Abstract
Previous research has established that age-related decline occurs in measures of cerebral white matter integrity, but the role of this decline in age-related cognitive changes is not clear. To conclude that white matter integrity has a mediating (causal) contribution, it is necessary to demonstrate that statistical control of the white matter-cognition relation reduces the magnitude of age-cognition relation. In this research, we tested the mediating role of white matter integrity, in the context of a task switching paradigm involving word categorization. Participants were 20 healthy, community-dwelling older adults (60–85 years), and 20 younger adults (18–27 years). From diffusion tensor imaging (DTI) tractography, we obtained fractional anisotropy (FA) as an index of white matter integrity in the genu and splenium of the corpus callosum and the superior longitudinal fasciculus (SLF). Mean FA values exhibited age-related decline consistent with a decrease in white matter integrity. From a model of reaction time distributions, we obtained independent estimates of the decisional and nondecisional (perceptual-motor) components of task performance. Age-related decline was evident in both components. Critically, age differences in task performance were mediated by FA in two regions: the central portion of the genu, and splenium-parietal fibers in the right hemisphere. This relation held only for the decisional component and was not evident in the nondecisional component. This result is the first demonstration that the integrity of specific white matter tracts is a mediator of age-related changes in cognitive performance.
Neuroimaging studies have identified several forms of age-related change in brain structure that are relevant as potential mediators of age-related changes in cognitive performance. Prominent among these is age-related decline in the volume of cerebral gray matter, particularly in prefrontal regions (Raz, 2005; Raz et al., 2005; Resnick, Pham, Kraut, Zonderman, & Davatzikos, 2003; Tisserand et al., 2002). Age-related decline is also evident in cerebral white matter volume, although with a different trajectory. Whereas the age-related decline in gray matter volume is relatively linear from younger adulthood, the corresponding decline in white matter tends to be nonlinear, with a plateau in middle-age and additional decline, beyond that of gray matter, in later adulthood (Guttmann et al., 1998; Salat, Kaye, & Janowsky, 1999; but cf. Marner, Nyengaard, Tang, & Pakkenberg, 2003).
Diffusion tensor imaging (DTI) can identify age-related changes in the microstructure of white matter that may not be detectable as a volumetric decline. This imaging modality is a form of magnetic resonance imaging (MRI) that measures both the rate and directionality of the displacement distribution of water molecules across tissue components (Basser & Jones, 2002; Beaulieu, 2002; Le Bihan, 2003; A. W. Song, Wong, Tan, & Hyde, 1996). Diffusion can be described as occurring equally in all directions (isotropic) or restricted primarily to one direction (anisotropic). Diffusion tends to be more anisotropic in white matter than in gray matter due to many factors, such as the degree of compactness of white matter tracts, their myelination, and the number of axons within a specific region.
For each voxel, DTI estimates diffusion along multiple axes, typically separated into one major axis (eigenvector) of diffusion and two minor axes that are orthogonal to the primary eigenvector. The most frequently used measure of white matter integrity, fractional anisotropy (FA), is calculated on the basis of all three diffusivities (the eigenvalues λ1, λ2, λ3). The FA is a scalar measure (0–1.0) representing the relative directionality of diffusion, independent of its rate, with higher FA values representing a higher degree of directionality. Each eigenvalue represents the rate of diffusion, independent of directionality. Diffusion tensor imaging studies using animal models, confirmed by histology, have suggested that diffusivity parallel to the primary direction (axial diffusivity; λ1) is more sensitive to the effects of axonal loss or degradation than to demyelination, whereas diffusivity perpendicular to the primary direction (radial diffusivity; the mean of λ2 and λ3) is differentially sensitive to demyelination-related effects (S. K. Song, Sun, Ju, et al., 2003; S. K. Song, Sun, Ramsbottom, et al., 2002; Sun et al., 2006).
Researchers are actively applying DTI to investigate age-related changes in white matter microstructure (Moseley, 2002; Salat, Tuch, Greve et al., 2005; Sullivan & Pfefferbaum, 2006). The DTI results complement those of the volumetric studies and consistently indicate an age-related decline in FA during adulthood, suggesting a decline in white matter integrity. Sullivan et al. (2001) first reported that age-related decline in FA is relatively more pronounced in prefrontal regions, and this pattern has been confirmed in later studies (Head et al., 2004; Madden et al., 2007; Salat, Tuch, Hevelone et al., 2005; Sullivan, Adalsteinsson, & Pfefferbaum, 2006). There is regional variability in the degree of age-related decline in FA within the frontal lobe, however, and decline is also notable in the posterior limb of the internal capsule and posterior periventricular white matter (Salat, Tuch, Greve et al., 2005). Analyses of the individual eigenvalues of the diffusion tensor suggest that the age-related decline in prefrontal FA is due almost entirely to increased radial diffusivity, that is, in λ2 and λ3 (Bhagat & Beaulieu, 2004; Sullivan et al., 2006), implicating histopathological mechanisms that pertain to age-related changes in the structure or myelin, rather than a frank loss of axons.
The goal of this research was to determine whether white matter integrity, within specific fiber tracts, mediates age-related changes in cognitive performance. Age-related decline in white matter integrity may contribute to older adults’ cognitive performance by disrupting the white matter pathways connecting widely distributed cortical networks (Bartzokis et al., 2004; Makris et al., 2007; Pfefferbaum, Adalsteinsson, & Sullivan, 2005). O’Sullivan et al. (2001) and Sullivan et al. (2001) reported significant correlations between DTI measures of white matter integrity and cognitive performance. Subsequent studies (Bucur et al., in press; Grieve, Williams, Paul, Clark, & Gordon, 2007; Madden et al., 2004; Schulte, Sullivan, Muller-Oehring, Adalsteinsson, & Pfefferbaum, 2005) have confirmed this pattern, which is most often expressed as a positive relation between performance and FA; that is, individuals with higher levels of white matter integrity exhibit higher levels of task performance.
To conclude that white matter integrity is a mediator of the relation between age and cognitive performance, it is not sufficient to merely demonstrate that a correlation between white matter integrity and a cognitive measure exists, or even that the correlation differs as a function of age group. To establish that variation in white matter integrity operates as a mediating, causal mechanism between age and cognition, rather than as a moderator variable with an associative but not necessarily causal relation, three conditions are necessary (Baron & Kenny, 1986). First, age differences should exist in the measure of cognitive performance; second, age differences should exist in the measure of white matter integrity; and third, white matter integrity should account for significant variance in cognitive performance, when age is also included in the regression model. These conditions can be tested statistically within the general linear model. A mediating relation exists when the variance in cognitive performance attributable specifically to age is less in the third condition than in the first.
When the amount of age-related variance in a dependent variable (e.g., cognition) is substantially attenuated by the presence of an additional independent variable (e.g., white matter integrity), relative to when age is the sole independent variable, then the additional variable can be interpreted as a mediator of the relation between age and the dependent variable (Salthouse, 1992a; Salthouse, Atkinson, & Berish, 2003). Using this type of analytic approach with T1-weighted MRI, Brickman et al. (2006) demonstrated that white matter volume in regions of the frontal lobe mediated adult age differences in neuropsychological tests of executive functioning and memory. To our knowledge, the only DTI study demonstrating a mediational effect of white matter integrity is that of Bucur et al. (in press), who found that the average FA within two regions of interest, the genu of the corpus callosum and a pericallosal frontal region, mediated the relation between elementary perceptual speed and memory retrieval, for younger and older adults combined. The Bucur et al. study, however, did not assess the mediation of age-related variance in the behavioral measures.
