in OVA-sensitized WTand TIMP-1 KO groups (n=9 each group). OVA sensitization resulted in significantly greater relative (to β-actin) expression of IL-5 (p=0.036), IL-6 (p=0.023), and IL-10 (p=0.018) in TIMP-1 KO mice compared to WT. TIMP-1 expression in TIMP-1 KO mice was lower than in WT (p<0.001) Data represented as mean±SEM.
Lung cytokine expression
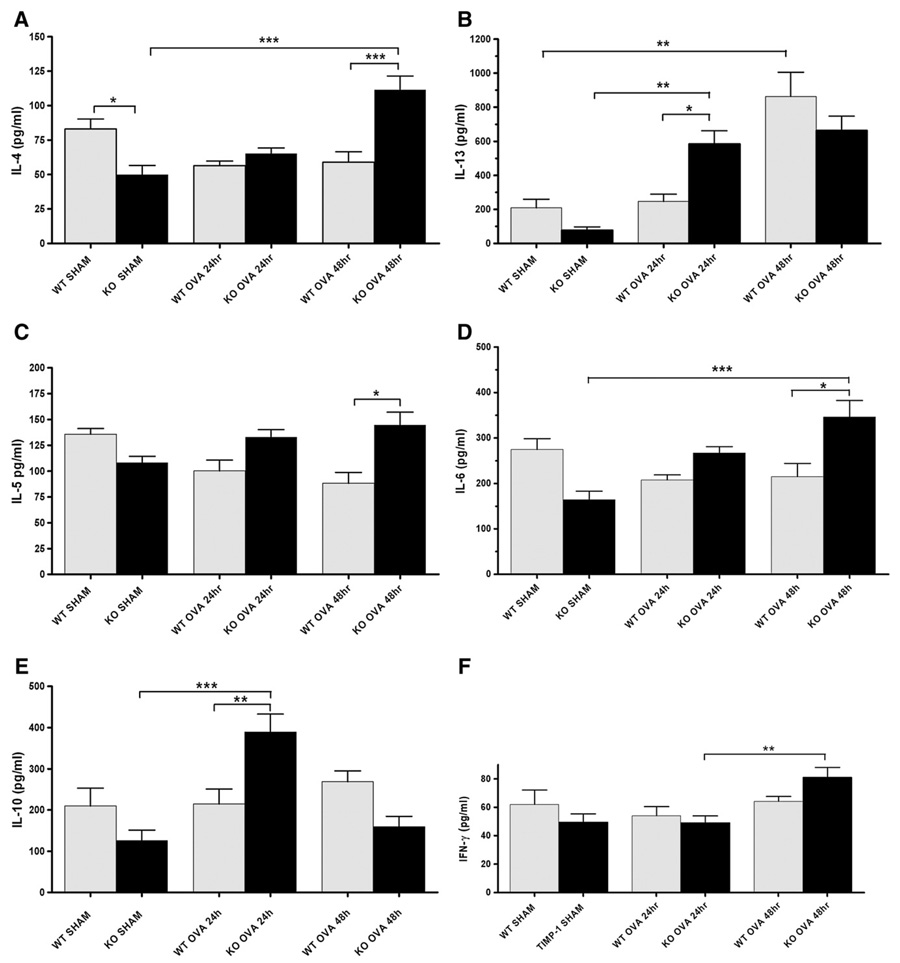
Th1 and Th2 cytokine measurements of lung homogenates were obtained in sham-sensitized as well as OVA-sensitized mice at 24 and 48 h after completion of day 7 nebulization (saline or ovalbumin respectively) for both WTand TIMP-1 KO groups. Cytokines analyzed include: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, TNFα, eotaxin, MIP-1α, MIP-2, INF-γ, and RANTES. Significantly greater expression of Th2 cytokines was demonstrated in sensitized TIMP-1 KO compared to sensitized WT mice (Fig. 8).
Figure 8.
