Abstract
Group B Streptococcus commonly colonises healthy adults without symptoms, yet under certain circumstances displays the ability to invade host tissues, evade immune detection and cause serious invasive disease. Consequently, Group B Streptococcus remains a leading cause of neonatal pneumonia, sepsis and meningitis. Here we review recent information on the bacterial factors and mechanisms that direct host–pathogen interactions involved in the pathogenesis of Group B Streptococcus infection. New research on host signalling and inflammatory responses to Group B Streptococcus infection is summarised. An understanding of the complex interplay between Group B Streptococcus and host provides valuable insight into pathogen evolution and highlights molecular targets for therapeutic intervention.
GBS (Group B Streptococcus/-cocci) is a leading agent of severe, invasive bacterial infection in human newborns. Neonatal infection with this opportunistic pathogen can present as early-onset or late-onset disease. In early-onset cases, bacteria are transferred from the mother to the infant in utero, following ascending infection of the placental membranes, or during passage through the birth canal, by aspiration of infected vaginal fluids. Early-onset neonatal infection manifests within the first few hours or days of life, often presenting as pneumonia and respiratory failure, which can quickly progress to bacteraemia and septic shock. By contrast, late-onset GBS disease can occur in infants up to several months old, and is distinguished by bloodstream infection with a high rate (40–60%) of progression to meningitis (Ref. 1). Infants that survive GBS meningitis can suffer serious long-term neurological consequences, such as seizures, hearing loss and cognitive impairment. Serious GBS infections are increasingly recognised in adult populations, particularly in the elderly and individuals compromised by underlying medical conditions. More than 40% of all invasive GBS cases in the USA occur past infancy (Ref. 2).
The development of GBS disease reflects successful bacterial colonisation of the vaginal epithelium, penetration of placental or epithelial barriers, resistance to immune clearance allowing bloodstream survival and, in cases of meningitis, the ability to breach the endothelial blood–brain barrier (BBB). In overcoming these obstacles, GBS expresses a diverse array of surface-associated and secreted virulence factors that mediate specific host-cell interactions and interfere with innate immune clearance mechanisms. The present review explores knowledge of GBS virulence mechanisms at each key step of disease progression, with particular emphasis on the most recent molecular insights gained from studies of isogenic bacterial mutants using in vitro and in vivo models of GBS infection.
Adherence to host epithelial surfaces
The pathogenesis of GBS disease can first be traced to asymptomatic mucosal colonisation, particularly of the maternal urogenital tract. Approximately 25% of healthy adults carry GBS, and the majority of babies born to a mother who harbours the bacteria will also become colonised (Ref. 3). GBS bind avidly to human vaginal epithelial cells under the low pH conditions characteristic of vaginal mucosa, through the low avidity interactions of cell-wall-associated lipoteichoic acid (LTA) and via higher-affinity interactions mediated by hydrophobic GBS surface proteins. Many of these GBS–host-cell interactions involve attachment of the bacterium to extracellular matrix (ECM) molecules such as fibronectin, fibrinogen and laminin, which in turn bind host-cell-surface proteins such as integrins (Fig. 1).
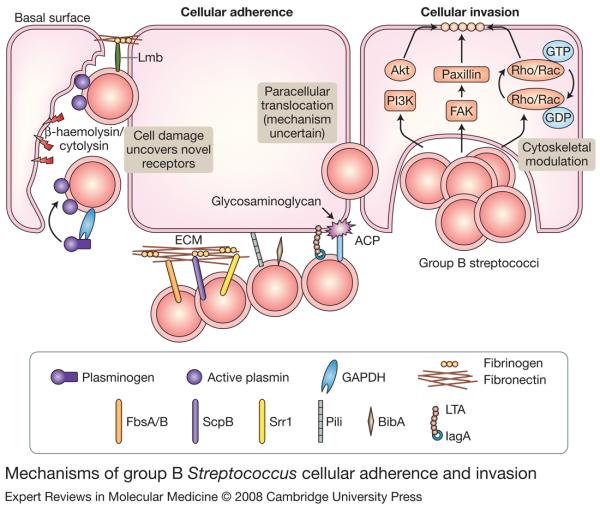
Figure 1. Mechanisms of group B Streptococcus cellular adherence and invasion.
Surface-expressed proteins FbsA/B, ScpB, Srr1, pili, BibA, LTA and ACP mediate group B Streptococcus (GBS) binding to host cells and ECM components, such as fibrinogen and fibronectin. Secreted β-haemolysin/cytolysin promotes GBS invasion, possibly by breaking down host barriers to reveal novel receptors on the basement membrane, such as laminin. GBS also use GAPDH to activate host plasminogen and degrade the ECM. Intracellular GBS invasion is enhanced by bacterial-dependent cytoskeletal rearrangements triggered by host PI3K/AKT- and FAK-signalling pathways and the Rho family of GTPases. Alternatively, GBS can also use an unknown mechanism to cross host epithelial barrier by a paracellular route. Several GBS adhesins, including FbsB, ScpB, pili, LTA and ACP, also contribute to cellular invasion. Abbreviations: ACP, alpha C protein; BibA, GBS immunogenic bacterial adhesin; ECM, extracellular matrix; FAK, focal adhesion kinase; FbsA/B, fibrinogen-binding proteins A and B; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GDP, guanosine diphosphate; GTP, guanosine triphosphate; Lmb, laminin-binding protein, LTA, lipoteichoic acid; PI3K, phosphoinositide 3-kinase; ScpB, C5a peptidase; Srr1, serine-rich repeat domain protein 1.
ScpB, a GBS cell-surface protein previously characterised for its ability to cleave the complement-derived chemoattractant C5a, was identified in a phage-display screen for fibronectin binding (Ref. 4). The dual functionality of ScpB was confirmed by decreased fibronectin binding of isogenic GBS ScpB deletion mutants and the direct interaction of recombinant ScpB with solid-phase fibronectin (Ref. 5). ScpB contains five distinct domains, including an N-terminal protease domain and three fibronectin type III domains (Fn1–Fn3) at the C-terminus. RGD motifs in the protease domain and between Fn1 and Fn2 bind to integrins, which may promote both cellular adherence and complement proteolysis by stabilising ScpB to allow C5a binding (Ref. 6). Naturally occurring ScpB variants with a deletion that destroys peptidase function retain the capacity to bind fibronectin (Refs 7, 8). Further targeted-mutagenesis studies demonstrate that GBS adherence to laminin involves the adhesin Lmb (Ref. 9); attachment to fibrinogen is mediated by repetitive motifs within the surface-anchored protein FbsA (Ref. 10), and the serine-rich repeat domain protein Srr-1 binds human keratin 4 (Ref. 11). In each case, these receptor–ligand interactions promote GBS adherence to epithelial cells. A recombinant form of GBS surface protein LrrG, containing the leucine-rich-repeat (LRR) motifs found in many bacterial invasins, binds to epithelial cells in a dose-dependent manner, suggesting that it may also serve an adhesin function during GBS infection (Ref. 12).
GBS were recently revealed to express pili (Ref. 13), filamentous cell-surface appendages better studied in Gram-negative bacteria, where they are known to facilitate host-cell attachment and colonisation (Ref. 14). Among eight sequenced GBS genomes, two genetic loci encoding pili were identified, the second existing in one of two variants, although not all genomes contain both loci (Ref. 15). ‘GBS pilus island 2’ includes the genes encoding PilB, an LP(x)TG-motif-containing protein that polymerises to form a pilus backbone, and accessory pilus proteins PilA and PilC (Refs 16, 17). Epithelial cell adherence was reduced in isogenic GBS mutants lacking PilA or PilC, but not those lacking PilB (Ref. 16). The crystal structure of the PilC homologue in GBS pilus island 1 reveals two IgG-like fold domains (N1 and N2), the latter of which is required for epithelial cell binding (Ref. 18).
Invasion across host epithelial barriers
Following cellular adherence and colonisation, GBS can use secreted toxins or employ cell-surface virulence factors, known as invasins, to promote bacterial entry and survival within host cells (Fig. 1). Some of these factors promote invasion by exploiting the ECM and/or host cellular signal transduction pathways–mechanisms that are just beginning to be understood in the context of GBS infection. Ultimately, entry into epithelial cells provides GBS with an intracellular niche for survival, but can also result in breakdown of host tissue integrity and inflammatory activation, both of which may contribute to disease pathology. Importantly, infection of placental cells can promote ascending in utero infection, whereas invasion of pulmonary epithelium and endothelium promote systemic dissemination.