In the present analyses, we sought to determine whether white matter integrity mediated age-related performance differences in a task-switching paradigm. On each trial, a cue indicated which of two categorization decisions should be performed on an upcoming target word. Performance in this type of task is typically slower and less accurate when the categorization on the current trial switches from the previous trial, relative to when it repeats, reflecting many factors involved in reconfiguring the task set (Meiran, Chorev, & Sapir, 2000; Monsell, 2003). The effects of task switching are more pronounced for older adults than for younger adults (Meiran, Gotler, & Perlman, 2001; Verhaeghen & Cerella, 2002), although the age effects are greater at the global level (i.e., a mixed-task trial block relative to a single-task trial block) than at the local level (i.e., switch trials versus repeat trials). Neuroimaging studies of younger adults suggest that task switching relies on a frontoparietal network that is activated during attention-demanding visual tasks (Braver, Reynolds, & Donaldson, 2003; Kimberg, Aguirre, & D’Esposito, 2000; Sohn, Ursu, Anderson, Stenger, & Carter, 2000).
Analyses of age differences in behavioral measures can be complicated by the overall increase in older adults’ reaction time (RT), as well as by individual differences in the relative emphasis on speed versus accuracy. To address this issue, and to isolate different components of cognitive performance, analyses of the behavioral data were based on the Ratcliff model of two-choice discrimination (Ratcliff, 1978; Ratcliff, Spieler, & McKoon, 2000; Wagenmakers, van der Maas, & Grasman, 2007). This model uses the RT distributions of both correct and incorrect responses to separate the decisional and nondecisional components of performance. The model yields several parameters, the most relevant of which, for the present purposes, are drift rate (v; the rate of accumulation towards a decision) and nondecision time (T-er; perceptual-motor response time). Thus, we examined the potential role of white matter integrity in these separate aspects of cognitive performance. In our word categorization task, for example, drift rate would reflect the retrieval of semantic information associated with the categorization response, independent of perceptual-motor speed.
We used DTI tractography (Catani, Howard, Pajevic, & Jones, 2002; Gerig, Gouttard, & Corouge, 2004; Mori & van Zijl, 2002) to characterize the diffusion properties of white matter pathways within the frontoparietal network. We performed tractography analyses for each participant (i.e., in untransformed, native space), to reduce the effect of individual differences in anatomy on tract estimation. The selected pathways were the genu and splenium of the corpus callosum and the superior longitudinal fasciculus (SLF). The genu and splenium serve as interhemispheric connection pathways for prefrontal cortex and occipitoparietal association cortex, respectively, whereas the SLF is a set of pathways connecting the frontal and parietal lobes within each hemisphere (Makris et al., 2005; Schmahmann & Pandya, 2006). Previous DTI studies have reported correlations between FA in these pathways and performance speed (Bucur et al., in press; Madden et al., 2004; Schulte et al., 2005; Sullivan et al., 2001), but the role of FA as a mediator of age-related variance in performance not been examined. In view of the central role of the frontoparietal network in task switching (Braver et al., 2003; Kimberg et al., 2000; Sohn et al., 2000), we hypothesized that FA in this network would be a mediator of age-related change in task performance. In addition, if white matter integrity within these frontoparietal regions influences the functioning of task-relevant attentional networks, then FA should mediate age-related changes in the quality of decisional information (drift rate parameter; v) rather than perceptual-motor speed (nondecision time parameter; T-er).
Methods
Participants
The research procedures were approved by the Institutional Review Board of the Duke University Medical Center, and all participants provided written informed consent. The participants were 20 younger adults (18–27 years of age; 10 women) and 20 older adults (60–85 years of age; 10 women). The younger adults were recruited from the Duke University campus, and the older adults were healthy, community-dwelling individuals recruited from the Duke Aging Center Research Participant Registry. All participants reported that they were right-handed, free of significant disease such as atherosclerosis and hypertension, and not taking medication known to affect cognitive functioning or cerebral blood flow. Review of each participant’s T2-weighted structural images by a neuroradiologist (one of the authors, J.M.P.) indicated that all were within normal limits with regard to abnormalities related to atrophy, ventricular dilation, and the presence of white matter hyperintensities.
Demographic and psychometric data (Table 1) were obtained approximately two weeks prior to the scanning session. All participants possessed corrected visual acuity of at least 20/40 and possessed at least a secondary school education (12 years). Participants scored a minimum of 27 on the Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975) and a maximum of 9 on the Beck Depression Inventory (Beck, 1978). During the psychometric testing session, participants also completed a practice version of the task-switching test (with different stimuli) that was used in the subsequent scanning session.
Table 1.
Participant Variables by Age Group
|
M |
SD |
|||
|---|---|---|---|---|
| Younger | Older | Younger | Older | |
| Age (years) | 22.40a | 69.60b | 2.50 | 6.05 |
| Education (years) | 15.75a | 16.40a | 2.22 | 2.82 |
| MMSE | 29.32a | 28.47b | 0.60 | 1.12 |
| BDI | 2.65a | 2.90a | 2.74 | 2.77 |
| Vocabulary | 65.60a | 65.05a | 3.15 | 3.80 |
| Digit Symbol Acc | 98.52a | 95.62b | 1.46 | 1.89 |
| Digit Symbol RT | 1338.40a | 1836.20b | 249.75 | 314.16 |
Note. n = 20 per age group. Vocabulary = raw score (maximum of 70) on the Wechsler Adult Intelligence Scale-Revised (Wechsler, 1981); BDI = points above zero on the Beck Depression Inventory (Beck, 1978); Digit Symbol Acc and Digit Symbol RT = percentage correct and reaction time (ms), respectively, on a computer test of digit-symbol coding (Salthouse, 1992b); MMSE = raw score (maximum 30) on the Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975). Means in the same row that do not share subscripts differ by t-test at p < .05.
Behavioral Task
The behavioral task that participants performed during scanning was a version of task switching, modeled after that of Braver et al. (2003). Participants completed six blocks of 51 test trials, corresponding to six fMRI runs. The fMRI data will be reported in a separate article. Participants viewed the task events with MRI-compatible goggles and responded manually (with the index finger of each hand) via a fiber-optic response box. Within each run, there were three on-task periods, separated by four off-task periods (one of which always occurred at the beginning of the scanner run). This is a mixed blocked/event-related design (Visscher et al., 2003). Each run was approximately 7 min 40 s in duration, comprised of the four off-task periods (19.5 s each) and three on-task periods (approximately 2 min 7 s each).