Cytokine (protein) expression in WT and TIMP-1 KO mice in lung homogenates at 24 and 48 h. Panels A–E represent Th2 cytokines, panel F representative of Th1 cytokine pattern. (Panel A) IL-4 levels were greater at sham conditions in WT than TIMP-1 KO mice (*p<0.05). With sensitization, TIMP-1 KO mice showed a significant increase (***p<0.001) in IL-4 by 48 h after the 7th day of OVA aerosol treatment compared to SHAM and also WT sensitized groups. (Panel B) IL-13 levels were significantly greater at 24 h in sensitized TIMP-1 KO mice compared to WT (**p<0.01). IL-13 levels increased in sensitized WT mice from baseline at 48 h only (**p<0.01). (Panel C) IL-5 increased only in TIMP-1 KO mice after sensitization, and was greater in TIMP-1 KO mice than WT at 48 h (*p<0.05). (Panel D) IL-6 is greater following sensitization only in TIMP-1 KO mice compared to TIMP-1 SHAM (***p<0.001) and WT (*p<0.05). (Panel E) IL-10 levels increase following sensitization only in TIMP-1 KO mice: increase relative to SHAM (***p<0.001) and WT (**p<0.01). (Panel F) IFN-γ (typical of Th1 cytokine responses) levels were not different at basal or 24 h between any groups. A slight increase was noted in TIMP-1 OVA mice at 48 h compared to 24 h (**p<0.01). No difference between WTand TIMP-1 KO groups were seen. Data represented as mean±SEM.
Cytokines highly associated with asthmatic lung inflammation and airway hyperreactivity (AHR) include IL-4 and IL-13. Mean IL-4 levels increased in KO mice at 48 h by more than 200% (p<0.001), whereas IL-4 did not increase relative to SHAM controls in WT mice at either 24 h or 48 h after 7 daily OVA aerosol treatments. Furthermore, IL-4 was significantly greater (p<0.001) at 48 h in TIMP-KO mice than WT at 48 h (Fig. 8; panel A). IL-13 levels (Fig. 8; panel B) were significantly greater in TIMP-1 KO mice at 24 h than in WT (p<0.01). Both genotypes responded with significant increases from baseline by 48 h, with no significant difference between genotype at this interval (p>0.05). For both IL-4 and IL-13, KO-SHAM (vs WT-SHAM) mice evidenced lower cytokine levels, resulting in greater percentage increases as well. Analysis of IL-5 response to sensitization revealed a similar pattern of TIMP-1 KO enhanced expression (Fig. 8; panel C).
IL-5 was significantly greater in TIMP-1 KO than WT 48 h after OVA nebulization (p<0.05), trending upwards from sham, through 24 to 48 h time points, whereas the opposite pattern appeared in the WT. IL-6 analysis (Fig. 8; panel D) showed the same pattern in TIMP-1 KO mice, with significantly enhanced expression at 48 h, relative to SHAM (p<0.001), and relative to WT sensitized mice (p<0.05).
IL-10 expression was again enhanced only in the TIMP-1 KO mice (Fig. 8; panel E). At 24 h the TIMP-1 KO mice demonstrated an almost 400% rise (p<0.001) relative to SHAM, and almost 2-fold greater quantities than WT sensitized mice (p<0.01). In contrast, WT mice had no demonstrable rise in IL-10 relative to SHAM at either time point. This pattern of enhanced cytokine expression in response to sensitization was not seen in other cytokines, including RANTES or eotaxin (data not shown). Furthermore, in contrast to Th2 cytokines, Th1 cytokines such as INF-γ(Fig. 8; panel F) showed minimal or no response to sensitization (compared to SHAM groups) in either genotype, and no effect of genotype.
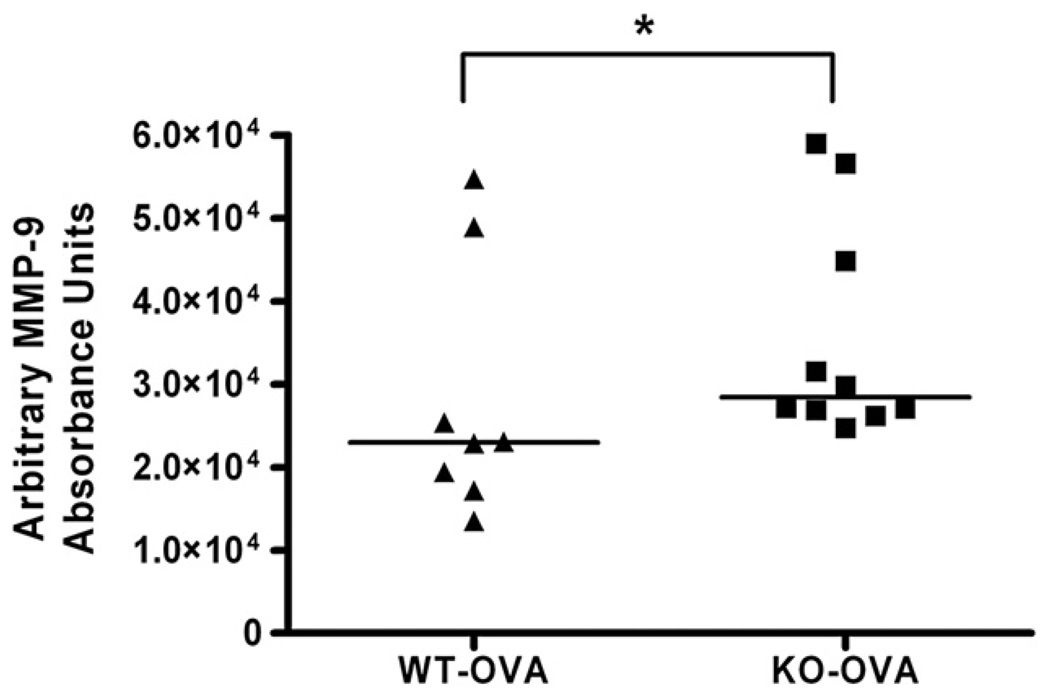
