Abstract
Melanoma growth stimulatory activity/growth-regulated protein (MGSA/GRO), a CXC chemokine, plays an important role in inflammation, wound healing, growth regulation, angiogenesis, and tumorigenesis. Constitutive expression of MGSA/GROα in melanoma tumors is associated with constitutive nuclear factor (NF)-κB activity. We show here that either exogenous addition or continuous expression of MGSA/GROα in immortalized melanocytes enhances NF-κB activation, as well as mitogen-activated protein (MAP) kinase kinase kinase (MEKK) 1, MAP kinase kinase (MEK) 3/6, and p38 MAP kinase activation. Expression of dominant negative M-Ras (S27N), dominant negative MEKK1 (K432M), or specific chemical inhibitors for p38 MAP kinase (SB202190 and SB203580) block MGSA/GROα-induced NF-κB transactivation, demonstrating that Ras, MEKK1, and p38 are involved in the signal pathways of MGSA/GROα activation of NF-κB. Expression of dominant active Ras or dominant active MEKK1 alone can also stimulate NF-κB activation. The expression of dominant negative MEKK1 inhibits the Ras-induced NF-κB activation, suggesting that MEKK1 is a downstream target of Ras. Moreover, MGSA/GROα induction of NF-κB is independent of the MEK1/ERK cascade, because MGSA/GROα failed to increase ERK and ELK activation, and specific chemical inhibitors for MEK1 (PD98059) had no effect on MGSA/GROα-enhanced NF-κB activation. These data demonstrate that NF-κB activation is required for MGSA/GROα-induced melanocyte transformation through a Ras/MEKK1/p38 cascade in melanocytes.
Melanoma growth stimulatory activity/growth-regulated protein (MGSA/GRO)1 is a member of the CXC chemokine (chemokine with the first two conserved cysteine residues separated by an intervening amino acid) family. MGSA/GRO plays a fundamental role in recruitment and activation of neutrophils, lymphocytes, and monocytes in host defense (1). There are four genes for MGSA/GRO. Three of them encode closely related proteins (MGSA/GROα, β, and γ) (2–5), and the other is a pseudogene (MGSA/GROδ) (6). All three MGSA/GRO proteins bind to the CXC chemokine receptor designated CXCR2, which is a seven-transmembrane G protein-coupled receptor. The order of potency for these three chemokines regarding neutrophil and basophil chemotactic activity, Ca+2 flux, respiratory burst, exocytosis, shape change, and receptor binding is MGSA/GROα > γ > β (7).
MGSA/GROα is expressed in 70% of the human melanoma tumors, but there is little expression of MGSA/GROβ or γ (8). It has become apparent that MGSA/GRO plays an important role in tumorigenesis and angiogenesis (8, 9). Aberrant overexpression of MGSA/GROα has been implicated in melanoma tumor progression both in vitro and in vivo (10–13). Mouse immortalized melanocytes (parental melan-a) stably transfected with MGSA/GROα, β, or γ exhibit an enhanced ability to form large colonies in soft agar and form melanoma tumors in nude mice (10, 11), as compared with parental melan-a cells that do not form tumors in nude mice or in C57B1/6 syngenic mice (14).
Activation of the phosphatidylinositol 3-kinase/Ras/Raf/Soc/MEK1/ERK pathway is common for G protein-coupled receptors (15–17). Receptors for chemokines are traditionally considered to be responsible for the activation of special leukocyte functions such as chemotaxis, degranulation, and the release of superoxide anions. For example, SDF-1α induces tyrosine phosphorylation and association of components of focal adhesion complexes and activates phosphatidylinositol 3-kinase, ERK, and NF-κB in a model of a murine pre-B cell line transfected with the human CXCR4 receptor (18). Interleukin-8 activation of the phosphatidylinositol 3-kinase/Ras/Raf pathway is required for human neutrophil migration. However, the regulation of cell migration by interleukin-8 is independent of ERK activation (19). For some cell types, MGSA/GRO activation of ERK can also be through a Ras/Raf1-independent pathway (20). However, the underlying mechanisms for signal transduction from CXCR2 receptors to transcription factors are poorly understood.