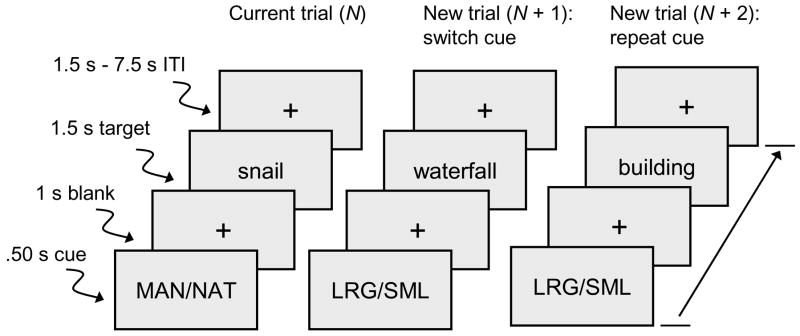
As illustrated in Figure 1, two types of trials occurred during each on-task period: cue-plus-target (12 per on-task period), and cue-only (4 per on-task period). On cue-plus-target trials, participants viewed a cue message and then made a two-choice categorization response to a subsequent target word (a concrete noun). The cue, which was either MAN/NAT or LRG/SML, indicated the type of category decision to be made regarding the target word on the current trial, either manmade/natural or large/small. Manmade/natural referred to artificial (e.g., sailboat) and natural (e.g., lemon) object categories, and large/small referred to whether the referent of the word was larger (e.g., deer) or smaller (e.g., bullet) than a computer monitor. Words were selected to be familiar concrete nouns that could be easily categorized in terms of these criteria. On cue-only trials, the cue message was not followed by a target. These latter trials were included to allow estimation of cue-specific hemodynamic activity and are not reported in the results.
Figure 1.
Sequence of events on each trial. The cue display indicated the type of category decision (manmade/natural or larger/smaller than the computer monitor) to be made regarding the target word. Participants responded at the onset of target. Trials are defined as repeat or switch in relation to the cue on the immediately preceding trial. ITI = inter-trial interval.
Each cue-plus-target trial was coded as either a repeat or a switch trial, on the basis of whether the associated cue was the same as, or different from, the cue on the immediately preceding trial (even if the preceding trial was cue-only). Within each of the six runs, there were 36 cue-plus-target trials, comprising 18 switch trials and 18 repeat trials. The number of successive trials with the same cue was limited to five. Across the six runs, the two types of categorization decisions, and the two alternative responses within each category, occurred an approximately equal number of times, on both switch and repeat trials.
At the beginning of each run, during the first off-task period, a row of three white fixation crosses appeared in the center of the viewing area for 19.5 s. These three crosses also remained in view during the other three off-task periods. During the on-task periods (Figure 1), each trial began with a 0.50 s cue and was followed by a 1.0 s single fixation cross; the target word then occurred for 1.50 s. Following the offset of the target, a variable blank period (with a single fixation cross) occurred for either 1.50 s, 3.0 s, 4.50 s, 6.0 s, or 7.50 s. The words were presented in large (56 point) white Arial font against a black background. During target word presentation, the two response options were presented at the bottom of the viewing area, at the left and right sides, corresponding to the location of the assigned buttons on the response box. The fixation cross was red following the cue but white following the target, to provide a clear indication of cue-only trials (i.e., the fixation cross changed from red to white). An off-task period followed each (randomized) set of 12 cue-plus-target trials and 4 cue-only trials.
Participants completed one block of 51 practice trials, while situated in the scanner, before performing the test trials. The instructions were to read the cue and to respond as quickly as possible to the target while still being correct. The manmade and large responses were always assigned to one response button and the natural and small responses were always assigned to the other button, but the assignment of the response-pairs to the individual buttons was varied across participants within each age group, as was the presentation order of the six blocks of test trials.
Imaging Protocol
Acquisition parameters
Scanning was conducted on a 3T GE Signa Excite MRI scanner (GE Healthcare, Waukesha, WI) with 50mT/m gradients, in concert with an 8-channel head coil. Head motion was minimized with a vacuum-pack system molded to each participant’s head. Participants wore foam-insert earplugs to counteract scanner noise. The scanning session began with a T1-weighted sagittal localizer series, spanning the midline, followed by four other series, in the following order: T1-weighted, T2-weighted, six runs of T2*-weighted (functional), and two runs of DTI. With the exception of the T1-weighted localizer series, slice orientation was near-axial, parallel to the anterior-posterior commissure (AC-PC) plane.
The near-axial T1-weighted images were acquired with a high-resolution, 3D fast inverse-recovery-prepared spoiled gradient recalled (SPGR) sequence (256 × 256 matrix; TR = 7.4 ms; TE = 3 ms; flip angle = 12°; FOV = 25.6 cm), yielding 104 contiguous slices of 1.2 mm (1 mm2 in-plane resolution). Each of the DTI sequences used a dual spin echo base sequence (128 × 128 matrix; TR = 17000 ms; TE = 86.7 ms; flip angle = 90°; FOV = 25.6 cm), yielding 52 contiguous slices of 2.4 mm (1 mm2 in-plane resolution). Diffusion was measured from 15 non-collinear directions at a b factor of 1000 s/mm2; one reference image had no diffusion weighting. To improve the signal-to-noise ratio, the two DTI runs were merged to a single data set for each participant. The resulting images were skull-stripped in order to remove non-brain and ambient noise. We calculated diffusion tensors from the 15 diffusion weighted images, based on a least squares fit of the tensor model to the diffusion data (Basser & Jones, 2002). Diagonalization of the tensor yielded three voxel-specific eigenvalues (λ1 > λ2 > λ3), which represent diffusivities along the three principal directions of the tensor.
Fiber tracking
The derivation of white matter fiber tracts from the initial DTI datasets was conducted at the individual participant level, in untransformed (native) space, with FiberTracking 2.2.1 and FiberViewer 1.2.3 software (http://www.ia.unc.edu/dev/; Gerig et al., 2004). The software employs a backtracking method (Mori & van Zijl, 2002) that estimates the direction of greatest diffusivity (λ1) between operator-defined target and source regions, while eliminating fibers terminating operator-defined exclusion regions. Because the 2.4 mm slice thickness of the DTI images was a multiple of the 1.2 mm slice thickness of the T1-weighted SPGR images, the latter were resampled to align with the tensors. Four trained operators, blind to participant age, used ITK-SNAP software (http://www.itksnap.org/; Yushkevich et al., 2006) to draw target, source, and exclusion regions on the axial slices of each participant’s SPGR image. In the case of significant head movement between SPGR and DTI sequence, regions were drawn on the tensor b0 image. The fiber tracking output comprises representations of fiber bundles that are estimated to pass through the target regions from the source regions, eliminating fibers from the exclusion regions.
We performed tractography for four pathways: fibers coursing through the genu of the corpus callosum, fibers coursing through the splenium of the corpus callosum connecting to the occipital lobe, fibers of the splenium connecting to the parietal lobe, and fibers associated with the superior longitudinal fasciculus (SLF). The target regions in the genu and splenium were drawn to generate fiber tracts from bilateral sources, and the target regions in the SLF were drawn in the left and right hemispheres separately. Fiber tracking parameters for the genu and splenium included a minimum FA of 0.15, a fiber tracking threshold (neighborhood voxel coherence) of 0.125, a minimum fiber number of 50, and an angle of maximum deviation of 35 degrees. Because the SLF is more difficult to distinguish from neighboring gray matter than is the corpus callosum, the minimum FA was set to 0.35 for the SLF. Occasional extraneous fibers were eliminated through length-based fiber set thresholding and Hausdorff (distance-based fiber set clustering) methods incorporated into the FiberViewer software.