Migration of GBS through freshly isolated chorioamniotic membranes has been documented by electron microscopy (Ref. 19). GBS invade primary chorion cells efficiently in vitro, and are capable of transcytosing through intact chorion cell monolayers without disruption of intracellular junctions (Ref. 20). GBS also secrete hyaluronate lyase, which is capable of degrading an important ECM component that is abundant in placental tissues (Ref. 21). Intracellular invasion of both alveolar epithelial and pulmonary endothelial cells by GBS was first noted in newborn macaques following intra-amniotic challenge (Ref. 22), and later confirmed in human tissue culture lines (Refs 23, 24). Electron microscopy studies demonstrate that host cytoskeletal changes are triggered by GBS, which lead to endocytotic uptake of the bacterium within a membrane-bound vacuole (Refs 25, 26).
FbsB, the proven surface-anchored GBS epithelial cell adhesin, which binds fibrinogen via its N-terminal domain (Ref. 10), Lmb, which mediates ECM adherence (Refs 9, 27) and ScpB, which interacts with fibronectin (Ref. 5), each play a demonstrable role in promoting efficient epithelial or endothelial cell invasion. Another GBS surface protein, Spb1, was identified by subtractive hybridisation to play a specific role in serotype III GBS invasion of epithelial cells (Ref. 28). In addition, the surface-anchored alpha C protein (ACP) is known to mediate GBS invasion of human cervical epithelial cells, and ACP deletion renders GBS less virulent in a neonatal mouse model of infection (Refs 29, 30). ACP specifically interacts with host cell glycosaminoglycan (GAG) on the epithelial cell surface to promote bacterial internalisation (Ref. 31). A GBS strain expressing an ACP variant with a charge-neutralising mutation in the GAG-binding residue cluster was deficient in invasion of cervical epithelial cells (Ref. 32). In a second possible mechanism, one of two N-terminal ACP domains promotes GBS invasion by binding α1β1-integrins on the epithelial cell surface (Ref. 33).
The intracellular uptake of GBS involves activation of cytoskeletal rearrangements in the target cell. Rho family GTPases, which are small, ubiquitous signalling molecules found in the eukaryotic cytosol, are known to be manipulated by pathogenic bacteria at the cell surface to trigger downstream regulation of actin polymerisation and cytoskeletal rearrangement (Ref. 34). GBS infection of epithelial cells increases activated levels of Rho family members RhoA, Rac1 and Cdc42, and GBS invasion can be inhibited by dominant-negative expression of these proteins and by Rho family GTPase inhibitors (Ref. 35). Furthermore, Rac1 and integrin-β1 are also involved in macrophage phagocytosis of GBS and subsequent phagosome maturation (Ref. 36). GBS invasion mediated by the ACP surface protein proceeds in a Rho-GTPase-dependent manner (Ref. 31). Another host signal transduction pathway involved in GBS uptake involves phosphoinositide-3 kinase (PI3K)/Akt. PI3K is a lipid kinase that catalyses the recruitment, phosphorylation and activation of the intracellular effector Akt, which in turn triggers downstream signalling to modulate cytoskeletal activities. Akt phosphorylation is demonstrated in the epithelial cell response to GBS infection, and chemical inhibition of PI3K or Akt and genetic inactivation of PI3K results in reduced GBS invasion (Ref. 37).
Although cellular invasion may play a principal role in bloodstream penetration in late-onset GBS infection, extensive lung epithelial and endothelial destruction may be evident in severe early-onset cases. Cellular damage results largely from the actions of the GBS β-haemolysin/cytolysin, a pore-forming toxin that lyses lung epithelial and endothelial cells and compromises their barrier function (Refs 38, 39). Even at subcytolytic doses, GBS β-haemolysin/cytolysin promotes lung epithelial cell invasion and triggers release of interleukin-8 (IL-8), a principal neutrophil chemoattractant (Ref. 40). GBS mutants lacking β-haemolysin/cytolysin expression are less able to penetrate pulmonary barriers and produce systemic infection than wild-type strains in a rabbit model of GBS pneumonia (Ref. 41). The cytolytic, proinvasive and proinflammatory effects of the GBS β-haemolysin/cytolysin are all neutralised by dipalmotyl phosphatidylcholine (DPPC), the major phospholipid constituent of lung surfactant (Ref. 38). This finding may help to explain the increased risk of premature, surfactant-deficient neonates to develop severe lung injury and invasive disease upon GBS infection.
The glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has been implicated in virulence of a number of bacterial pathogens including group A Streptococcus (GAS), through unanticipated dual functionalities that include binding and activation of host plasminogen (Ref. 42). GAS acquisition of surface plasmin activity promotes host invasion and systemic spread (Ref. 43). GBS GAPDH shares homology with GAS GAPDH and is expressed on the cell surface. GBS can bind lysine residues of host plasminogen via GAPDH, activate the bound proenzyme to plasmin, and thereby gain the ability to degrade host matrix proteins such as fibronectin (Ref. 44). Pretreatment of GBS with plasminogen and exogenous tissue plasminogen activator enhances virulence in are mouse model of infection, possibly because of a plasmin-mediated increase in bacterial invasiveness in host tissues (Ref. 45).
Finally, in addition to penetration of host cell barriers by intracellular invasion or direct damage to cells and extracellular matrix, new evidence indicates that GBS can cross cell monolayers via a paracellular route. GBS have been shown to associate with junctional protein complexes in electron microscopic studies. In a Transwell™ model of epithelial cell barrier function, GBS transcytosis proceeded with active and transient junction opening without altering transepithelial electrical resistance (Ref. 46). The GBS strain expressing a GAG-binding-deficient ACP variant could not invade cervical epithelial cells, but could still accomplish transcytosis, indicating that the two processes can occur independently (Ref. 32).
Resistance to innate immune clearance
Once GBS penetrates cellular barriers to reach the bloodstream or deep tissues, a broader immunological response is activated to clear the infection, in which host phagocytic cells including neutrophils and macrophages play a critical role. Effective uptake and clearance of GBS by these cells depends upon opsonisation by specific antibodies or serum complement, factors that can be quantitatively and qualitatively deficient in newborns, especially those born prematurely. The propensity of GBS to produce invasive infections further reflects many virulence factors that allow the bacteria to resist opsonophagocytosis or neutralise the bactericidal activities of neutrophils and macrophages (Fig. 2).
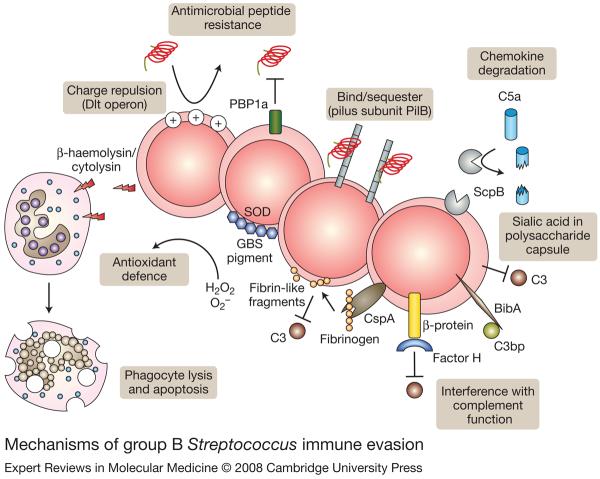
Figure 2. Mechanisms of group B Streptococcus immune evasion.
Group B Streptococcus (GBS) express several surface-expressed or secreted factors to evade host immune defences and promote survival. The Dlt operon is responsible for increasing incorporation of D-alanine residues in cell-wall teichoic acids, thereby reducing electronegativity and affinity for cationic antimicrobial peptides. PBP1a and the pilB subunit of GBS pili also contribute to antimicrobial peptide resistance. ScpB, the sialic acid capsule, BibA, β protein and CspA all inhibit host clearance of GBS by interfering with complement components C5a, C3 and C3bp. SOD properties of the orange carotenoid pigment shield GBS from killing by phagocyte-generated reactive oxygen species. Alternatively, β-haemolysin/cytolysin can boost GBS survival by cytolytic or proapoptotic injury to host phagocytes. Abbreviations: BibA, GBS immunogenic bacterial adhesin; CspA, cell-surface protease A; PBP1a, penicillin-binding protein 1a; ScpB, C5a peptidase; SOD, superoxide dismutase.