MAP kinase pathways are cellular signaling pathways that enable cells to transduce extracellular signals to an intracellular response. To date, four separate MAP kinases have been identified in mammalian cells: ERK, JNK, p38, and BMK1/ERK5. MAP kinases are activated by phosphorylation of Thr and Tyr amino acids by dual specificity MAP kinase kinases (MEKs), which are themselves activated by MAP kinase kinase kinases (MEKKs). MEKKs are the protein serine-threonine kinases that have regulatory sequences for binding the small guanine nucleotide-binding proteins Ras (21) and Cdc42/Rac (22). It has been reported that MEKK1 is one of several downstream effectors of Ras (21). Except for MEK4, MEKs are, in general, very specific for downstream MAP kinases. MEK1 and MEK2 selectively phosphorylate and activate the ERK subgroup (23), whereas MEK3 and MEK6 selectively activate p38 (24–26). MEK4 does not activate the ERK subgroup but activates both p38 and JNK (24, 27), whereas MEK5 activates BMK1/ERK5 (28). MEK7 has been identified as a specific activator of the JNK subgroup (29, 30). In contrast, the activities of MEKKs show less specificity, and each of the MEKKs transduce signals to more than one MAP kinase cascade.
Activation of Ras also has been implicated in the control of NF-κB activities in fibroblasts (31). This NF-κB activation is required for Ras-initiated cellular transformation in NIH 3T3 cells (31, 32). Inhibition of NF-κB by IκBα expression blocks focus formation induced by oncogenic Ras in NIH 3T3 cells (31). In addition, the expression of antisense p65 blocks cellular transformation (33, 34). It has been reported that there is enhanced NF-κB transcriptional activity through Raf-dependent and Raf-independent mitogen-activated protein kinase signaling pathways when oncogenic Ras is overexpressed in NIH 3T3 cells (35). However, these experiments were done under conditions in which the Ras or the Ras target is overexpressed. In our mouse immortalized melanocytes system, we could investigate these Ras signaling components in a normal cellular context in response to chemokine stimulation.
Our earlier studies showed that MGSA/GRO-induced melanocyte transformation depends on the ability of MGSA/GROα to activate Ras activation through the CXCR2 receptor (36). However, the downstream components of the Ras-affected pathways in melanoma have not been fully elucidated. Thus, we performed experiments to define the MGSA/GRO intracellular signaling pathways in murine melanoma cells. Our data show that MGSA/GROα induces NF-κB activation as well as MEKK1, MEK3/6, and p38 activity. This MGSA/GROα induction of NF-κB is dependent on activation of the Ras/MEKK/p38 cascade, based upon experiments using dominant negative Ras, dominant negative MEKK1 or p38 inhibitors (SB202190 and SB203580). However, MGSA/GROα activation of NF-κB is independent of the MEK1/ERK/ELK and JNK pathways, because MGSA/GROα fails to induce activation of ERK/ELK and JNK, and the inhibitor for MEK1 (PD98059) has no effect on MGSA/GROα-enhanced NF-κB activation. Finally, we report that this NF-κB activation is required for MGSA/GROα-induced melanocyte transformation.
MATERIALS AND METHODS
Cell Culture
The nontransformed mouse immortalized melanocyte cell line, parental melan-a (gift of Dr. Dorthea Bennett), was cultured in Dulbecco’s modified Eagle’s medium supplemented with 50 units/ml penicillin, 50 μg/ml streptomycin, 3 mM glutamine, 10% heat-inactivated fetal bovine serum (Life Technologies, Inc.) and 200 nM 12-O-tetradecanoylphorbol-13-acetate (Sigma). The V1, γ3-14, Mel-a-6, E6A, L7A, and R8A clones were cultured in the same medium supplemented with 800 μg/ml G418 (Sigma) as described previously (Ref. 11 and Table I). The equal expression level of MGSA/GROα or CXCR2 receptor in these clones has been previously verified (11, 36). The MEK1 inhibitor (PD98059), the p38 inhibitors (SB202190 and SB203580), and the CXCR2 inhibitor (SB 225002) (Calbiochem-Novabiochem) were prepared as a stock in Me2SO (10 mM). The pertussis toxin was purchased from Sigma. Purified recombinant human MGSA/GROα (a kind gift of Repligen Corp., Needham, MA) was used at 50 ng/ml.