Lung zymography for MMP-9
To determine whether absence of TIMP-1 would result in measurable differences in a principal target, MMP-9, sensitized WT and TIMP-1 KO mice lung homogenates (n=15 per group) (protocol day 29) were analyzed by zymography. MMP-9 activity (band intensity) and identity was normalized against a recombinant MMP-9 standard (see Methods). WT sensitized mice demonstrated lower (p=0.0217) MMP-9 activity (22,975 AU) than TIMP-1 KO activity (28,434 AU). (Fig. 9).
Figure 9.
MMP-9 zymography. MMP-9 enzyme activity was greater in sensitized TIMP-1 KO than WT mice (p=0.02). Bar represents median for group.
Discussion
To assess the role of TIMP-1 in the pathogenesis of allergic/asthmatic inflammation, we utilized the TIMP-1 KO mouse in an OVA asthma model. We speculated that the absence of TIMP-1 would create a ‘permissive’ environment wherein pro-inflammatory/remodeling activity would occur. We hypothesized specifically, that TIMP-1 KO mice, once sensitized would manifest enhanced airway hyperreactivity, inflammation, and evidence for perturbations in remodeling when compared to WT mice in which TIMP-1 could modulate the effects of target MMPs (MMP-9, MMP-2 and MMP-12). We demonstrated that TIMP-1 KO mice, compared to WT controls, when sensitized with ovalbumin, evidence increased airway reactivity to methacholine, and underwent greater reduction of lung compliance. Additionally, in these KO mice, lung inflammation was more intense, as shown by extensive peribronchial cellular infiltrates. Additionally, TIMP-1 KO sensitized mice have increased gene expression of several Th2 cytokines relative to WT controls. Specifically Th2 cytokines, IL-4, IL-13, IL-5, IL-6, and IL-10, were expressed in greater amounts in sensitized TIMP-1 KO mice relative to WT sensitized controls. Quantities of cytokines identified are consistent with others' findings [14,32–37]. Taken together, our findings support the hypothesis that TIMP-1 deficiency (allowing enhanced MMP-9 activity) results in an asthma phenotype, including airway hyperreactivity, enhanced cellular peribronchial inflammation, Th2 cytokine gene expression, Th2 skewing in cytokine protein expression, and greater reduction of dynamic lung compliance following sensitization with ovalbumin.
Airway hyperreactivity and lung inflammation
The mechanism underlying our findings of AHR and inflammation appears to be an augmented Th2 inflammatory response. Several key cytokines have been implicated in the generation of AHR. IL-4 and IL-13 are causally linked to AHR in murine models. [38,39]. Bronchial hyperreactivity and allergic inflammation have been associated with IL-5 in animal models [40–42]. Additionally, our findings are consistent with previous reports in which increased IL-10 levels are associated with allergic lung inflammation, and IL-10 administration has been linked, in the presence of IL-13, to AHR [43,44].
Lung homogenate cytokine data was further supported by gene expression data. Enhanced gene expression (mRNA) for IL-5, IL-6, IL-10 with trends toward enhanced IL-4 and IL-13 production was observed in the TIMP-1 KO (versus WT) sensitized groups. Increases in IL-6 have been associated, in other studies, with allergic asthmatic inflammation, similar to our TIMP-1 deficient sensitized mice. Epithelial cell IL-6 gene expression is increased in response to histamine [45,46].