Upon penetration of GBS into the lung tissue or bloodstream of the newborn infant, an immunological response is recruited to clear the microorganism. Central to this response are host phagocytic cells, including neutrophils and macrophages. Effective uptake and killing by neutrophils requires opsonisation of the bacterium by specific antibodies in the presence of complement. However, complement deposition does not affect GBS survival or uptake by macrophages, probably because GBS protect themselves by binding factor H, a host counter-regulator of complement (Ref. 47). Neonates are particularly prone to invasive disease because of their quantitative or qualitative deficiencies in phagocytic cell function, specific antibody, or the classic and alternative complement pathways. In addition to these newborn host susceptibilities, GBS possess a number of virulence determinants that seek to thwart each of the key components of effective opsonophagocytic killing. The sialylated GBS capsular polysaccharide (CPS) represents one such defence factors.
Complement is a system of enzymatic reactions used by the innate immune system to recognise microbes and coat their surfaces with host proteins, making them more easily detected and engulfed by phagocytic cells bearing complement receptors, while simultaneously amplifying other aspects of the inflammatory response. The thick CPS is critical for limiting the effectiveness of host complement defence. The serotype-specific epitopes of ten known GBS CPSs (Ia, Ib, II-VIII and more recently IX) are created by different arrangements of four monosaccharides (glucose, galactose, N-acetylglucosamine and sialic acid) into unique repeating units, but unfailingly these structures contain a terminal sialic acid bound to galactose in an α2 → 3 linkage (Refs 48, 49, 50, 51, 52, 53, 54). This sialic acid molecule provides antiphagocytic protection by impairing surface deposition of opsonically active complement C3 on the bacterial surface. GBS subjected to sialidase treatment, or isogenic GBS mutants lacking capsular sialylation, are more susceptible to neutrophil killing and are less virulent in animal models of infection (Refs 55, 56). However, since others have shown that encapsulated and unencapsulated GBS are equally susceptible to macrophage uptake, the role of CPS in resisting phagocytosis per se versus other aspects of immune cell killing remains unclear (Ref. 57).
Sialic-acid-dependent reduction in C3 deposition is correlated with diminished production of C5a, an important complement-derived chemoattractant (Ref. 58), which works synergistically with ScpB-mediated proteolytic inactivation of C5a to reduce host neutrophil mobilisation. Additionally, a new cell-surface GBS immunogenic bacterial adhesin (BibA) was recently determined to mediate inhibition of other complement components. BibA binds human C3bp, a component of the classical complement pathway, promotes resistance to phagocytic killing, mediates adherence to epithelial cells and contributes to virulence in a mouse model of infection (Ref. 59). GBS β-protein was shown to prevent opsonophagocytosis by binding short consensus repeats found in the middle region of factor H, enabling the unbound active region to block C3b deposition on the bacterial cell surface (Ref. 60). The beta antigen of C protein binds human IgA antibody (Ref. 61), and IgA deposited nonspecifically on the bacterial surface probably inhibits interactions with complement. Finally, a cell-surface protease, CspA, targets host fibrinogen, producing adherent fibrin-like cleavage products that coat the bacterial surface and interfere with complement-mediated opsonophagocytic clearance (Ref. 62).
Once engulfed and contained in the phagosome, GBS face the rapid release of toxic reactive oxygen species (ROS) produced in the oxidative burst. Unlike Staphylococcus aureus, GBS do not produce catalase, yet are still able to resist killing by ROS and survive inside macrophage phagolysosomes (Refs 63, 64, 65). GBS possess a endogenous source of the oxygen-metabolite scavenger glutathione (Ref. 65), and the GBS SodA enzyme can neutralise superoxide anions (Ref. 66). GBS also produce an orange carotenoid pigment, a property unique among haemolytic streptococci, that is genetically linked to the cyl operon encoding the β-haemolysin/cytolysin cytotoxin (Ref. 67). The free-radical scavenging properties of this carotenoid neutralise hydrogen peroxide, superoxide, hypochlorite and singlet oxygen, and thereby provide a shield against several elements of phagocyte ROS killing (Ref. 68).
Antimicrobial peptides (AMPs) that exhibit broad-spectrum activities, such as cathelicidins and defensins, are produced by many immune and epithelial cell types. The small, cationic nature of most AMPs supports the assumption that their mechanism of killing involves their electrostatic attraction to negatively charged microbial cell surfaces, followed by their assembly to create membrane pores or otherwise disrupt membrane integrity (Ref. 69). GBS increase their intrinsic resistance to AMPs by incorporation of positively charged D-alanine residues into their cell-wall teichoic acids, thereby reducing surface electronegativity and affinity for the cationic peptides (Ref. 70). A surface-anchored penicillin-binding protein, PBP1a, enhances GBS resistance to cathelicidins and defensins, thereby reducing GBS susceptibility to killing by alveolar macrophages and neutrophils (Ref. 71), and promoting bacterial survival in a neonatal rat model of aerosolised lung infection (Ref. 72). Similarly, expression of the pilus backbone protein PilB renders GBS more resistant to killing by cathelicidin AMPs, and is associated with enhanced phagocyte resistance and systemic virulence (Ref. 73).
Induction of phagocyte apoptosis, or programmed cell death, represents an alternative bacterial defence mechanism to avoid phagocytic clearance. Apoptosis is a carefully regulated signal cascade involving a group of cysteine proteases known as caspases and several pro- and anti-apoptotic regulators belonging to the Bcl-2 family. In contrast to some cell-death ligands, macrophage apoptosis triggered by GBS requires caspase-3 activation and utilises unique changes in regulation and localisation of Bcl-2 family members (Ref. 74). GBS-induced macrophage apoptosis can also progress independently of caspases. Here, calpains, which belong to a different class of cytosolic cysteine proteases, are recruited to cleave and activate Bcl-2 family members and relay the death signal; the dual pathways for phagocyte destruction increase the chances that host defences will be circumvented (Ref. 75). The complete role of GBS β-haemolysin/ cytolysin in the induction of apoptosis and/or necrotic macrophage cell death remains unclear. Production of this cytolysin was shown to enhance GBS survival in mouse and human blood and this pro-survival phenotype was linked to its ability to induce cytolysis and apoptosis of phagocytes (Ref. 68). Furthermore, growth of GBS in high glucose concentrations, which minimises β-haemolysin/cytolysin production, also reduces macrophage apoptosis (Ref. 76). However, it was also demonstrated that in vitro macrophage infection with either wild-type GBS or a GBS mutant lacking β-haemolysin/cytolysin resulted in similar levels of viability, indicating that GBS-induced macrophage apoptosis can also occur by a β-haemolysin/cytolysin-independent mechanism regulated, at least in part, by glucose (Ref. 76).
Finally, a new understanding of GBS immune avoidance by molecular mimicry is emerging. The conserved GBS terminal α2→3 linked sialic acid capsular component is identical to a sugar epitope widely displayed on the surface of all mammalian cells. Compared with wild-type strains, capsule-deficient GBS mutants elicit greater degrees of proinflammatory cytokine release from human cells. Like human sialic acids, GBS capsular sialic acids have been demonstrated to engage sialic-acid-recognising immunoglobulin superfamily lectins (Siglecs) on human leukocytes, a family of cell-surface receptors with intracellular domains that send negative signals to limit host cell activation. This interaction suggests that bacterial surface sialylation may have evolved to mimic host `self' antigens, allowing GBS to disguise themselves from immune detection, manipulate phagocyte function and dampen the immune response to GBS infection (Ref. 77).
Inflammatory activation and the sepsis syndrome
When failures in epithelial barrier function and immunological clearance allow GBS to establish bacteraemia in the neonate, development of septicaemia may ensue. Animal models in which GBS are infused intravenously demonstrate a biphasic host inflammatory response (Ref. 78). The acute phase (<1 hour) is manifested by increased pulmonary artery pressure and decreased arterial oxygenation, and is associated with a rise in serum levels of thromboxanes. Pulmonary hypertension and hypoxaemia persist through the late phase (2–4 hours), in which a progressive pattern of systemic hypotension, decreased cardiac output and metabolic acidosis develops together with haematological abnormalities, organ system dysfunction and an increase in inflammatory markers, such as thromboxanes, prostacyclins, tumor necrosis factor-alpha (TNF-α), IL-1 and IL-6.
IL-1, a known stimulator of cyclo-oxygenase and lipo-oxygenase pathways, appears to occupy a proximal position in the deleterious cytokine cascade of septic shock. Treatment with an IL-1 receptor antagonist improves cardiac output and mean arterial pressure and improves survival in piglets receiving a continuous infusion of GBS (Ref. 79). Conversely, the cytokine IL-12, which is elevated 12–72 hours after challenge in animal models, has an important role in regulating the systemic response to GBS infection. Pretreatment with a monoclonal antibody against IL-12 results in greater mortality and intensity of bacteraemia, whereas therapeutic administration of IL-12 is associated with lower mortality and bloodstream replication of the organism (Ref. 80).