Table I.
Nomenclature of melan-a clones
| Clone name | Vector | Expressing gene | Cells transfected | Cellular transformation |
|---|---|---|---|---|
| V1 | pRC/CMV | Parental melan-a cells | No | |
| V4 | ||||
| V6 | ||||
| γ3-14 | pRC/CMV | MGSA/GROγ (ELR) | Parental melan-a cells | Yes |
| Mel-a-6 | pRC/CMV | MGSA/GROα (ELR) | Parental melan-a cells | Yes |
| Mel-a-4 | ||||
| Mel-a-9 | ||||
| E6A | pRC/CMV | MGSA/GROα (ALR) | Parental melan-a cells | No |
| L7A | pRC/CMV | MGSA/GROα (EAR) | Parental melan-a cells | No |
| R8A | pRC/CMV | MGSA/GROα (ELA) | Parental melan-a cells | No |
Nuclear Extracts and Mobility Shift Assay
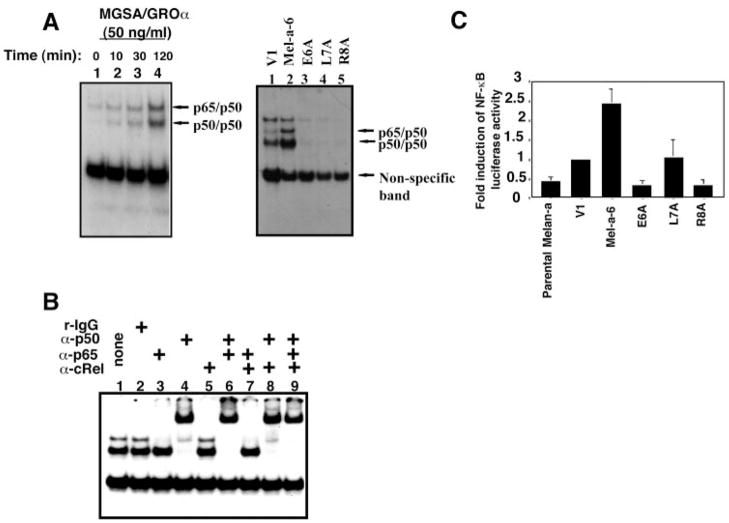
Cells were lysed with buffer (10 mM HEPES, 10 mM sodium chloride, 1.5 mM magnesium chloride, 0.5 mM dithiothreitol, 5 mM β-mercaptoethanol, 100 μM phenylmethylsulfonyl fluoride) with 0.5% Nonidet P-40. The nuclear proteins were extracted from the nuclear pellets using high salt extraction (25% glycerol, 0.2 mM EDTA, 20 mM HEPES, 1.5 mM magnesium chloride, 0.5 mM dithiothreitol, 10 mM potassium chloride, and 240 mM sodium chloride) with protease inhibitors and phosphatase inhibitors, as described previously (37). EMSA were performed by incubating 0.25 ng of double-stranded oligonucleotide end-labeled with 32P by polynucleotide kinase (New England Biolabs, Beverly, CA) and equal amounts of nuclear protein (5 μg) for 30 min at room temperature, as described previously (37). The binding reaction mixtures were resolved in a 6% nondenaturing polyacrylamide gel. Antibody supershift EMSA were performed by incubating nuclear extract proteins with antibodies (1 μg) against p65, p50, c-Rel, or control antibody r-IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at room temperature for 1 h before adding the labeled probe.