The nature of the cellular infiltrates in the TIMP-1 KO sensitized mice is consistent with cytokine expression in our model. Specifically, lung eosinophilia may be explained, in part, by enhanced IL-5 elaboration by Th2 lymphocytes. However, the differential counts showed more absolute cellularity, but primarily on the basis of increased eosinophils themselves in KO vs WT groups. An alternative explanation is that enhanced MMP-9 activity is enhancing eosinophil trafficking. There is in-vitro evidence to support this as a possible mechanism [47]. In turn, the eosinophils may be contributing additional IL-5, amplifying eosinophil chemotaxis and survival. Also, eosinophils may be, in addition to macrophages, further generating MMP-9, perpetuating the amplification loop [48].
Lung remodeling
Lung remodeling, as evidenced by collagen deposition, was not fully consistent with our initial hypothesis. Specifically, we expected that the pro-inflammatory state would favor significantly more collagen deposition in sensitized TIMP-1 deficient animals compared to WT, which was not observed. Both genotypes did evidence histological remodeling with collagen deposition in the sub-epithelial regions, particularly in the broncho-vascular bundles. Although there was an increase in collagen I α-2 mRNA, a collagen precursor, in sensitized TIMP-1 KO versus WT sensitized mice, sensitized TIMP-1 KO mice did not evidence more (hydroxyproline) collagen. In fact TIMP-1 KO mice in both SHAM and OVA-sensitized states evidenced less hydroxyproline content than WT-SHAM and OVA-sensitized controls respectively (Fig. 6).
This suggests that collagen matrix is reduced in the TIMP-1 deficient animals in basal or stimulated conditions, and can be explained by enhanced MMP degradation/turnover of collagen during normal development as well as during inflammation. Enhanced MMP-9 activity was confirmed (zymography) in TIMP-1 KO mice relative to WT, supporting the mechanism for this. This may overshadow any relative increase in collagen production resulting from the enhanced inflammatory response. Of interest was the finding that KO-sham-sensitized mice had greater lung dynamic compliance than WT-sham mice. Furthermore, the change in dynamic compliance between sham and sensitized TIMP-1 KO mice was twice as great as in WT. This suggests that, under identical allergic inflammatory stimuli, TIMP-1 deficient mice may, in fact, undergo a greater change in remodeling. Since the change in compliance was greater, but hydroxypro-line assay demonstrated less collagen in sensitized KO mice, there are several possible explanations. The simplest is that the alteration in compliance merely reflects more cellular infiltration, and is independent of collagen deposition in the matrix. An alternate explanation is that these mice might, in fact, be potentially capable of more collagen or elastin remodeling, but the model, being acute (only 7 days of antigen challenge to the lung, with tissue harvested 9 days after initial OVA lung exposure) does not allow enough time for the inflammation-linked collagen component of remodeling to become fully evident. Alternatively, hydroxyproline assay does not reflect collagen sub-types or geometric deposition differences that might have produced subtle changes in lung mechanics not reflected in protein assays. One additional possibility is that the KO-sham mice, evidencing hyper-compliant lungs relative to WT-sham, are doing so by a mechanism not related to collagen. This might be explained by alterations in elastin content of the lungs. Since MMP-12 (macrophage metalloelastase) has elastin as its principal substrate, and TIMP-1 inhibits MMP-12, [49] it is possible that enhanced MMP-12 activity during growth and development resulted in reduced elastin content, hence enhanced lung compliance. There was no sign of emphysematous changes within these lungs, which is seen in other MMP-12-driven pathologic states such as emphysema [49].
We observed that sensitized TIMP-1 KO mice (versus sensitized WT controls) developed much more severe peribronchial and perivascular lymphocytic, eosinophilic and macrophage infiltrates. Gene expression and confirmatory protein expression analysis of lung homogenate (as noted above) supports that these recruited cells were responsible for contributing inflammatory mediators resulting in observed alterations in airway reactivity and lung compliance. These findings are similar to those of Lee et al. [44]. Utilizing an IL-10-over-expressor mouse asthma model they demonstrated increased peribronchial and perivascular T and B cell infiltrates, which is similar to our findings. Additionally, MMP-9 has been identified as an important facilitator of eosinophil chemotaxis in matrix in vitro [47].