Since the release of TNF-α, IL-l and IL-6 are stimulated by soluble GBS cell-wall antigens (Ref. 81), studies have sought to identify the specific GBS component(s) of the cell wall that trigger the host cytokine cascade. GBS peptidoglycan is more effective than lipoteichoic acid or capsular polysaccharide as a stimulator of cytokine release from monocytes (Ref. 82). Knockout studies in mice indicate that cell wall peptidoglycan-induced activation of p38 and NF-κB depends upon the cytoplasmic toll-like receptor (TLR) adaptor protein MyD88, but does not proceed via the pattern recognition receptors TLR2 or TLR4 (Ref. 83). GBS activation of TLR2 was shown to depend on surface expression of lipoproteins, which also play a significant role in the development of GBS sepsis (Ref. 84). Structural differences in the linkage, anchoring and backbone of GBS lipoteichoic acid compared with those present in other Gram-positive bacteria may account for its diminished immune activation properties (Ref. 85).
Inhibitor studies have revealed that the mitogen-activated protein kinase (MAPK)/c-Jun N-terminal kinase (JNK) signalling pathway is essential for the NF-κB-dependent inflammatory response of phagocytes to GBS. Since phagocytosis and oxidative killing of GBS were not affected by inhibition of this pathway, JNK may represent a viable therapeutic target for GBS sepsis (Ref. 86). The nitric oxide (NO) pathway has also been implicated in the overproduction of proinflammatory cytokines, such as IL-6, and initiation of cellular injury during GBS infection of lung tissue (Ref. 87). The GBS cell wall and β-haemolysin/cytolysin act synergistically to upregulate inducible nitric oxide synthase (iNOS) in murine macrophages (Ref. 88). The inducible cyclo-oxygenase COX2 is also activated upon GBS infection in human monocytes, probably through MAPK pathway signalling (Ref. 89). GBS infection was also shown to stimulate COX2 and prostaglandin E2 (PGE2) expression in lung tissue in vitro and in vivo. GBS-induced COX2/PGE2 inflammatory response was reduced by treatment with an iNOS inhibitor and restored by addition of a NO donor, indicating that it is at least partially regulated by the NO pathway (Ref. 90).
The role of complement in GBS-mediated inflammation remains controversial. In one study, whole blood derived from C3 or complement receptor 3 (CR3/CD11b/CD18) knockout mice infected with GBS revealed a tempered TNF-α response (Ref. 91). NO was also shown to depend on CR3 expression in macrophages exposed to GBS (Ref. 92). However, others have shown that macrophages lacking CR3 demonstrate a normal cytokine response to GBS infection (Ref. 93).
The proinflammatory effects of the GBS β-haemolysin/cytolysin also contribute to sepsis pathophysiology. The toxin acts to stimulate iNOS and NO release in macrophages (Ref. 88). In a mouse model of bacteraemia and arthritis, β-haemolysin/cytolysin expression is associated with higher mortality, increased bacterial loads, greater degrees of joint injury and intraarticular and systemic release of the proinflammatory cytokines IL-1 and IL-6 (Ref. 94). Challenge of rabbits with isogenic GBS mutants showed that β-haemolysin/cytolysin production was associated with significantly higher degrees of hypotension, increased mortality and evidence of liver necrosis with hepatocyte apoptosis (Ref. 95). Partially purified GBS β-haemolysin/cytolysin preparations produce significant hypotensive actions when infused in rats and rabbits, including death due to shock (Ref. 96). The β-haemolysin/cytolysin toxin contributes directly to cardiomyocyte dysfunction and apoptosis, which may augment its role in the pathophysiological abnormalities of GBS sepsis (Ref. 97).
GBS blood-brain barrier penetration and meningitis
The pathophysiology of GBS meningitis varies according to age of onset. In early-onset disease, autopsy studies demonstrate little or no evidence of leptomeningeal inflammation, despite the presence of abundant bacteria, vascular thrombosis and parenchymal haemorrhage (Ref. 98). By contrast, infants with late-onset disease usually have diffuse purulent arachnoiditis with prominent involvement of the base of the brain (Ref. 99). Similar age-related differences in central nervous system (CNS) pathology are evident in the infant rat model of invasive disease (Ref. 100). These histopathological differences reflect underdevelopment of the host immunological response in the immediate neonatal period, with a higher proportion of deaths resulting from overwhelming septicaemia.
To produce meningitis, GBS must penetrate the BBB, a specialised structural and functional barrier that maintains homeostasis of the CNS. The BBB consists largely of specialised brain microvascular endothelial cells (BMECs), which guard the brain from circulating toxins and microbes by maintaining tight intercellular junctions and prohibiting pinocytosis. Intracellular invasion and transcytosis of human BMEC tissue culture monolayers has been shown in vitro, and this model has been used to probe the potential role(s) of individual GBS virulence determinants in the initial pathogenesis of GBS CNS infection.
When a GBS transposon mutant library was screened for reduced BMEC invasion, a particularly hypoinvasive mutant was found to harbour a disruption of a gene (iagA) encodes an enzyme for biosynthesis of diglucosyldiacylglycerol, a membrane glycolipid that functions as an anchor for lipoteichoic acid. Deletion of iagA yielded a GBS mutant that sheds lipoteichoic acid into the medium, exhibits decreased BMEC invasion in vitro and is attenuated in a murine model of meningitis (Ref. 101). In separate avenues of research, GBS mutants lacking the GBS fibrinogen receptor FbsA, laminin-binding protein Lmb, or pilus backbone subunit protein PilB also demonstrated reduced adherence or invasion of BMECs in vitro (Refs 17, 27, 102). At high bacterial densities, human BMEC invasion by GBS is accompanied by evidence of β-haemolysin/cytolysin-induced cellular injury (Ref. 26). Correspondingly, β-hemolysin/ cytolysin-knockout mutants show decreased BBB penetration and decreased lethality from meningitis in vivo (Ref. 103).
GBS invasion of human BMECs can be blocked by inhibition of actin polymerisation, suggesting that GBS trigger rearrangement of the host cytoskeleton and induce their own uptake (Ref. 26). This process may be accomplished, at least in part, by tyrosine phosphorylation of focal adhesion kinase (FAK), which occurs upon GBS infection. Phosphorylation of FAK induces its association with PI3K and paxillin, an actin filament adaptor protein (Ref. 104), and is required for efficient GBS BMEC invasion. GBS-infected BMECs also exhibit increased levels of activated Rho family members RhoA and Rac1. Rho family GTPase inhibitors and dominant-negative expression of RhoA and Rac1 are effective in blocking GBS BMEC invasion (Ref. 105).
The host inflammatory response to GBS contributes significantly to the pathogenesis of meningitis and CNS injury. The initiation of the inflammatory response is triggered through the sentinel function of the BBB endothelium, which activates a specific pattern of gene transcription for neutrophil recruitment, including production of chemokines (e.g. IL-8, Groα), endothelial receptors (intracellular cell-adhesion molecule 1, ICAM-1) and neutrophil activators (GM-CSF) (Ref. 103). A vascular distribution of cortical lesions in neonatal rats with GBS meningitis indicates that disturbances of cerebral blood flow contribute to neuronal damage (Ref. 106). Inflammation of individual brain vessels can lead to focal lesions, whereas diffuse alterations of cerebral blood flow could cause generalised hypoxic/ischaemic injury and cerebral oedema (Refs 106, 107). In porcine BMECs, iNOS production is upregulated in response to GBS infection in a NF-κB-dependent manner (Ref. 108). Further arteriolar dysfunction is associated with the presence of oxygen free radicals thought to be a byproduct of infiltrating neutrophils (Ref. 109). GBS β-haemolysin/cytolysin induces IL-8 and the neutrophil receptor ICAM-1, thereby promoting neutrophil migration across polar BMEC monolayers, suggesting that the toxin is crucial to this particular manifestation of GBS CNS disease (Ref. 103).