Whole Cell Extracts and Western Blot
Whole cell extracts were prepared from control, MGSA/GRO-treated parental melan-a cells, and MGSA/GRO-expressing melan-a cells after serum-free starvation for 4 h. Western blots were performed following protocols provided by Santa Cruz Biotechnology Inc. The cells were washed at 4 °C with 1× phosphate-buffered saline and lysed in 0.25–0.5 ml of RIPA buffer (1× phosphate-buffered saline, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) with protease inhibitor mixture tablets (Roche Molecular Biochemicals). 50 μg of soluble protein was boiled, loaded, and electrophoretically separated on a 10% SDS-PAGE and then electrophoretically transferred to a 0.45-μm nitrocellulose membrane (BIO-RAD, Hercules, CA). The membrane was blocked with 5% dry milk in TBS-T buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.05% Tween-20) for 1 h and then incubated for 12–16 h at 4 °C in a 1:200 dilution (1 μg/ml) of the anti-p-MEK3/6 (B-9) antibody, the anti-p-p38 antibody (D-8), the anti-p-ERK antibody (E-4), the anti-p-JNK antibody (G-7) (Santa Cruz Biotechnology, Inc.), or the anti-p-ELK1 (9181) (New England BioLabs), respectively. Then the membrane was incubated in a 1:3000 dilution of the appropriate anti-mouse, anti-goat, or anti-rabbit immunoglobulin conjugated with horseradish peroxidase (Roche Molecular Biochemicals) in TBS-T buffer with 5% dry milk for 1 h at room temperature. The protein bands were detected with the ECL Western blotting detection reagents (Amersham Pharmacia Biotech) according to the manufacturer’s instructions. The blots were stripped and reprobed with anti-MEK3 (N-20) and MEK6 (N-19) antibodies, anti-p38 antibody (A-12), or anti-ELK1 antibody (9182) (New England BioLabs).
Transient Transfection Assays
Wild type M-Ras, constitutively active M-Ras (G22V), and dominant negative M-Ras (S27N) expression constructs and pBK-CMV expression vector were gifts from Dr. Takeshi Endo. The dominant active MEKK1 and dominant negative MEKK1 (dN) expression constructs and pCMV5Myc expression vector were gifts from Dr. Melanie Cobb. The NF-κB luciferase reporter gene construct was constructed by inserting two copies of the NF-κB consensus DNA binding sequence into the pGL-3 promoter vector (Promega, Madison, WI). The pSV-β-galactosidase expression construct was obtained from Promega. 4 × 105 cells (6-well plates) were transiently cotransfected with 0.2 μg of NF-κB luciferase reporter gene and 0.4 μg of expression constructs indicated in figures, together with either 0.4 μg of pSV-β-galactosidase or pBL-CAT3 expression construct by the LipofectAMINE Plus reagent following manufacturer’s protocol (Life Technologies, Inc.). Three hours later, the cells were incubated with complete medium at, or 37 °C/5% CO2. The cells were treated with inhibitors, MGSA/GROα both, after serum-free starving for 4 h. Two days later, the cells were washed with cold phosphate-buffered saline and lysed in 1× reporter lysis buffer (Promega) for 15 min at room temperature, and the lysate was cleared by centrifugation. The luciferase, β-galactosidase, or CAT activity was measured according to standard protocols (Promega) using a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego, CA) or Beckman LS 3801. Transfection efficiency was monitored by assaying for CAT or β-galactosidase activity derived from a cotransfected expression constructs. All p values were obtained by using the Student’s two-tailed t test.
Immune Complex Kinase Assays
Whole cell extracts were prepared from control and MGSA/GROα-treated parental melan-a cells after serum-free starvation for 4 h. MEKK kinase assays were performed as described in the manufacturer’s protocol (Upstate Biotechnology, Lake Placid, NY). 400 μg of protein of each whole cell extract was immunoprecipitated with MEKK1 antibody (Santa Cruz). Immunoprecipitated MEKK1 activity was assayed using the MEKK1 substrate, GST fusion protein MEK4 (inactivated) (Upstate Biotechnology). Kinase reactions were initiated by addition of 2 μg of GST-MEK4 and kinase buffer containing 500 μM cold ATP and 10 μCi of [γ-32P]ATP. Reactions were incubated for 30 min at 30 °C and terminated by the addition of an equal volume of 2× SDS loading buffer followed by boiling for 5 min. Phosphorylated proteins were resolved by 10% SDS-PAGE and transferred to a 0.45-μm nitrocellulose membrane (Bio-Rad). The phosphorylated bands were visualized by autoradiography. The blot was probed with MEKK1 antibody to monitor equal loading of MEKK1.
Transformation Assays
Focus formation assays were performed in triplicate in three independent experiments. Mel-a-6 cells were cotransfected with 0.2 μg of a puromycin resistant vector (pBABE) and either 2 μg of pCMV4 (vector) or IκB-α (wild type or ΔN) by LipofectAMINE Plus reagent (Life Technologies, Inc.). The cells were cultured in 5% fetal bovine serum/Dulbecco’s modified Eagle’s medium with 0.8 mg/ml G418 and 0.5 mg/ml puromycin, and the foci of transformed cells were counted 18 days after transfection. All p values were obtained by using the Student’s two-tailed t test.