We initially hypothesized that the enhanced allergic inflammation in the TIMP-1 KO mice would be coupled to enhanced collagen deposition. Consistent with the work of Kim et al. [50], (in which a bleomycin pulmonary fibrosis model was employed) hydroxyproline content of lung homogenates, increased in both genotypes after sensitization. However, their model did not show effect of genotype in either sham or sensitized groups. In contrast, when our animals were given a Th2 stimulus (versus a Th1 PMN-driven stimulus), we did observe an effect of genotype in both sham and sensitized conditions. It should be emphasized that our hydroxyproline findings are perfectly consistent with the lung compliance data. This discrepancy might, in part, be explained by the fact that Kim et al. utilized a model, which was Th1 skewed and PMN-driven, generating a different, possibly more intense or altered, stimulus to collagen deposition, and our primary observational time points were more acute. Both models did demonstrate augmented inflammation in the TIMP-1 KO mice relative to WT, whether Th1 or Th2 (our study). These model-dependent differences underscore two concepts. First, that the model and injury, being a surrogate for (human) disease, behave as a model for that intervention, and may not be generalizable to other models or disease states. In other words, a pulmonary fibrosis model cannot fully predict allergic model findings. Second, TIMP-1, being ‘multifacial’ likely will perform differing modulating roles in differing inflammatory injuries. Stated otherwise, TIMP-1 performs many functions, and appears to modulate inflammation and repair differentially depending upon the type of injury.
Our study was limited in several regards. A majority of KO mice, including this TIMP-1 KO, are crossed onto the C57BL/6 background, which is difficult, compared to the BALB/c strain, to allergically sensitize [26,51]. As a result, Th2-driven (acute and chronic) models are more difficult to generate with C57BL/6 animals. Despite this limitation, the TIMP-1 KO mice in our study manifested enhanced TH2 responses (IL-4, IL-5, IL-6, IL-13, and IL-10), supporting the role of TIMP-1 in modulating the response to allergic sensitization in this acute asthma model. The acute allergic injury model might not reflect the effects of chronic allergic stimulation, thus we may have excluded time points that may have demonstrated genotype-specific alterations in inflammation, and remodeling. Also, we identified unique lung compliance properties in the TIMP-1 KO-SHAM mice suggesting that they undergo unique developmental changes. While these are potentially important and novel findings, a conditional knockout model, in which the TIMP-1 gene could be silenced after lung development might allow more precise definition of pathophysiologic findings related to the acute allergic insult. Finally, because TIMP-1 targets MMP-2, MMP-9, and MMP-12, as well as possessing unique immunomodulatory effects independent of MMPs [52], it is difficult to determine which targets are primarily responsible for the phenotype observed without silencing them simultaneously.
Overall, these data are of significance in the context of several related observations. TIMP-1 polymorphisms have been shown, through linkage analysis in Australian females, to be associated with increased risk of AHR and asthma [19]. This suggests that TIMP-1 has relevance to modulating clinical airway hyperreactivity, if not the asthma phenotype. It is possible that TIMP-1 is important not only in its ability to regulate MMP activity, but also through MMP-independent functions (including regulation of apoptosis, cell survival, and promotion of steroidogenesis, and angiogenesis) [3,4,52].
In conclusion, our data demonstrate that genetic TIMP-1 deficiency results in an asthma phenotype, including airway hyperreactivity, enhanced cellular peribronchial (eosinophilic) inflammation, Th2 cytokine gene expression, Th2 skewing in cytokine protein production, and greater reduction of dynamic lung compliance following sensitization with ovalbumin. This supports the hypothesis that TIMP-1 contributes to the modulation of allergic lung inflammation in this murine model, perhaps independent of collagen-matrix remodeling. Changes in baseline lung compliance and the presence of enhanced collagen I precursor mRNA elaboration in TIMP-1 deficient mice suggest a need to better define the role of TIMP-1 in both lung development, and in chronic asthma models. Further investigation into the contribution of TIMP-1 to the interface between inflammation and remodeling in lung matrix is warranted. This study suggests the need to determine if TIMP-1 plays a role not only in post-transcriptional regulation of inflammation, through altered cell trafficking, but also as a modulator of more proximal regulatory mechanisms at the RNA level.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.clim.2008.08.029.
Acknowledgments
This work was supported by the following grants: IRCAF #28466-1-1036640 — University at Buffalo (MFS, PJO, SAS); American Lung Association Research Grant RG-1198-N (MFS, PJO, SAS); Veteran's Administration RCD Award RCD-012-05 S (MFS); NIH Grant HL 48889 (PRK). The authors thank Paul D. Soloway Ph.D., for initially providing the TIMP-1 KO mice utilized in this study.