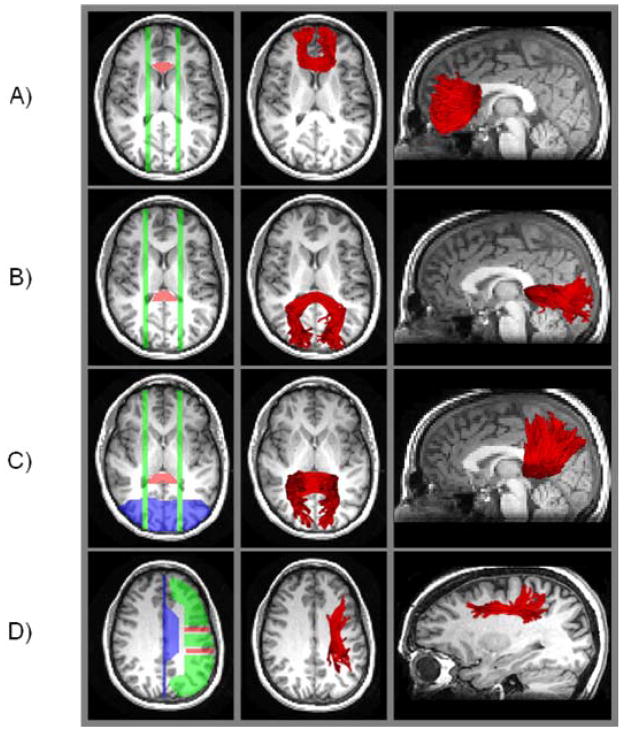
Examples of target regions, source regions, exclusion regions, and fiber tracts for a single participant are presented in Figure 2. The target regions were defined using anatomical landmarks (Duvernoy, 1999; Makris et al., 2005; Schmahmann & Pandya, 2006).For the genu and splenium, we used a method similar to that of Sullivan et al. (2006). We located the target region for the genu using the anterior horns of the lateral ventricle as lateral boundaries (in the axial view), with the anterior cingulate gyrus as the anterior boundary. Operators drew this target region on 3–5 axial slices, using the presence of the body of the corpus callosum in the axial view as a superior boundary. Source regions for the genu were two parasagittal planes (one per hemisphere; each three slices wide), placed at a point 4–6 sagittal slices from the axial midline (at approximately the lateral boundaries of the target region), spanning the entire brain in the sagittal view.
Figure 2.
Examples of fiber tracts (in red) generated by target and source region placement, for a single participant. The orange areas are target regions, the green areas are source regions, and the blue areas are exclusion regions. All of these regions are operator defined, for each participant, using anatomical boundaries. The fiber tracking algorithm estimates tracts that pass through the target regions from the source regions, eliminating any fibers terminating in the exclusion regions. The approximate locations of output fiber tracts are illustrated by overlaying on a single-slice T1-weighted image. Panel A = genu; Panel B = splenium-occipital; Panel C = splenium-parietal; Panel D = superior longitudinal fasciculus.
The target for the splenium-occipital region was also located on 3–5 axial slices, using the presence of the body of the corpus callosum in the axial view as a superior boundary, although these slices were not constrained to correspond exactly to those used for the genu. In the axial view, the splenium-occipital target region was defined to be a section that is just medial to the posterior horns of the lateral ventricle. The posterior boundary was the posterior cingulate gyrus. Source regions were two parasagittal planes, located similarly to those for the genu, but slightly more lateral, 6–8 sagittal slices from the axial midline. To isolate fibers connecting the splenium and occipital lobe, tract estimation was constrained to exclude any fibers terminating in the parietal lobe.
The splenium-parietal target and source regions were defined similarly to the splenium-occipital case, except that tract estimation was constrained to exclude any fibers terminating in the occipital lobe. As a result of these constraints implemented in the fiber tracking algorithm, the fibers estimated for the splenium-occipital and splenium-parietal regions, though passing through the splenium in each case, were mutually exclusive.
The SLF comprises four anatomical subcomponents (Makris et al., 2005; Schmahmann & Pandya, 2006). We attempted to limit analysis, as much as possible, to SLF II, which occupies the central core of white matter, above the insula, between the frontal and parietal lobes of each hemisphere. Each SLF target region (per hemisphere) spanned three consecutive coronal slices. To define the first, posterior target region, the central sulcus was located in the upper axial slices, and three consecutive coronal slices were selected for the target, with the middle slice positioned at the most lateral point of the central sulcus. The target region on these three coronal slices included gray and white matter from the top of the brain to the to the lateral fissure, excluding the cingulate gyrus. The second, anterior target region, was also located in the axial view, at the slice just superior to the ventricles. This region comprised three coronal slices, with the middle one positioned at the most lateral point of the precentral sulcus. This region also included the gray and white matter between the top of the brain and the lateral fissure, excluding the cingulate gyrus.
Operators drew three source regions for the SLF; these spanned the axial slices between the top of the brain and the top of the ventricles, excluding the cingulate gyrus, within each hemisphere. The first source comprised frontal lobe gray and white matter anterior to the anterior target region, the second source was the gray and white matter between the anterior and posterior target regions; and the third source was gray and white matter between the posterior target region and the parieto-occipital sulcus. Tract estimation was additionally constrained to exclude fibers terminating in the temporal or occipital lobe.
Within the genu and splenium regions, tract length was limited to 18 mm on either side of the axial midline. We divided the genu and splenium into center, left, and right segments, using a point 5 mm on either side of the midline 0 value as the lateral boundaries of the center segment, and the 6–18 mm on either side as the left and right segments. For the SLF, tract length was limited to 18 mm anterior and posterior to the central sulcus (i.e., anterior and posterior segments of 18 mm each).
Results
Task Switching Performance
Mean reaction time and error rate
We eliminated those cue-plus-target trials on which the participant either did not respond or responded with a RT < 300 ms or > 2500 ms (< 2% of trials within each age group). The mean RT for correct responses (across all six runs) and the mean percentage accuracy are presented in Table 2. Analysis of variance (ANOVA) of the mean RT data, using age group as a between-subjects variable and task condition (switch, repeat) as a within-subjects variable, yielded only a main effect of age group, F(1, 38) = 15.56, p < .001, reflecting the age-related increase in RT. For accuracy, only the main effect of condition was significant, F(1, 38) = 44.90, p < .0001, reflecting the higher accuracy for repeat trials than for switch trials.
Table 2.
Mean Reaction Time and Error Rate, in Task Switching, by Age Group and Task Condition
|
M |
SD |
|||
|---|---|---|---|---|
| Younger | Older | Younger | Older | |
| Repeat Trials | ||||
| Reaction time (ms) | 897.30 | 1038.12 | 105.34 | 124.97 |
| Accuracy (%) | 95.46 | 93.47 | 2.22 | 3.71 |
|
| ||||
| Switch Trials | ||||
| Reaction time (ms) | 899.06 | 1046.09 | 115.40 | 118.04 |
| Accuracy (%) | 92.27 | 91.44 | 2.92 | 2.68 |
Note. n = 20 per age group.