RESULTS
MGSA/GRO Increases NF-κB Activation
Earlier results showed high constitutive nuclear NF-κB activity in human Hs294T melanoma cells as compared with normal retinal pigment epithelial cells (37). Hs294T cells secrete a high level of MGSA/GROα protein, whereas retinal pigment epithelial cells cells do not express MGSA/GROα protein (37). Here, we investigated whether MGSA/GROα induces the activation of NF-κB in mouse melanocytes (parental melan-a cells). First, we performed EMSA to evaluate DNA binding activity in parental melan-a cells treated with MGSA/GROα for the indicated times by using a 32P-labeled consensus NF-κB DNA binding sequence as the probe. As seen in Fig. 1A (left panel), there are two shifted NF-κB nuclear complexes bound to the probe, and MGSA/GROα induces an increase in the formation of these complexes over time (10–120 min). Based on supershift EMSA as shown in Fig. 1B, the lower band consisted of the p50 homodimer, whereas the upper band is the p65/p50 heterodimer. The antibody to c-Rel did not affect the mobility of the NF-κB complexes (Fig. 1B). When the ELR motif of MGSA/GROα is mutated, the ligand exhibits marked reduction in its affinity for receptor, CXCR2 (38). EMSA analysis of nuclear extracts from MGSA/GROα-expressing clones (Mel-a-6), and each of three ELR motif mutant forms of MGSA/GROα-expressing clones (E6A/ELR, L7A/ELR and R8A/ELR) revealed that overexpression of MGSA/GROα increased the NF-κB p65/p50 heterodimer binding activity, whereas all three ELR motif mutant forms failed to enhance NF-κB binding activity (Fig. 1A, right panel). The V1 clone exhibited higher basal p50 homodimer binding activity than the parental melan-a cells. This low level of p50 homodimer NF-κB in V1 cells correlates with a slightly greater constitutive secretion of MIP-2 (138 pg/ml) and KC (49 pg/ml) in these cells, as compared with the parental melan-a cells (MIP-2 (0 pg/ml) and KC (21 pg/ml); Table II). Taken together, our data suggest that exogenous addition or overexpression of MGSA/GROα in the parental melan-a cells enhances NF-κB binding activity, and the ELR motif of MGSA/GROα is required for this induction.
Fig. 1. MGSA/GRO increases NF-κB activation.
A, NF-κB binding activity. In the left panel, the radiolabeled double-stranded NF-κB consensus oligonucleotide (AGTTGAGGGGACTTTCCCAGGC) was mixed with nuclear extracts (5 μg) prepared from parental melan-a cells untreated (lane 1) or 50 ng/ml MGSA/GROα for the indicated times (lanes 2–4). Then EMSA was performed as described under “Experimental Procedures.” The positions of the NF-κB subunits are indicated. In the right panel, EMSA was performed on the nuclear extracts from the following B supershift. Nuclear extract (5 μg) from the melan-a clones: V1 (lane 1), Mel-a-6 (lane 2), E6A (lane 3), L7A (lane 4), and R8A (lane 5). B, NF-κ parental melan-a cells treated with 50 ng/ml MGSA/GROα for 120 min were preincubated with 1 μg of rabbit IgG (lane 2), α-p65 (lane 3), α-p50 (lane 4), α-c-Rel (lane 5), α-p50 and α-p65 (lane 6), α-p65 and α-c-Rel (lane 7), α-p50 and α-c-Rel (lane 8), or α-p50, p65 and α-c-Rel (lane 9) at room temperature for 1 h. Then EMSA was performed as described for A. C, NF-κB transactivation. Parental melan-a, V1, Mel-a-6, E6A, L7A, or R8A cells were transiently cotransfected with 0.2 μg of NF-κB luciferase reporter gene and 0.4 μg of pSV-β-galactosidase expression construct. Luciferase and β-galactosidase activity were measured 48 h later. The relative luciferase activity represents the luciferase activity normalized by β-galactosidase activity. The bar graph shows a mean (± S.E.) of fold induction from four independent transfections (the relative luciferase activity of V1 cells was arbitrarily set to 1, all other activities were given relative to this standard).