References
- 1.Atkinson JJ, Senior RM, Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am. J. Resir. Cell Mol. Biol. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 2.Gueders MM, Balbin M, Rocks N, Foidart JM, Gosset P, Louis R, Shapiro S, Lopez-Otin C, Noel A, Cataldo DD. Matrix metalloproteinase-8 deficiency promotes granulocytic allergen-induced airway inflammation. J. Immunol. 2005;175:2589–2597. doi: 10.4049/jimmunol.175.4.2589. [DOI] [PubMed] [Google Scholar]
- 3.Kelly EA, Jarjour NN. Role of matrix metalloproteinases in asthma. Curr. Opin. Pulm. Med. 2003;9:28–33. doi: 10.1097/00063198-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Lambert E, Dasse E, Haye B, Petitfrere E. TIMPs as multifacial proteins. Crit. Rev. Oncol. Hematol. 2004;49:187–198. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Kumagai K, Ohno I, Okada S, Ohkawara Y, Suzuki K, Shinya T, Nagase H, Iwata K, Shirato K. Inhibition of matrix metalloproteinases prevents allergen-induced airway inflammation in a murine model of asthma. J. Immunol. 1999;162:4212–4219. [PubMed] [Google Scholar]
- 6.Cataldo DD, Tournoy KG, Vermaelen K, Munaut C, Foidart JM, Louis R, Noel A, Pauwels RA. Matrix metalloproteinase-9 deficiency impairs cellular infiltration and bronchial hyperresponsiveness during allergen-induced airway inflammation. Am. J. Pathol. 2002;161:491–498. doi: 10.1016/S0002-9440(10)64205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corry DB, Kiss A, Song LZ, Song L, Xu J, Lee SH, Werb Z, Kheradmand F. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J. 2004;18:995–997. doi: 10.1096/fj.03-1412fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMillan SJ, Kearley J, Campbell JD, Zhu XW, Larbi KY, Shipley JM, Senior RM, Nourshargh S, Lloyd CM. Matrix metallo-proteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J. Immunol. 2004;172:2586–2594. doi: 10.4049/jimmunol.172.4.2586. [DOI] [PubMed] [Google Scholar]
- 9.Corry DB, Rishi K, Kanellis J, Kiss A, Song Lz LZ, Xu J, Feng L, Werb Z, Kheradmand F. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat. Immunol. 2002;3:347–353. doi: 10.1038/ni773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosse M, Chakir J, Rouabhia M, Boulet LP, Audette M, Laviolette M. Serum matrix metalloproteinase-9: tissue inhibitor of metalloproteinase-1 ratio correlates with steroid responsiveness in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 1999;159:596–602. doi: 10.1164/ajrccm.159.2.9802045. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino M, Takahashi M, Takai Y, Sim J. Inhaled corticosteroids decrease subepithelial collagen deposition by modulation of the balance between matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 expression in asthma. J. Allergy Clin. Immunol. 1999;104:356–363. doi: 10.1016/s0091-6749(99)70379-9. [DOI] [PubMed] [Google Scholar]
- 12.Dahlen B, Shute J, Howarth P. Immunohistochemical localisation of the matrix metalloproteinases MMP-3 and MMP-9 within the airways in asthma. Thorax. 1999;54:590–596. doi: 10.1136/thx.54.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J. Allergy Clin. Immunol. 1998;102:783–788. doi: 10.1016/s0091-6749(98)70018-1. [DOI] [PubMed] [Google Scholar]
- 14.Cataldo DD, Bettiol J, Noel A, Bartsch P, Foidart JM, Louis R. Matrix metalloproteinase-9, but not tissue inhibitor of matrix metalloproteinase-1, increases in the sputum from allergic asthmatic patients after allergen challenge. Chest. 2002;122:1553–1559. doi: 10.1378/chest.122.5.1553. [DOI] [PubMed] [Google Scholar]
- 15.Vignola AM, Riccobono L, Mirabella A, Profita M, Chanez P, Bellia V, Mautino G, D'Accardi P, Bousquet J, Bonsignore G. Sputum metalloproteinase-9/tissue inhibitor of metalloproteinase- 1 ratio correlates with airflow obstruction in asthma and chronic bronchitis. Am. J. Respir. Crit. Care Med. 1998;158:1945–1950. doi: 10.1164/ajrccm.158.6.9803014. [DOI] [PubMed] [Google Scholar]