Reaction time model
We separated the decisional and nondecisional components of performance with a simplified version of the Ratcliff model of two-choice discrimination (Ratcliff, 1978; Ratcliff, Spieler, & McKoon, 2000) developed by Wagenmakers et al. (2007). The Wagenmakers et al. implementation combines the information from the mean and variance of RT for correct responses, and the mean accuracy, for each participant, to estimate three parameters: the quality of decision information (drift rate; v), the time required for perceptual-motor processes (nondecision time; T-er), and response conservativeness (cautiousness; a). We obtained separate estimates of drift rate and nondecision time for the repeat and switch trials. The drift rate, representing the accumulation of information, can vary as a function of the decision process on each trial. Although nondecision time should not, in theory, vary across different types of trials intermixed within a block, we analyzed nondecision time for switch and repeat trials separately because there is evidence that task difficulty can influence the nondecision time parameter (Spaniol, Madden, & Voss, 2006). Analysis of the cautiousness parameter was limited to the participant level, because the participants’ response conservativeness (i.e., amount of evidence required for a particular response) should not fluctuate systematically within a block of trials.
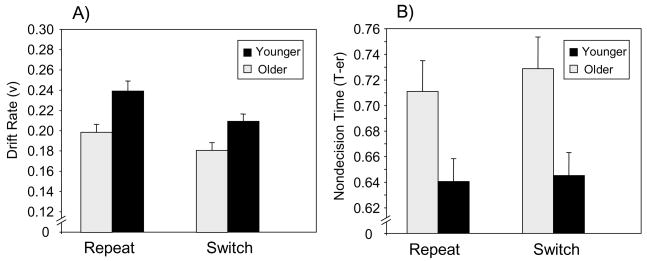
Analysis of variance of the drift rate and nondecision time data (Figure 3) treated age group as a between-subjects variable and task condition (repeat vs. switch) as a within-subjects variable. Significant main effects of age group indicated that older adults exhibited a lower drift rate, F(1, 38) = 10.74, p < .01, and higher nondecision time, F(1, 38) = 6.84, p < .05, than younger adults. Switch trials were associated with a lower drift rate, F(1, 38) = 36.52, p < .0001, and higher nondecision time, F(1, 38) = 5.80, p < .05, than repeat trials. The Age Group × Task Condition interaction was not significant for either drift rate or nondecision time. The value of the a parameter was higher for older adults (M = 0.14; SD = 0.02) than for younger adults (M = 0.13; SD = 0.02), t(38) = 2.65, SE = 0.005, p < .01, indicating an age-related increase in cautiousness.
Figure 3.
Mean parameter estimates (Wagenmakers, van der Maas, & Grasman, 2007) of task switching performance, as a function of age group and repeat versus switch trials. Panel A = the drift rate parameter (v), representing the quality of decision processing; Panel B = the nondecision time parameter (T-er), representing perceptual encoding and response time.
Fiber Tracking Data
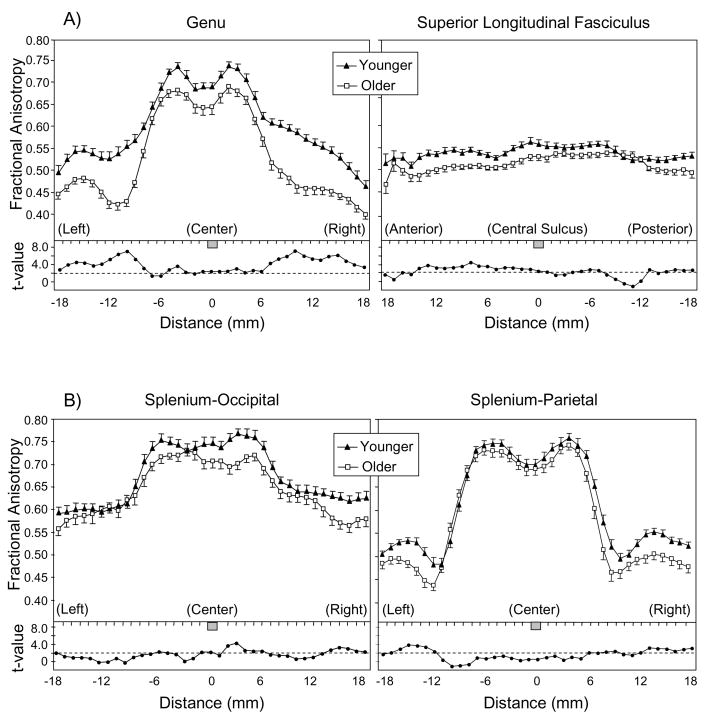
Mean FA values within the estimated fiber tracts, at millimeter intervals along the estimated tract, are presented in Figure 4. (Splenium-parietal data for one older adult were eliminated because the fiber tracking algorithm did not generate the minimum number of 50 fibers.) We performed ANOVAs of FA for each region, using age group as a between-subjects variable, and segment as a within-subjects variable. For the SLF, hemisphere was an additional within-subjects variable. The results of these analyses (Table 3) confirm the pattern evident in Figure 4: For each of the four regions, there was a significant main effect of age group, reflecting an age-related decline in FA. Each region also exhibited a significant effect of segment. In the case of the genu and both splenium regions, this effect represented a decline in FA for the peripheral segments relative to the central segment. For the SLF, the segment effect represented lower FA values anterior to the central sulcus than posterior to it.
Figure 4.
Mean fractional anisotropy (FA) as a function of age group and interval along the tract. Error bars represent 1 SE. Panel A = genu and superior longitudinal fasciculus; Panel B = splenium-occipital and splenium-parietal. For genu and splenium, the tracts are oriented left-right, with 0 = axial midline. For the superior longitudinal fasciculus, the tracts are oriented anterior-posterior and 0 = central sulcus. Below the mean FA data, t-values are plotted for the age group comparison at each point along the tract. The dotted line represents the significant t(38) value for p < .05, two-tailed.
Table 3.
Significant ANOVA Effects for Fractional Anisotropy
| Region | Effect | df | F | p |
|---|---|---|---|---|
| Genu | ||||
| Grp | 1, 38 | 40.03 | <.0001 | |
| Segment | 2, 76 | 578.58 | <.0001 | |
| Grp × Segment | 2, 76 | 7.39 | 0.0012 | |
|
| ||||
| SLF | ||||
| Grp | 1, 38 | 22.18 | <.0001 | |
| Segment | 1, 38 | 13.34 | 0.0008 | |
| Grp × Segment | 1, 38 | 6.43 | 0.0155 | |
| Hem × Segment | 1, 38 | 5.65 | 0.0226 | |
|
| ||||
| Splenium-occipital | ||||
| Grp | 1, 38 | 8.93 | 0.0049 | |
| Segment | 2, 76 | 172.52 | <.0001 | |
|
| ||||
| Splenium-parietal | ||||
| Grp | 1, 37 | 8.31 | 0.0065 | |
| Segment | 2, 74 | 480.09 | <.0001 | |
| Grp × Segment | 2, 74 | 2.77 | 0.0693 | |
Note. SLF = superior longitudinal fasciculus; Grp = age group; Hem = hemisphere; Segment = center, left, right for genu and splenium regions; Segment = anterior, posterior for SLF; n = 20 per age group except for older adults’ Splenium-Parietal, where n = 19.
In the FA analyses of the regions, two Age Group × Segment interactions were significant, indicating variation in the age difference as a function of the segment within the region. The first Age Group × Segment interaction, for the genu, resulted from a more pronounced age-related decline in FA for the peripheral segments than for the central segment. (The splenium-parietal data exhibited a marginally significant trend towards a similar pattern.) The second Age Group × Segment effect, for the SLF, occurred as the result of greater age-related decline in FA anterior to the central sulcus than in the more posterior segment of this tract. The SLF also exhibited a Hemisphere × Segment interaction, because the lower mean FA for the anterior segment, relative to the posterior segment, was present for both hemispheres but was greater for the left hemisphere (M difference in FA of 0.0179) than for the right hemisphere (M difference in FA of 0.0026).