Table II. Basal chemokine expression, ras activity, and NF-κB activity in different clones.
Endogenous MIP-2 and KC levels were determined by enzyme-linked immunosorbent assay from conditioned medium collected from parental melan-a cells or V1 cells grown in the absence of serum. The mean result from two individual assays performed in duplicate are shown in ng/ml. B activation was determined as described under The basal level of activated Ras in parental and V1 cells is shown from our previous work (36). NF-κ “Materials and Methods.”
| Clone name | Endogenous MIP-2 | Endogenous KC | Ras activity | NF-κB activation |
|---|---|---|---|---|
| pg/ml | pg/ml | |||
| Parental melan-a cells | 0 | 21 ± 0 | 0.5 | 1 (a relative value of luciferase activity for parental cells) |
| V1 | 138 ± 23 | 49 ± 2 | 1 | 2-fold increase as compared to the parental melan-a cells |
| V1 transfected with pBK-CMV | 1 | 1 (a relative value of luciferase activity for V1 transfected with pBK-CMV) | ||
| V1 transfected with Ras (wild type) | 3-fold | 2.8-fold increase as compared to the V1 transfected with pBK-CMV |
Second, we tested the transactivation of these MGSA/GROα-enhanced NF-κB complexes. The cells were cotransfected with a luciferase reporter gene driven by the SV40 promoter containing two copies of the NF-κB consensus element and pSV-β-galactosidase expression construct for internal control. MGSA/GROα-expressing cells (Mel-a-6) exhibited significantly increased NF-κB transactivation (2.5-fold, p < 0.01) compared with the V1 cell. Constitutive expression of ELR mutant forms of MGSA/GROα failed to increase the NF-κB transactivation (Fig. 1C). The V1 cells exhibited 2-fold higher basal NF-κB transactivation than the parental cells (Fig. 1C). The observation is consistent with the higher NF-κB binding activity in V1 cells than in the parental melan-a cells. To further confirm that the high basal NF-κB activation in V1 cells is mediated by CXCR2, we tested the ability of a selective CXCR2 inhibitor, SB 225002 (39), as well as the ability of pertussis toxin (PTx) to inhibit this high basal NF-κB activation. Signal transduction mediated by ligand binding of CXCR2 has been shown to be dependent on the interaction of the receptor with the PTx-sensitive Gi proteins in neutrophils and 293 cells stably transfected to express CXCR2 (40). V1 cells were cotransfected with an NF-κB-dependent luciferase reporter and the pSV-β-galactosidase expression construct and then treated with highly specific chemical inhibitors for CXCR2 (SB225002). The results showed that NF-κB transactivation enhanced by endogenous MIP-2 and KC is blocked 29, 35, or 49% by these inhibitors at 10, 50, or 100 nM, respectively (Table III). Similarly, PTx inhibits this basal NF-κB transactivation by 32, 52, or 63% at 0.5, 1, or 2 μg/ml, respectively (Table III). These data support the concept that the higher basal NF-κB transactivation in V1 cells is mediated by CXCR2 and PTx-sensitive Gi proteins. Taken together, our data demonstrate that MGSA/GROα enhances NF-κB activation. This activation requires the ELR motif, suggesting the ligand must activate its receptor, CXCR2, on melanocytes.
Table III. A selective CXCR2 inhibitor, SB 225002, or PTx inhibits the high basal NF-κB transactivation in VI cells.
The mean results from two individual experiments performed in duplicate are shown in fold induction.