- 16.Selman M, Ruiz V, Cabrera S, Segura L, Ramirez R, Barrios R, Pardo A. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L562–L574. doi: 10.1152/ajplung.2000.279.3.L562. [DOI] [PubMed] [Google Scholar]
- 17.Madtes DK, Elston AL, Kaback LA, Clark JG. Selective induction of tissue inhibitor of metalloproteinase-1 in bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2001;24:599–607. doi: 10.1165/ajrcmb.24.5.4192. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Stetler-Stevenson WG, Fleming MV, Fishback N, Koss MN, Liotta LA, Ferrans VJ, Travis WD. Immunohisto-chemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am. J. Pathol. 1996;149:1241–1256. [PMC free article] [PubMed] [Google Scholar]
- 19.Lose F, Thompson PJ, Duffy D, Stewart GA, Kedda MA. A novel tissue inhibitor of metalloproteinase-1 (TIMP-1) polymorphism associated with asthma in Australian women. Thorax. 2005;60:623–628. doi: 10.1136/thx.2004.026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sands MF, Ohtake PJ, Mahajan S, Soloway P, Blume J, Schwartz S. Tissue inhibitor of metalloproteinase-1 (TIMP-1) modulates murine airway hyperreactivity (AHR) Am. J. Respir. Crit. Care Med. 2004;169:A702. [Google Scholar]
- 21.Sands MF, Ohtake PJ, Mahajan S, Fang Y, Takyar S, Soloway P, Mullan B, Blume J, Lachina S, Knight P, Schwartz SA. Tissue inhibitor of metalloproteinase-1 (TIMP-1) modulates lung hyperreactivity and inflammation in a murine asthma model. Ann. Allergy Immun. 2007;96:A82. [Google Scholar]
- 22.Osiewicz K, McGarry M, Soloway PD. Hyper-resistance to infection in TIMP-1-deficient mice is neutrophil dependent but not immune cell autonomous. Ann. N. Y. Acad. Sci. 1999;878:494–496. doi: 10.1111/j.1749-6632.1999.tb07706.x. [DOI] [PubMed] [Google Scholar]
- 23.Soloway PD, Alexander CM, Werb Z, Jaenisch R. Targeted mutagenesis of Timp-1 reveals that lung tumor invasion is influenced by Timp-1 genotype of the tumor but not by that of the host. Oncogene. 1996;13:2307–2314. [PubMed] [Google Scholar]
- 24.Nothnick WB, Soloway P, Curry TE., Jr Assessment of the role of tissue inhibitor of metalloproteinase-1 (TIMP-1) during the periovulatory period in female mice lacking a functional TIMP-1 gene. Biol. Reprod. 1997;56:1181–1188. doi: 10.1095/biolreprod56.5.1181. [DOI] [PubMed] [Google Scholar]
- 25.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brewer JP, Kisselgof AB, Martin TR. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am. J. Respir. Crit. Care Med. 1999;160:1150–1156. doi: 10.1164/ajrccm.160.4.9806034. [DOI] [PubMed] [Google Scholar]
- 27.Ellis R, Leigh R, Southam D, O'Byrne PM, Inman MD. Morphometric analysis of mouse airways after chronic allergen challenge. Lab. Invest. 2003;83:1285–1291. doi: 10.1097/01.lab.0000087586.25982.b5. [DOI] [PubMed] [Google Scholar]
- 28.Tournoy KG, Kips JC, Schou C, Pauwels RA. Airway eosinophilia is not a requirement for allergen-induced airway hyperresponsiveness. Clin. Exp. Allergy. 2000;30:79–85. doi: 10.1046/j.1365-2222.2000.00772.x. [DOI] [PubMed] [Google Scholar]
- 29.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin. Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 30.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Knight PR, Sreekumar A, Siddiqui J, Laxman B, Copeland S, Chinnaiyan A, Remick DG, Knight PR, Sreekumar A, Siddiqui J, Laxman B, Copeland S, Chinnaiyan A, Remick DG. Development of a sensitive microarray immunoassay and comparison with standard enzyme-linked immunoassay for cytokine analysis. Shock. 2004;21:26–30. doi: 10.1097/01.shk.0000101668.49265.19. [DOI] [PubMed] [Google Scholar]
- 33.Knight PR, Davidson BA, Nader ND, Helinski JD, Marschke CJ, Russo TA, Hutson AD, Notter RH, Holm BA. Progressive, severe lung injury secondary to the interaction of insults in gastric aspiration. Exp. Lung Res. 2004;30:535–557. doi: 10.1080/01902140490489162. [DOI] [PubMed] [Google Scholar]