In the neonatal rat model of meningitis, TNF-α production by astrocytes, microglia and infiltrating leukocytes appears to contribute to apoptosis of hippocampal neurons (Ref. 110) and further increases in BBB permeability (Ref. 111). GBS signal through TLR2 to activate and stimulate NO production by microglia cells, resulting in neuronal destruction (Ref. 112). Microglial apoptosis is triggered by GBS cell death via the cysteine protease caspase-8, and is hypothesised to represent a self-dampening mechanism that prevents over-stimulation of CNS inflammation (Ref. 113). Intraventricular inoculation of newborn piglets with GBS results in an early sharp rise in cerebrospinal fluid TNF-α levels, followed shortly by prostaglandin release and subarachnoid inflammation (Ref. 114). In the neonatal rat, simultaneous intracisternal administration of dexamethasone with GBS challenge markedly reduces the magnitude of subarachnoid inflammation, vasculopathy, and neuronal injury (Ref. 106).
Clinical applications for vaccine development
An effective vaccine against GBS would represent a major public health advance for newborn infants and other high-risk populations. Purified GBS capsular polysaccharide antigens modelled on the effective campaigns for Haemophilus influenzae type B (HiB) and pneumococcal vaccination in childhood have been coupled to an immunogenic protein carrier. Such glycoconjugate vaccines against serotypes Ia, Ib and II–VIII GBS have been synthesised and found to be immunogenic in preclinical trails in mice, rabbits and/or baboons. Several of these have advanced to Phase I and Phase II clinical trails in healthy adults with an excellent safety profile (Refs 115, 116, 117). Immunised humans develop serotype-specific anti-CPS antibodies that function well to promote GBS killing during in vitro opsonophagocytic assays. The recent discovery of frequent O-acetyl modifications of the immunodominant terminal α2 →α3-linked sialic acid moiety on several GBS serotypes may provide important insight for optimising CPS purification to retain native structure and maximum immunogenicity (Ref. 118). One challenge faced by the glycoconjugate approach is to develop combination products that would provide appropriately broad-spectrum antigenic coverage for the diverse GBS serotypes associated with disease in any particular demographic group or geographic area.
The investigation of candidate surface-expressed protein antigens distributed more broadly (or ideally universally) among strains of different GBS serotypes has intensified in recent years (Ref. 1). The C5a peptidase ScpB is universally expressed by GBS strains capable of eliciting protective IgG antibodies, and may be deliverable in recombinant form within a biodegradable polymer (Refs 7, 119). Similarly, surface proteins LrrB and Sip are highly conserved across GBS strains of diverse serotypes and each induces protective immunity in mice (Refs 12, 120). The component proteins of newly discovered GBS pili may also represent candidates for a universal vaccine antigen and have been explored as classical antigens and recombinantly expressed in Lactococcus lactis as a live-attenuated vaccine concept (Refs 13, 121). Although universal protein vaccine antigens may overcome some limitations associated with capsule-based vaccines, introduction of any vaccine during pregnancy (one model for GBS prevention) will meet a concerned and apprehensive target population and will no doubt demand a variety of intensive risk assessment and educational interventions (Ref. 122).
Research in progress and outstanding research questions
Each new year of GBS research heralds the discovery of novel virulence determinants or new functions for previously identified surface proteins or secreted factors. Advances can be attributed to the application of new methodologies, such as the use of reverse vaccinology to screen genomes for immunogenic surface antigens, which led to the identification multifunctional immunogenic adhesin BibA and unveiled the surface pili that had been overlooked for more than 50 years of GBS research (Refs 13, 59). Functional screening and careful reexamination of previously identified surface structures have also revealed secondary functions, such as a role for the GBS C5a peptidase ScpB in epithelial adherence or capsular sialic acid in engagement of host Siglec receptors. Furthermore, its is likely that GBS will remain a useful model organism for Gram-positive bacterial pathogenesis and for probing the developmental regulation of newborn immune function.
Rising incidences of invasive disease in adults and emerging patterns of antibiotic resistance (Ref. 123) indicate that further attention must be paid to elucidate GBS virulence factors and the mechanisms by which they interact with host cells and our immune system. Indeed, the emergence of GBS strains with decreased susceptibility to β-lactam antibiotics has now been reported in both the USA and Japan (Refs 124, 125). These GBS isolates harbour mutations in penicillin-binding protein 2x (PBP2x), which is similar to the first-step mutations on the pathway to full β-lactam resistance seen in pneumococcal isolates a few decades ago. Enhanced understanding of the molecular basis of GBS pathogenesis may pinpoint novel bacterial and host molecules that can represent novel therapeutic or immunoprophylactic targets against disease caused by this foremost of neonatal pathogens.
Acknowledgements
The authors appreciate the contribution of anonymous peer reviewers to the completeness of the manuscript. The authors' own research on GBS molecular pathogenesis has been supported by the National Institutes of Health (V.N., K.S.D.), an American Heart Association Established Investigator Award (V.N.), a Burroughs-Wellcome Fund Career Award in the Biomedical Sciences (K.S.D.), and a Graduate Research Fellowship from the National Science Foundation (H.C.M.).
References
- 1.Johri AK, et al. Group B Streptococcus: global incidence and vaccine development. Nat Rev Microbiol. 2006;4:932–942. doi: 10.1038/nrmicro1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards MS, Baker CJ. Group B streptococcal infections in elderly adults. Clin Infect Dis. 2005;41:839–847. doi: 10.1086/432804. [DOI] [PubMed] [Google Scholar]
- 3.Campbell JR, et al. Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstet Gynecol. 2000;96:498–503. doi: 10.1016/s0029-7844(00)00977-7. [DOI] [PubMed] [Google Scholar]
- 4.Beckmann C, et al. Identification of novel adhesins from Group B streptococci by use of phage display reveals that C5a peptidase mediates fibronectin binding. Infect Immun. 2002;70:2869–2876. doi: 10.1128/IAI.70.6.2869-2876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Q, et al. The group B streptococcal C5a peptidase is both a specific protease and an invasin. Infect Immun. 2002;70:2408–2413. doi: 10.1128/IAI.70.5.2408-2413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CK, et al. Structure of the streptococcal cell wall C5a peptidase. Proc Natl Acad Sci U S A. 2005;102:18391–18396. doi: 10.1073/pnas.0504954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleary PP, et al. Immunization with C5a peptidase from either group A or B streptococci enhances clearance of group A streptococci from intranasally infected mice. Vaccine. 2004;22:4332–4341. doi: 10.1016/j.vaccine.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Tamura GS, et al. High-affinity interaction between fibronectin and the group B streptococcal C5a peptidase is unaffected by a naturally occurring four-amino-acid deletion that eliminates peptidase activity. Infect Immun. 2006;74:5739–5746. doi: 10.1128/IAI.00241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spellerberg B, et al. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect Immun. 1999;67:871–878. doi: 10.1128/iai.67.2.871-878.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schubert A, et al. The fibrinogen receptor FbsA promotes adherence of Streptococcus agalactiae to human epithelial cells. Infect Immun. 2004;72:6197–6205. doi: 10.1128/IAI.72.11.6197-6205.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samen U, et al. The surface protein Srr-1 of Streptococcus agalactiae binds human keratin 4 and promotes adherence to epithelial HEp-2 cells. Infect Immun. 2007;75:5405–5414. doi: 10.1128/IAI.00717-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seepersaud R, et al. Characterization of a novel leucine-rich repeat protein antigen from group B streptococci that elicits protective immunity. Infect Immun. 2005;73:1671–1683. doi: 10.1128/IAI.73.3.1671-1683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauer P, et al. Genome analysis reveals pili in Group B Streptococcus. Science. 2005;309:105. doi: 10.1126/science.1111563. [DOI] [PubMed] [Google Scholar]