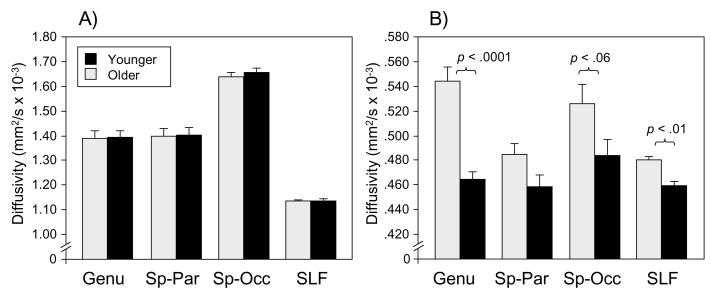
We also conducted analyses of the age difference for axial diffusivity (λ1) and radial diffusivity (mean of λ2 and λ3) separately (averaged over within-tract segments), to test previous observations that age-related change is specific to radial diffusivity (Bhagat & Beaulieu, 2004; Sullivan et al., 2006). The most important aspect of these results, illustrated in Figure 5, is that no region exhibited a significant age difference in axial diffusivity. Significant age-related increases in radial diffusivity occurred for the genu, F(1, 38) = 19.15, p < .0001, and the SLF, F(1, 38) = 8.62, p < .01,with a similar though marginal effect in the splenium-occipital region F(1, 38) = 3.76, p < .06.
Figure 5.
Diffusivity as a function of age group and region. Panel A = axial diffusivity (the first eigenvalue, λ1); Panel B = radial diffusivity (the mean of the second and third eigenvalues, λ2, and λ3); Sp-Par = splenium-parietal; Sp-Occ = splenium-occipital; SLF = superior longitudinal fasciculus.
Mediation of Task Switching by White Matter Integrity
We limited the mediation analyses to the performance measures of drift rate and nondecision time, because these latter measures (unlike the cautiousness parameter) reflect the quality of information processing for individual decisions, which in turn would rely on the integrity of task-relevant neural networks. In addition, because our goal was to determine the mediation of age-related effects, and the magnitude of the age group differences in drift rate and nondecision time did not vary significantly as a function of switch versus repeat trials, we averaged over these different trial types and obtained a single drift rate and nondecision time for each participant.
For both nondecision time and drift rate, regression models were developed for each regional FA value as a predictor of performance, with age group included in the model (i.e., covaried). Three fiber-tract segments (splenium-occipital left, splenium-parietal center, and SLF left-posterior) were excluded from further analyses because they did not exhibit a significant age-related change (Table 4). For nondecision time, none of the 10 candidate FA values was significant as a predictor when covaried for age group. For drift rate, age-covaried FA values from two regions were significant as predictors: genu center, β = 0.249, SE = 0.124, p = .05, and splenium-parietal right, β = 0.348, SE = 0.151, p < .05. Parameter estimates for both regions were positive, indicating that individuals with higher FA values also had higher drift rates (i.e, increased white matter integrity was associated with more efficient accumulation of evidence).
Table 4.
Test of Age Group Difference in Mean Fractional Anisotropy, by Region
| Region | Younger | Older | Younger | Older | |||
|---|---|---|---|---|---|---|---|
| M | SD | t | df | p | |||
| Genu center | 0.7088 | 0.6628 | 0.0272 | 0.0567 | 3.27 | 38 | 0.0023 |
| Genu left | 0.5624 | 0.4915 | 0.0277 | 0.0414 | 6.36 | 38 | <.0001 |
| Genu right | 0.5570 | 0.4677 | 0.0286 | 0.0489 | 7.05 | 38 | <.0001 |
|
| |||||||
| Splenium-occipital center | 0.7490 | 0.7129 | 0.0454 | 0.0417 | 2.62 | 38 | 0.0125 |
| Splenium-occipital left | 0.6357 | 0.6178 | 0.0303 | 0.0398 | 1.59 | 38 | 0.1195 |
| Splenium-occipital right | 0.6481 | 0.6129 | 0.0345 | 0.0409 | 2.94 | 38 | 0.0056 |
|
| |||||||
| Splenium-parietal center | 0.7275 | 0.7138 | 0.0482 | 0.0412 | 0.95 | 37 | 0.3466 |
| Splenium-parietal left | 0.5674 | 0.5468 | 0.0269 | 0.0311 | 2.21 | 37 | 0.0332 |
| Splenium-parietal right | 0.5552 | 0.5119 | 0.0343 | 0.0344 | 3.94 | 37 | 0.0004 |
|
| |||||||
| SLF right-anterior | 0.5449 | 0.5108 | 0.0174 | 0.0248 | 5.05 | 38 | <.0001 |
| SLF right-posterior | 0.5420 | 0.5189 | 0.0225 | 0.0192 | 3.50 | 38 | 0.0012 |
| SLF left-anterior | 0.5393 | 0.5101 | 0.0356 | 0.0229 | 3.08 | 38 | 0.0038 |
| SLF left-posterior | 0.5485 | 0.5368 | 0.0250 | 0.0136 | 1.85 | 38 | 0.0721 |
Note. SLF = superior longitudinal fasciculus. n = 20 per age group except for the older adults’ Splenium-Parietal region, where n = 19.
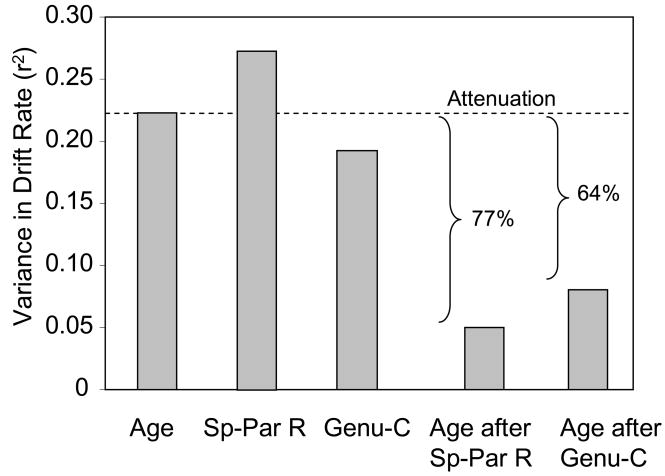
We used hierarchical regression to estimate the amount of age-related variance in drift rate, when FA was entered into the model before age group, relative to when age group was the only predictor (Salthouse, 1992a). The unique variance in drift rate associated with age group, following FA, divided by the variance associated with age group as the sole predictor, is an indicator of the degree to which FA attenuates the age-related variance.
The results, illustrated in Figure 6, indicate that when used as sole predictors, there were significant components of variance in drift rate for age group, β = −0.174, SE = 0.005, p < .01, genu center, β = 0.367, SE = 0.116, p < .01, and splenium-parietal right, β = 0.482, SE = 0.130, p < .001. When entered before age group, the splenium-parietal right FA variable led to a substantial attenuation in age-related variance, and age group did not remain significant as a predictor of drift rate. The genu center FA variable, when entered before age group, also led to a pronounced attenuation in the age-related variance in drift rate, though age group remained significant as a predictor, β = −0.123, SE = 0.006, p < .05.