- 34.Henderson WR, Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am. J. Respir. Crit. Care Med. 2002;165:108–116. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 35.Cataldo DD, Tournoy KG, Vermaelen K, Munaut C, Foidart JM, Louis R, Noel A, Pauwels RA, Cataldo DD, Tournoy KG, Vermaelen K, Munaut C, Foidart J-M, Louis R, Noel A, Pauwels RA. Matrix metalloproteinase-9 deficiency impairs cellular infiltration and bronchial hyperresponsiveness during allergen-induced airway inflammation. Am. J. Pathol. 2002;161:491–498. doi: 10.1016/S0002-9440(10)64205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wert SE, Yoshida M, LeVine AM, Ikegami M, Jones T, Ross GF, Fisher JH, Korfhagen TR, Whitsett JA. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5972–5977. doi: 10.1073/pnas.100448997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elias JA, Lee CG, Zheng T, Shim Y, Zhu Z. Interleukin-13 and leukotrienes: an intersection of pathogenetic schema. Am. J. Respir. Cell. Mol. Biol. 2003;28:401–404. doi: 10.1165/rcmb.F264. [DOI] [PubMed] [Google Scholar]
- 40.Shardonofsky FR, Venzor J, III, Barrios R, Leong KP, Huston DP. Therapeutic efficacy of an anti-IL-5 monoclonal anti-body delivered into the respiratory tract in a murine model of asthma. J. Allergy Clin. Immunol. 1999;104:215–221. doi: 10.1016/s0091-6749(99)70138-7. [DOI] [PubMed] [Google Scholar]
- 41.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, Leikauf GD, Lee NA. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J. Exp. Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J. Clin. Invest. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Scott MR, Justice JP, Bradfield JF, Enright E, Sigounas A, Sur S. IL-10 reduces Th2 cytokine production and eosinophilia but augments airway reactivity in allergic mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L667–L674. doi: 10.1152/ajplung.2000.278.4.L667. [DOI] [PubMed] [Google Scholar]
- 44.Lee CG, Homer RJ, Cohn L, Link H, Jung S, Craft JE, Graham BS, Johnson TR, Elias JA. Transgenic over-expression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J. Biol. Chem. 2002;277:35466–35474. doi: 10.1074/jbc.M206395200. [DOI] [PubMed] [Google Scholar]
- 45.Noah TL, Paradiso AM, Madden MC, McKinnon KP, Devlin RB. The response of a human bronchial epithelial cell line to histamine: intracellular calcium changes and extracellular release of inflammatory mediators. Am. J. Respir. Cell Mol. Biol. 1991;5:484–492. doi: 10.1165/ajrcmb/5.5.484. [DOI] [PubMed] [Google Scholar]
- 46.Marini M, Vittori E, Hollemborg J, Mattoli S. Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J. Allergy Clin. Immunol. 1992;89:1001–1009. doi: 10.1016/0091-6749(92)90223-o. [DOI] [PubMed] [Google Scholar]
- 47.Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am. J. Respir. Cell Mol. Biol. 1997;17:519–528. doi: 10.1165/ajrcmb.17.4.2877. [DOI] [PubMed] [Google Scholar]
- 48.Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, Isoyama S, Tanno Y, Tamura G, Yamauchi K, Nagura H, Shirato K, Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, Isoyama S, Tanno Y, Tamura G, Yamauchi K, Nagura H, Shirato K. Eosinophils as a source of matrix metallo-proteinase-9 in asthmatic airway inflammation. Am. J. Resir. Cell Mol. Biol. 1997;16:212–219. doi: 10.1165/ajrcmb.16.3.9070604. [DOI] [PubMed] [Google Scholar]
- 49.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 50.Kim KH, Burkhart K, Chen P, Frevert CW, Randolph-Habecker J, Hackman RC, Soloway PD, Madtes DK. Tissue inhibitor of metalloproteinase-1 deficiency amplifies acute lung injury in bleomycin-exposed mice. Am. J. Respir. Cell Mol. Biol. 2005;33:271–279. doi: 10.1165/rcmb.2005-0111OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Lamm WJ, Albert RK, Chi EY, Henderson WR, Jr, Lewis DB. Influence of the route of allergen administration and genetic background on the murine allergic pulmonary response. Am. J. Respir. Crit. Care Med. 1997;155:661–669. doi: 10.1164/ajrccm.155.2.9032210. [DOI] [PubMed] [Google Scholar]
- 52.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.clim.2008.08.029.