- 14.Sauer FG, et al. Bacterial pili: molecular mechanisms of pathogenesis. Curr Opin Microbiol. 2000;3:65–72. doi: 10.1016/s1369-5274(99)00053-3. [DOI] [PubMed] [Google Scholar]
- 15.Rosini R, et al. Identification of novel genomic islands coding for antigenic pilus-like structures in Streptococcus agalactiae. Mol Microbiol. 2006;61:126–141. doi: 10.1111/j.1365-2958.2006.05225.x. [DOI] [PubMed] [Google Scholar]
- 16.Dramsi S, et al. Assembly and role of pili in group B streptococci. Mol Microbiol. 2006;60:1401–1413. doi: 10.1111/j.1365-2958.2006.05190.x. [DOI] [PubMed] [Google Scholar]
- 17.Maisey HC, et al. Group B streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. J Bacteriol. 2007;189:1464–1467. doi: 10.1128/JB.01153-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan V, et al. An IgG-like domain in the minor pilin GBS52 of Streptococcus agalactiae mediates lung epithelial cell adhesion. Structure. 2007;15:893–903. doi: 10.1016/j.str.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galask RP, et al. Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol. 1984;148:915–928. doi: 10.1016/0002-9378(84)90534-9. [DOI] [PubMed] [Google Scholar]
- 20.Winram SB, et al. Characterization of group B streptococcal invasion of human chorion and amnion epithelial cells In vitro. Infect Immun. 1998;66:4932–4941. doi: 10.1128/iai.66.10.4932-4941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin B, et al. Cloning and expression of the gene for group B streptococcal hyaluronate lyase. J Biol Chem. 1994;269:30113–30116. [PubMed] [Google Scholar]
- 22.Rubens CE, et al. Pathophysiology and histopathology of group B streptococcal sepsis in Macaca nemestrina primates induced after intraamniotic inoculation: evidence for bacterial cellular invasion. J Infect Dis. 1991;164:320–330. doi: 10.1093/infdis/164.2.320. [DOI] [PubMed] [Google Scholar]
- 23.Rubens CE, et al. Respiratory epithelial cell invasion by group B streptococci. Infect Immun. 1992;60:5157–5163. doi: 10.1128/iai.60.12.5157-5163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson RL, et al. Group B streptococci invade endothelial cells: type III capsular polysaccharide attenuates invasion. Infect Immun. 1993;61:478–485. doi: 10.1128/iai.61.2.478-485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valentin-Weigand P, et al. Characterization of group B streptococcal invasion in HEp-2 epithelial cells. FEMS Microbiol Lett. 1997;147:69–74. doi: 10.1111/j.1574-6968.1997.tb10222.x. [DOI] [PubMed] [Google Scholar]
- 26.Nizet V, et al. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenenbaum T, et al. Streptococcus agalactiae invasion of human brain microvascular endothelial cells is promoted by the laminin-binding protein Lmb. Microbes Infect. 2007;9:714–720. doi: 10.1016/j.micinf.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Adderson EE, et al. Subtractive hybridization identifies a novel predicted protein mediating epithelial cell invasion by virulent serotype III group B Streptococcus agalactiae. Infect Immun. 2003;71:6857–6863. doi: 10.1128/IAI.71.12.6857-6863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolduc GR, et al. The alpha C protein mediates internalization of group B Streptococcus within human cervical epithelial cells. Cell Microbiol. 2002;4:751–758. doi: 10.1046/j.1462-5822.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- 30.Li J, et al. Inactivation of the alpha C protein antigen gene, bca, by a novel shuttle/suicide vector results in attenuation of virulence and immunity in group B Streptococcus. Proc Natl Acad Sci U S A. 1997;94:13251–13256. doi: 10.1073/pnas.94.24.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baron MJ, et al. Alpha C protein of group B Streptococcus binds host cell surface glycosaminoglycan and enters cells by an actin-dependent mechanism. J Biol Chem. 2004;279:24714–24723. doi: 10.1074/jbc.M402164200. [DOI] [PubMed] [Google Scholar]
- 32.Baron MJ, et al. Identification of a glycosaminoglycan binding region of the alpha C protein that mediates entry of group B streptococci into host cells. J Biol Chem. 2007;282:10526–10536. doi: 10.1074/jbc.M608279200. [DOI] [PubMed] [Google Scholar]
- 33.Bolduc GR, Madoff LC. The group B streptococcal alpha C protein binds alpha1beta1-integrin through a novel KTD motif that promotes internalization of GBS within human epithelial cells. Microbiology. 2007;153:4039–4049. doi: 10.1099/mic.0.2007/009134-0. [DOI] [PubMed] [Google Scholar]
- 34.Dumenil G, Nassif X. Extracellular bacterial pathogens and small GTPases of the Rho family: an unexpected combination. Curr Top Microbiol Immunol. 2005;291:11–28. doi: 10.1007/3-540-27511-8_2. [DOI] [PubMed] [Google Scholar]
- 35.Burnham CA, Shokoples SE, Tyrrell GJ. Rac1, RhoA, and Cdc42 participate in HeLa cell invasion by group B streptococcus. FEMS Microbiol Lett. 2007;272:8–14. doi: 10.1111/j.1574-6968.2007.00768.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang QQ, et al. Integrin beta 1 regulates phagosome maturation in macrophages through Rac expression. J Immunol. 2008;180:2419–2428. doi: 10.4049/jimmunol.180.4.2419. [DOI] [PubMed] [Google Scholar]
- 37.Burnham CA, Shokoples SE, Tyrrell GJ. Invasion of HeLa cells by group B streptococcus requires the phosphoinositide-3-kinase signalling pathway and modulates phosphorylation of host-cell Akt and glycogen synthase kinase-3. Microbiology. 2007;153:4240–4252. doi: 10.1099/mic.0.2007/008417-0. [DOI] [PubMed] [Google Scholar]
- 38.Nizet V, et al. Group B streptococcal β-hemolysin expression is associated with injury of lung epithelial cells. Infect Immun. 1996;64:3818–3826. doi: 10.1128/iai.64.9.3818-3826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibson RL, Nizet V, Rubens CE. Group B streptococcal β-hemolysin promotes injury of lung microvascular endothelial cells. Pediatr Res. 1999;45:626–634. doi: 10.1203/00006450-199905010-00003. [DOI] [PubMed] [Google Scholar]
- 40.Doran KS, et al. Group B streptococcal β-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J Infect Dis. 2002;185:196–203. doi: 10.1086/338475. [DOI] [PubMed] [Google Scholar]
- 41.Hensler ME, et al. Virulence role of group B Streptococcus β-hemolysin/cytolysin in a neonatal rabbit model of early-onset pulmonary infection. J Infect Dis. 2005;191:1287–1291. doi: 10.1086/428946. [DOI] [PubMed] [Google Scholar]
- 42.Terao Y, et al. Multifunctional glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pyogenes is essential for evasion from neutrophils. J Biol Chem. 2006;281:14215–14223. doi: 10.1074/jbc.M513408200. [DOI] [PubMed] [Google Scholar]
- 43.Cole JN, et al. Trigger for group A streptococcal M1T1 invasive disease. FASEB J. 2006;20:1745–1747. doi: 10.1096/fj.06-5804fje. [DOI] [PubMed] [Google Scholar]
- 44.Seifert KN, et al. Characterization of group B streptococcal glyceraldehyde-3-phosphate dehydrogenase: surface localization, enzymatic activity, and protein-protein interactions. Can J Microbiol. 2003;49:350–356. doi: 10.1139/w03-042. [DOI] [PubMed] [Google Scholar]
- 45.Magalhaes V, et al. Interaction with human plasminogen system turns on proteolytic activity in Streptococcus agalactiae and enhances its virulence in a mouse model. Microbes Infect. 2007;9:1276–1284. doi: 10.1016/j.micinf.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Soriani M, et al. Group B Streptococcus crosses human epithelial cells by a paracellular route. J Infect Dis. 2006;193:241–250. doi: 10.1086/498982. [DOI] [PubMed] [Google Scholar]
- 47.Maruvada R, Blom AM, Prasadarao NV. Effects of complement regulators bound to Escherichia coli K1 and Group B Streptococcus on the interaction with host cells. Immunology. 2008;124:265–276. doi: 10.1111/j.1365-2567.2007.02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jennings HJ, et al. Structure of native polysaccharide antigens of type Ia and type Ib group B Streptococcus. Biochemistry. 1983;22:1258–1264. doi: 10.1021/bi00274a042. [DOI] [PubMed] [Google Scholar]
- 49.Wessels MR, et al. Isolation and characterization of type IV group B Streptococcus capsular polysaccharide. Infect Immun. 1989;57:1089–1094. doi: 10.1128/iai.57.4.1089-1094.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wessels MR, et al. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. A revised structure for the type III group B streptococcal polysaccharide antigen. J Biol Chem. 1987;262:8262–8267. [PubMed] [Google Scholar]