Figure 6.
Attenuation of age-related variance in drift rate (decisional processing) by regional FA. The first three bars (from left to right) in the graph illustrate the variance in drift rate associated with age group, FA in the right-hemisphere splenium-parietal tract (Sp-Par R), and FA in the central genu (Genu-C), when each of these variables is used as the sole predictor of drift rate. The last two bars in the graph illustrate the attenuation of age-related variance when either the splenium or genu FA variables are entered before age group in the regression model. The substantial attenuation indicates that these variables are mediators of age differences in this aspect of task performance.
Discussion
DTI Findings
Using DTI tractography, we characterized white matter integrity within several pathways of the frontoparietal network and tested whether integrity (as indexed by FA) in this network mediated adult age differences in cognitive performance during task switching. We relied on the definition of mediation by Baron and Kenny (1986), which required that several conditions be met: the existence of age differences in both cognitive performance and FA, and a significant relation between FA and cognition when age-related effects in cognition were controlled statistically. The main finding was that, as hypothesized, mean FA in two regions, the central portion of the genu, and the splenium-parietal fibers in the right hemisphere, was related to performance independently of age and provided substantial attenuation of the age-related variance in performance (Figure 6). In addition, as discussed below, FA was related to a measure of the quality of decisional information and not to the perceptual-motor (nondecisional) component. The present study is, to our knowledge, the first demonstration of the mediation of age-related differences in cognitive performance by white matter integrity.
Age-related decline in FA was distributed throughout nearly all of the selected pathways. The decline was numerically greatest the peripheral segments of the genu (Figure 4), consistent with previous observations of greater age-related decline in FA in anterior brain regions (Head et al., 2004; Sullivan et al., 2001, 2006; Sullivan & Pfefferbaum, 2006). The analysis of axial and radial diffusivity suggested that the age-related changes in FA were not equally apparent in all three eigenvalues but were instead limited entirely to radial diffusivity (Figure 5). This age-related increase in diffusivity perpendicular to the primary eigenvalue confirms previous findings (Bhagat & Beaulieu, 2004; Sullivan et al., 2006) and suggests that the primary neurobiological mechanism of age-related change in white matter integrity is decreased integrity of myelin rather than axonal damage or loss (S. K. Song, Sun, Ju, et al., 2003; S. K. Song, Sun, Ramsbottom, et al., 2002; Sun et al., 2006). However, the contributions of axonal loss (Marner et al., 2003) to changes in DTI assessments of cerebral white matter in the aging brain are not well understood and may also result in age-related increases in λ2 and λ3.
The two regions that we found to be mediators, the genu and splenium of the corpus callosum, have been identified previously in correlational studies of white matter and behavior. Previous studies have found that increased perceptual speed is associated with increased white matter integrity in the genu (Bucur et al., in press; Schulte et al., 2005; Sullivan et al., 2001). White matter integrity of the splenium has also exhibited similar correlations with speed-dependent measures (Madden et al., 2004; Sullivan et al., 2001). The present data suggest that splenium fibers leading to the parietal lobe, especially in the right hemisphere, are related more closely to age differences in cognitive performance than are splenium-occipital fibers. The cortical network that is active during task switching involves dorsolateral prefrontal and superior parietal regions (Braver et al., 2003; Kimberg et al., 2000; Sohn et al., 2000). Previous fMRI investigations of visual attention have also noted that attention-dependent parietal activations are relatively greater in the right hemisphere (Corbetta & Shulman, 2002; Kim et al., 1999). Age-related decline in white matter integrity would lead to some degree of graded, functional disconnection among task-relevant cortical regions in the frontoparietal network (Bartzokis et al., 2004; Makris et al., 2007; O’Sullivan et al., 2001; Pfefferbaum et al., 2005; Sullivan & Pfefferbaum, 2006).
Current models of age-related change in brain function frequently propose that age-related deficits in cognitive performance reflect the interactive effects of structural changes in the frontal lobe and the compensatory recruitment of these regions to support task performance (Cabeza, 2002; Davis, Dennis, Daselaar, Fleck, & Cabeza, in press; Dennis & Cabeza, 2008; Grady, 2000). In view of the mediational effects associated with the splenium as well as the genu, however, we emphasize that white matter integrity of posterior brain regions is also relevant. The cortical networks activated by attention-demanding tasks are distributed widely and vulnerable to disruption at posterior as well as anterior sites (Andrews-Hanna et al., 2007; Greenwood, 2000; Tisserand & Jolles, 2003). In addition, although age-related decline in FA for the SLF was greater in the portion of this pathway anterior to the central sulcus than posterior to it, the anterior SLF was not a significant mediator of age differences in performance. Thus, although age-related decline may be detected more frequently within anterior brain regions, whether a particular white matter region serves as a behavioral mediator is not dependent on the magnitude of age-related decline in FA for that region (Madden et al., 2004).
The tractography method that we used, applied at the individual participant level, has the advantage of taking individual differences in anatomy into account, but several sources of error exist, including the thresholding, the use of non-isotropic voxel dimensions, the presence of crossing fibers, and partial volume effects. It would consequently be valuable to have converging evidence, regarding the age differences in white matter integrity that we have observed, from tractography based on spatially normalized data and isotropic voxel dimensions.
Behavioral Findings
We separated the decisional and nondecisional components of performance by applying a quantitative model that combines information from the distribution of RT and accuracy across trials (Ratcliff, 1978; Ratcliff, Spieler, & McKoon, 2000; Wagenmakers et al., 2007). This model has been applied previously to age differences in other forms of two-choice discrimination (Ratcliff, Thapar, & McKoon, 2004; Spaniol, Madden, & Voss, 2006), but has not been investigated in the context of task switching. The results (Figure 3) demonstrated that, relative to younger adults, older adults exhibited both a decline in the quality of the decisional component (drift rate, v), and an increase in the time required by nondecisional, encoding and response processes (T-er). We also obtained an age-related increase in cautiousness (a). These findings confirm previous age-related effects applications of this model (Ratcliff, Thapar, & McKoon, 2004; Spaniol, Madden, & Voss, 2006). New findings were the decreased drift rate and increased nondecision time for switch trials, relative to repeat trials, reflecting the increased attentional demands of task switching as expressed in these model parameters. The effects of aging and task switching were not interactive, however, and the increased difficulty of the switch trials relative to repeat trials was similar for the two age groups. This pattern is consistent with previous studies of age differences in task switching. Global switch costs (i.e., the difference between tasks involving switching and those involving a single, constant decision) are typically higher for older adults than for younger adults, whereas local switch costs (i.e., the cost of switching across adjacent trials) is less age-dependent (Meiran et al., 2001; Verhaeghen & Cerella, 2002).