- 51.Jennings HJ, et al. Structural determination of the capsular polysaccharide antigen of type II group B Streptococcus. J Biol Chem. 1983;258:1793–1798. [PubMed] [Google Scholar]
- 52.Kogan G, et al. Structural elucidation of the novel type VII group B Streptococcus capsular polysaccharide by high resolution NMR spectroscopy. Carbohydr Res. 1995;277:1–9. doi: 10.1016/0008-6215(95)00195-y. [DOI] [PubMed] [Google Scholar]
- 53.Kogan G, et al. Structural and immunochemical characterization of the type VIII group B Streptococcus capsular polysaccharide. J Biol Chem. 1996;271:8786–8790. doi: 10.1074/jbc.271.15.8786. [DOI] [PubMed] [Google Scholar]
- 54.Slotved HC, et al. Serotype IX, a proposed new Streptococcus agalactiae serotype. J Clin Microbiol. 2007;45:2929–2936. doi: 10.1128/JCM.00117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell JR, Baker CJ, Edwards MS. Deposition and degradation of C3 on type III group B streptococci. Infect Immun. 1991;59:1978–1983. doi: 10.1128/iai.59.6.1978-1983.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marques MB, et al. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect Immun. 1992;60:3986–3993. doi: 10.1128/iai.60.10.3986-3993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segura MA, Cleroux P, Gottschalk M. Streptococcus suis and group B Streptococcus differ in their interactions with murine macrophages. FEMS Immunol Med Microbiol. 1998;21:189–195. doi: 10.1111/j.1574-695X.1998.tb01165.x. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi S, et al. Capsular sialic acid limits C5a production on type III group B streptococci. Infect Immun. 1999;67:1866–1870. doi: 10.1128/iai.67.4.1866-1870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santi I, et al. BibA: a novel immunogenic bacterial adhesin contributing to group B Streptococcus survival in human blood. Mol Microbiol. 2007;63:754–767. doi: 10.1111/j.1365-2958.2006.05555.x. [DOI] [PubMed] [Google Scholar]
- 60.Jarva H, et al. The group B streptococcal beta and pneumococcal Hic proteins are structurally related immune evasion molecules that bind the complement inhibitor factor H in an analogous fashion. J Immunol. 2004;172:3111–3118. doi: 10.4049/jimmunol.172.5.3111. [DOI] [PubMed] [Google Scholar]
- 61.Jerlstrom PG, Chhatwal GS, Timmis KN. The IgA-binding beta antigen of the c protein complex of Group B streptococci: sequence determination of its gene and detection of two binding regions. Mol Microbiol. 1991;5:843–849. doi: 10.1111/j.1365-2958.1991.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 62.Harris TO, et al. A novel streptococcal surface protease promotes virulence, resistance to opsonophagocytosis, and cleavage of human fibrinogen. J Clin Invest. 2003;111:61–70. doi: 10.1172/JCI16270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cornacchione P, et al. Group B streptococci persist inside macrophages. Immunology. 1998;93:86–95. doi: 10.1046/j.1365-2567.1998.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teixeira CF, et al. Cytochemical study of Streptococcus agalactiae and macrophage interaction. Microsc Res Tech. 2001;54:254–259. doi: 10.1002/jemt.1137. [DOI] [PubMed] [Google Scholar]
- 65.Wilson CB, Weaver WM. Comparative susceptibility of group B streptococci and Staphylococcus aureus to killing by oxygen metabolites. J Infect Dis. 1985;152:323–329. doi: 10.1093/infdis/152.2.323. [DOI] [PubMed] [Google Scholar]
- 66.Poyart C, et al. Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae. Infect Immun. 2001;69:5098–5106. doi: 10.1128/IAI.69.8.5098-5106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spellerberg B, et al. The cyl genes of Streptococcus agalactiae are involved in the production of pigment. FEMS Microbiol Lett. 2000;188:125–128. doi: 10.1111/j.1574-6968.2000.tb09182.x. [DOI] [PubMed] [Google Scholar]
- 68.Liu GY, et al. Sword and shield: linked group B streptococcal β-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci U S A. 2004;101:14491–14496. doi: 10.1073/pnas.0406143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gallo RL, Nizet V. Endogenous production of antimicrobial peptides in innate immunity and human disease. Curr Allergy Asthma Rep. 2003;3:402–409. doi: 10.1007/s11882-003-0074-x. [DOI] [PubMed] [Google Scholar]
- 70.Poyart C, et al. Regulation of D-alanyllipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J Bacteriol. 2001;183:6324–6334. doi: 10.1128/JB.183.21.6324-6334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamilton A, et al. Penicillin-binding protein 1a promotes resistance of group B streptococcus to antimicrobial peptides. Infect Immun. 2006;74:6179–6187. doi: 10.1128/IAI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones AL, et al. A streptococcal penicillin-binding protein is critical for resisting innate airway defenses in the neonatal lung. J Immunol. 2007;179:3196–3202. doi: 10.4049/jimmunol.179.5.3196. [DOI] [PubMed] [Google Scholar]
- 73.Maisey HC, et al. A group B streptococcal pilus protein promotes phagocyte resistance and systemic virulence. FASEB J. 2008;22:1715–1724. doi: 10.1096/fj.07-093963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ulett GC, et al. Mechanisms of group B streptococcal-induced apoptosis of murine macrophages. J Immunol. 2005;175:2555–2562. doi: 10.4049/jimmunol.175.4.2555. [DOI] [PubMed] [Google Scholar]
- 75.Fettucciari K, et al. Group B Streptococcus induces macrophage apoptosis by calpain activation. J Immunol. 2006;176:7542–7556. doi: 10.4049/jimmunol.176.12.7542. [DOI] [PubMed] [Google Scholar]
- 76.Ulett GC, et al. Beta-hemolysin-independent induction of apoptosis of macrophages infected with serotype III group B streptococcus. J Infect Dis. 2003;188:1049–1053. doi: 10.1086/378202. [DOI] [PubMed] [Google Scholar]
- 77.Carlin AF, et al. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J Bacteriol. 2007;189:1231–1237. doi: 10.1128/JB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rojas J, et al. Pulmonary hemodynamic and ultrastructural changes associated with Group B streptococcal toxemia in adult sheep and newborn lambs. Pediatr Res. 1983;17:1002–1008. doi: 10.1203/00006450-198312000-00015. [DOI] [PubMed] [Google Scholar]
- 79.Vallette JD, Jr., et al. Effect of an interleukin-1 receptor antagonist on the hemodynamic manifestations of group B streptococcal sepsis. Pediatr Res. 1995;38:704–708. doi: 10.1203/00006450-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 80.Mancuso G, et al. Role of interleukin 12 in experimental neonatal sepsis caused by group B streptococci. Infect Immun. 1997;65:3731–3735. doi: 10.1128/iai.65.9.3731-3735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vallejo JG, Baker CJ, Edwards MS. Roles of the bacterial cell wall and capsule in induction of tumor necrosis factor alpha by type III group B streptococci. Infect Immun. 1996;64:5042–5046. doi: 10.1128/iai.64.12.5042-5046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vallejo JG, Baker CJ, Edwards MS. Interleukin-6 production by human neonatal monocytes stimulated by type III group B streptococci. J Infect Dis. 1996;174:332–337. doi: 10.1093/infdis/174.2.332. [DOI] [PubMed] [Google Scholar]
- 83.Mancuso G, et al. Dual role of TLR2 and myeloid differentiation factor 88 in a mouse model of invasive group B streptococcal disease. J Immunol. 2004;172:6324–6329. doi: 10.4049/jimmunol.172.10.6324. [DOI] [PubMed] [Google Scholar]
- 84.Henneke P, et al. Lipoproteins are critical TLR2 activating toxins in group B streptococcal sepsis. J Immunol. 2008;180:6149–6158. doi: 10.4049/jimmunol.180.9.6149. [DOI] [PubMed] [Google Scholar]
- 85.Henneke P, et al. Role of lipoteichoic acid in the phagocyte response to group B streptococcus. J Immunol. 2005;174:6449–6455. doi: 10.4049/jimmunol.174.10.6449. [DOI] [PubMed] [Google Scholar]
- 86.Kenzel S, et al. c-Jun kinase is a critical signaling molecule in a neonatal model of group B streptococcal sepsis. J Immunol. 2006;176:3181–3188. doi: 10.4049/jimmunol.176.5.3181. [DOI] [PubMed] [Google Scholar]
- 87.Raykova VD, et al. Nitric oxide-dependent regulation of pro-inflammatory cytokines in group B streptococcal inflammation of rat lung. Ann Clin Lab Sci. 2003;33:62–67. [PubMed] [Google Scholar]
- 88.Ring A, et al. Synergistic action of nitric oxide release from murine macrophages caused by group B streptococcal cell wall and beta-hemolysin/cytolysin. J Infect Dis. 2002;186:1518–1521. doi: 10.1086/344895. [DOI] [PubMed] [Google Scholar]