Because the difference between switch and repeat trials was similar for the two age groups, we averaged across these trials to investigate the mediation of age-related effects in the drift rate and nondecision time measures. The mediational effect of white matter integrity that we observed (Figure 6) consequently refers to performance of the manmade/natural and large/small categorization decisions, independently of the effects of switching between these tasks. The role of white matter integrity, however, was specific to the decisional component of this categorization (e.g., retrieval of the requisite semantic information); mediation was not associated with time required for nondecisional processes. Paus et al. (2001) emphasized that, in studies of white matter-behavioral correlations, information processing tasks have an advantage, relative to standardized psychometric tests, by providing better access to the “building blocks” of cognitive abilities. The present findings support the Paus et al. proposal and suggest that correlations between FA and behavioral measures (Sullivan & Pfefferbaum, 2006) are not dependent entirely on perceptual-motor processing and may instead reflect decision-level processing. Application of tractography to pathways more directly involved with perceptual-motor processing (e.g., corticospinal tracts), may yield correlations between FA and nondecision time. From the present results, we conclude that some decision-related processes operate less efficiently for older adults than for younger adults, and that age-related decline in the white matter integrity within specific regions of the frontoparietal network (central genu and splenium-parietal fibers in the right hemisphere) has a causal role in this form of age-related cognitive change.
Acknowledgments
This research was supported by NIH research grants R01 AG011622 (DJM), R01 AG019731 and R01 AG23770 (RC), R21 AG30771 (SAH), and NIH training grant T32 AG000029 (MCC, BB, & NAD). We are grateful to Susanne M. Harris, Anne M. Shepler, and Leslie Crandell Dawes, for technical assistance, and to Allen Song for advice during this research.
Contributor Information
David J. Madden, Duke University Medical Center
Julia Spaniol, Duke University Medical Center.
Matthew C. Costello, Duke University Medical Center
Barbara Bucur, Duke University Medical Center.
Leonard E. White, Duke University Medical Center
Roberto Cabeza, Duke University.
Simon W. Davis, Duke University
Nancy A. Dennis, Duke University
James M. Provenzale, Duke University Medical Center
Scott A. Huettel, Duke University Medical Center
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: Implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiology of Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: Theory, experimental design and data analysis - a technical review. NMR in Biomedicine. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beck AT. The Beck depression inventory. New York: Psychological Corporation; 1978. [Google Scholar]
- Bhagat YA, Beaulieu C. Diffusion anisotropy in subcortical white matter and cortical gray matter: Changes with aging and the role of CSF-suppression. Journal of Magnetic Resonance Imaging. 2004;20:216–227. doi: 10.1002/jmri.20102. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, et al. Regional white matter and neuropsychological functioning across the adult lifespan. Biological Psychiatry. 2006;60:444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, White LE, Cabeza R, et al. Age-related slowing of memory retrieval: Contributions of perceptual speed and cerebral white matter integrity. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2007.02.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior anterior shift in aging. Cerebral Cortex. doi: 10.1093/cercor/bhm155. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3. New York: Psychology Press; 2008. pp. 1–54. [Google Scholar]
- Duvernoy HM. The human brain: Surface, three-dimensional sectional anatomy with MRI, and blood supply (2nd ed.) New York: Springer-Verlag; 1999. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gerig G, Gouttard S, Corouge I. Analysis of brain white matter via fiber tract modeling. Conference Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2004;6:4421–4424. doi: 10.1109/IEMBS.2004.1404229. [DOI] [PubMed] [Google Scholar]
- Grady CL. Functional brain imaging and age-related changes in cognition. Biological Psychology. 2000;54:259–281. doi: 10.1016/s0301-0511(00)00059-4. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. Journal of the International Neuropsychological Society. 2000;6:705–726. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: A diffusion tensor MR imaging study. AJNR American Journal of Neuroradiology. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, et al. White matter changes with normal aging. Neurology. 1998;50:972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Kim YH, Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Mesulam MM. The large-scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. NeuroImage. 1999;9:269–277. doi: 10.1006/nimg.1999.0408. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D’Esposito M. Modulation of task-related neural activity in task-switching: An fMRI study. Brain Research Cognitive Brain Research. 2000;10:189–196. doi: 10.1016/s0926-6410(00)00016-1. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nature Reviews Neuroscience. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, et al. Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study. Neurobiology of Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: Relation to response time. NeuroImage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, van der Kouwe A, Kennedy DN, Hodge SM, Dale A, et al. Frontal connections and cognitive changes in normal aging rhesus monkeys: A DTI study. Neurobiology of Aging. 2007:1556–1567. doi: 10.1016/j.neurobiolaging.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. Journal of Comparative Neurology. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Meiran N, Chorev Z, Sapir A. Component processes in task switching. Cognitive Psychology. 2000;41:211–253. doi: 10.1006/cogp.2000.0736. [DOI] [PubMed] [Google Scholar]
- Meiran N, Gotler A, Perlman A. Old age is associated with a pattern of relatively intact and relatively impaired task-set switching abilities. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2001;56:P88–102. doi: 10.1093/geronb/56.2.p88. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Science. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC. Fiber tracking: Principles and strategies - a technical review. NMR in Biomedicine. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Moseley M. Diffusion tensor imaging and aging - a review. NMR in Biomedicine. 2002;15:553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Research Bulletin. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. NeuroImage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. A theory of memory retrieval. Psychological Review. 1978;85:59–108. [Google Scholar]
- Ratcliff R, Spieler D, McKoon G. Explicitly modeling the effects of aging on response time. Psychonomic Bulletin and Review. 2000;7:1–25. doi: 10.3758/bf03210723. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Thapar A, McKoon G. A diffusion model analysis of the effects of aging on recognition memory. Journal of Memory and Language. 2004;50:408–424. [Google Scholar]
- Raz N. The aging brain observed in vivo: Differential changes and their modifiers. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. Oxford: Oxford University Press; 2005. pp. 19–57. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. Journal of Neuroscience. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimers disease. Archives of Neurology. 1999;56:338–344. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Annals of the New York Academy of Sciences. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Mechanisms of age-cognition relations in adulthood. Hillsdale: NJ: Erlbaum; 1992a. [Google Scholar]
- Salthouse TA. What do adult age differences in the Digit Symbol Substitution Test reflect? Journal of Gerontology. 1992b;47:P121–128. doi: 10.1093/geronj/47.3.p121. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford; 2006. [Google Scholar]
- Schulte T, Sullivan EV, Muller-Oehring EM, Adalsteinsson E, Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: A diffusion tensor imaging study. Cerebral Cortex. 2005;15:1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. Inaugural article: The role of prefrontal cortex and posterior parietal cortex in task switching. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AW, Wong EC, Tan SG, Hyde JS. Diffusion weighted fMRI at 1.5 T. Magnetic Resonance in Medicine. 1996;35:155–158. doi: 10.1002/mrm.1910350204. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Madden DJ, Voss A. A diffusion model analysis of adult age differences in episodic and semantic long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:101–117. doi: 10.1037/0278-7393.32.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, et al. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cerebral Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage. 2006;32:1195–1204. doi: 10.1016/j.neuroimage.2006.04.212. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex. 2003;39:1107–1128. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, et al. Regional frontal cortical volumes decrease differentially in aging: An MRI study to compare volumetric approaches and voxel-based morphometry. NeuroImage. 2002;17:657–669. [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention: A review of meta-analyses. Neuroscience and Biobehavioral Reviews. 2002;26:849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, et al. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. NeuroImage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Wagenmakers EJ, van der Maas HLJ, Grasman RPPP. An EZ-diffusion model for response time and accuracy. Psychonomic Bulletin & Review. 2007;14:3–22. doi: 10.3758/bf03194023. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]