- 89.Maloney CG, et al. Induction of cyclooxygenase-2 by human monocytes exposed to group B streptococci. J Leukoc Biol. 2000;67:615–621. doi: 10.1002/jlb.67.5.615. [DOI] [PubMed] [Google Scholar]
- 90.Natarajan G, et al. Nitric oxide and prostaglandin response to group B streptococcal infection in the lung. Ann Clin Lab Sci. 2007;37:170–176. [PubMed] [Google Scholar]
- 91.Levy O, et al. Critical role of the complement system in group B streptococcus-induced tumor necrosis factor alpha release. Infect Immun. 2003;71:6344–6353. doi: 10.1128/IAI.71.11.6344-6353.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goodrum KJ, McCormick LL, Schneider B. Group B streptococcus-induced nitric oxide production in murine macrophages is CR3 (CD11b/CD18) dependent. Infect Immun. 1994;62:3102–3107. doi: 10.1128/iai.62.8.3102-3107.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henneke P, et al. Cellular activation, phagocytosis, and bactericidal activity against group B streptococcus involve parallel myeloid differentiation factor 88-dependent and independent signaling pathways. J Immunol. 2002;169:3970–3977. doi: 10.4049/jimmunol.169.7.3970. [DOI] [PubMed] [Google Scholar]
- 94.Puliti M, et al. Severity of group B streptococcal arthritis is correlated with β-hemolysin expression. J Infect Dis. 2000;182:824–832. doi: 10.1086/315773. [DOI] [PubMed] [Google Scholar]
- 95.Ring A, et al. Group B streptococcal β-hemolysin induces mortality and liver injury in experimental sepsis. J Infect Dis. 2002;185:1745–1753. doi: 10.1086/340818. [DOI] [PubMed] [Google Scholar]
- 96.Griffiths BB, Rhee H. Effects of haemolysins of groups A and B streptococci on cardiovascular system. Microbios. 1992;69:17–27. [PubMed] [Google Scholar]
- 97.Hensler ME, Miyamoto S, Nizet V. Group B streptococcal β-hemolysin/cytolysin directly impairs cardiomyocyte viability and function PLoS One 3. 2008:e2446. doi: 10.1371/journal.pone.0002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Quirante J, Ceballos R, Cassady G. Group B β-hemolytic streptococcal infection in the newborn. I. Early onset infection. Am J Dis Child. 1974;128:659–665. doi: 10.1001/archpedi.1974.02110300069009. [DOI] [PubMed] [Google Scholar]
- 99.Berman PH, Banker BQ. Neonatal meningitis. A clinical and pathological study of 29 cases. Pediatrics. 1966;38:6–24. [PubMed] [Google Scholar]
- 100.Ferrieri P, Burke B, Nelson J. Production of bacteremia and meningitis in infant rats with group B streptococcal serotypes. Infect Immun. 1980;27:1023–1032. doi: 10.1128/iai.27.3.1023-1032.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doran KS, et al. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J Clin Invest. 2005;115:2499–2507. doi: 10.1172/JCI23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tenenbaum T, et al. Adherence to and invasion of human brain microvascular endothelial cells are promoted by fibrinogen-binding protein FbsA of Streptococcus agalactiae. Infect Immun. 2005;73:4404–4409. doi: 10.1128/IAI.73.7.4404-4409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Doran KS, Liu GY, Nizet V. Group B streptococcal β-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J Clin Invest. 2003;112:736–744. doi: 10.1172/JCI17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shin S, et al. Focal adhesion kinase is involved in type III group B streptococcal invasion of human brain microvascular endothelial cells. Microb Pathog. 2006;41:168–173. doi: 10.1016/j.micpath.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 105.Shin S, Kim KS. RhoA and Rac1 contribute to type III group B streptococcal invasion of human brain microvascular endothelial cells. Biochem Biophys Res Commun. 2006;345:538–542. doi: 10.1016/j.bbrc.2006.04.130. [DOI] [PubMed] [Google Scholar]
- 106.Kim YS, et al. Brain injury in experimental neonatal meningitis due to group B streptococci. J Neuropathol Exp Neurol. 1995;54:531–539. doi: 10.1097/00005072-199507000-00007. [DOI] [PubMed] [Google Scholar]
- 107.Wahl M, et al. Mediators of blood-brain barrier dysfunction and formation of vasogenic brain edema. J Cereb Blood Flow Metab. 1988;8:621–634. doi: 10.1038/jcbfm.1988.109. [DOI] [PubMed] [Google Scholar]
- 108.Glibetic M, et al. Group B Streptococci and inducible nitric oxide synthase: modulation by nuclear factor kappa B and ibuprofen. Semin Perinatol. 2001;25:65–69. doi: 10.1053/sper.2001.23181. [DOI] [PubMed] [Google Scholar]
- 109.McKnight AA, et al. Oxygen free radicals and the cerebral arteriolar response to group B streptococci. Pediatr Res. 1992;31:640–644. doi: 10.1203/00006450-199206000-00020. [DOI] [PubMed] [Google Scholar]
- 110.Bogdan I, et al. Tumor necrosis factor-alpha contributes to apoptosis in hippocampal neurons during experimental group B streptococcal meningitis. J Infect Dis. 1997;176:693–697. doi: 10.1086/514092. [DOI] [PubMed] [Google Scholar]
- 111.Kim KS, Wass CA, Cross AS. Blood-brain barrier permeability during the development of experimental bacterial meningitis in the rat. Exp Neurol. 1997;145:253–257. doi: 10.1006/exnr.1997.6458. [DOI] [PubMed] [Google Scholar]
- 112.Lehnardt S, et al. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol. 2006;177:583–592. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
- 113.Lehnardt S, et al. TLR2 and caspase-8 are essential for group B Streptococcus-induced apoptosis in microglia. J Immunol. 2007;179:6134–6143. doi: 10.4049/jimmunol.179.9.6134. [DOI] [PubMed] [Google Scholar]
- 114.Ling EW, et al. Biochemical mediators of meningeal inflammatory response to group B streptococcus in the newborn piglet model. Pediatr Res. 1995;38:981–987. doi: 10.1203/00006450-199512000-00025. [DOI] [PubMed] [Google Scholar]
- 115.Paoletti LC, Kasper DL. Conjugate vaccines against group B Streptococcus types IVand VII. J Infect Dis. 2002;186:123–126. doi: 10.1086/341073. [DOI] [PubMed] [Google Scholar]
- 116.Paoletti LC, Kasper DL. Glycoconjugate vaccines to prevent group B streptococcal infections. Expert Opin Biol Ther. 2003;3:975–984. doi: 10.1517/14712598.3.6.975. [DOI] [PubMed] [Google Scholar]
- 117.Baker CJ, Edwards MS. Group B streptococcal conjugate vaccines. Arch Dis Child. 2003;88:375–378. doi: 10.1136/adc.88.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lewis AL, Nizet V, Varki A. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc Natl Acad Sci U S A. 2004;101:11123–11128. doi: 10.1073/pnas.0403010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Santillan DA, Andracki ME, Hunter SK. Protective immunization in mice against group B streptococci using encapsulated C5a peptidase. Am J Obstet Gynecol. 2008;198:114 e111–116. doi: 10.1016/j.ajog.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 120.Brodeur BR, et al. Identification of group B streptococcal Sip protein, which elicits cross-protective immunity. Infect Immun. 2000;68:5610–5618. doi: 10.1128/iai.68.10.5610-5618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Buccato S, et al. Use of Lactococcus lactis expressing pili from group B Streptococcus as a broad-coverage vaccine against streptococcal disease. J Infect Dis. 2006;194:331–340. doi: 10.1086/505433. [DOI] [PubMed] [Google Scholar]
- 122.Patten S, et al. Vaccination for Group B Streptococcus during pregnancy: attitudes and concerns of women and health care providers. Soc Sci Med. 2006;63:347–358. doi: 10.1016/j.socscimed.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 123.Chohan L, et al. Patterns of antibiotic resistance among group B Streptococcus isolates: 2001–2004. Infect Dis Obstet Gynecol. 2006;2006:57492. doi: 10.1155/IDOG/2006/57492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dahesh S, et al. Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to beta-lactam antibiotics. Antimicrob Agents Chemother. 2008;52:2915–2918. doi: 10.1128/AAC.00461-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kimura K, et al. First molecular characterization of group B streptococci with reduced penicillin susceptibility. Antimicrob Agents Chemother. 2008;52:2890–2897. doi: 10.1128/AAC.00185-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading, resources and contacts
Websites
Clinical information on GBS infection can be found at the following websites:
- Centers for Disease Control (USA): http://www.cdc.gov/groupbstrep/
- eMedicine: http://www.emedicine.com/Med/topic2185.htm
- Group B Strep Support (UK): http://www.gbss.org.uk/
- Group B Strep Association (USA): http://www.groupbstrep.org/