| Inhibitors | Me2SO | SB 225002 (nM) |
Carrier | PTx (μg/ml) |
||||
|---|---|---|---|---|---|---|---|---|
| 10 | 50 | 100 | 0.5 | 1 | 2 | |||
| NF-κB activation | 1 | 0.71 ± 0.084 | 0.65 ± 0.065 | 0.51 ± 0.060 | 1 | 0.68 ± 0.10 | 0.48 ± 0.056 | 0.37 ± 0.052 |
Ras Mediates MGSA/GROα-enhanced NF-κB Activation
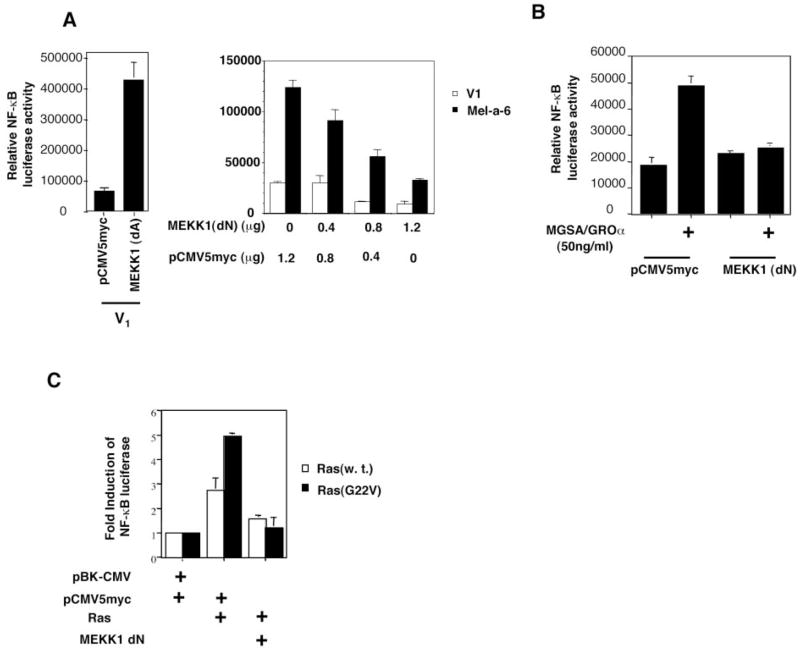
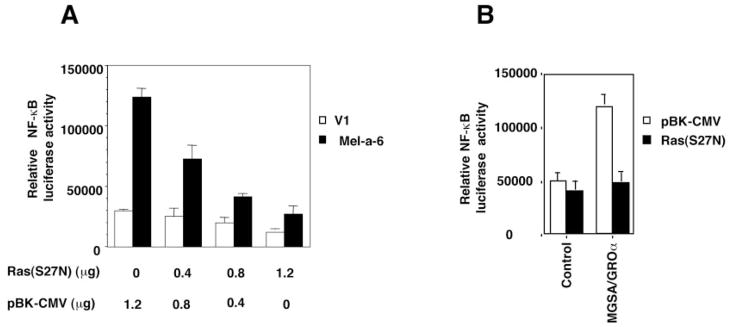
Our earlier results showed that an early response to MGSA/GROα involves activation of Ras, whereas an increase in the expression of Ras protein occurs as a later event (36). We postulated that the MGSA/GROα up-regulation of Ras may correlate with MGSA/GROα-induced NF-κB activation. To determine whether Ras could directly induce NF-κB activation in melanocytes, V1 cells were cotransfected with the NF-κB luciferase reporter construct and an M-Ras expression construct (either wild type, constitutively active, or control vector pBK-CMV). The expression of wild type M-Ras or a constitutively active M-Ras (G22V) increased basal NF-κB transactivation by 2.8- (p < 0.001) and 4.9-fold (p < 0.001) in V1 cells, respectively (see Fig. 4C). Because the expression of wild type M-Ras or dominant active M-Ras in melan-a clones did not affect the activation of the IP-10 promoter CAT reporter construct (data not shown), the effects of Ras on NF-κB are not the result of a general enhancement of metabolic activity or transcription activity. To understand why the expression of wild type M-Ras increased NF-κB activation even in the absence of exogenous MGSA/GROα, we examined the basal level of activated Ras in the V1 cells transfected with wild type M-Ras, using the GST-Raf Ras-binding domain, which precipitates only activated Ras (Ras-GTP but not Ras-GDP) (36). Ras transfected V1 cells exhibited 3-fold higher level of activated Ras than the V1 cells transfected with PBK-CMV vector alone (Table II). We postulate that the continuous exposure to endogenous MIP-2 and KC produced by the V1 cells is sufficient to activate Ras and enhance basal NF-κB transactivation in V1 cells. We have previously demonstrated that exogenous addition of MGSA/GROα to parental melan-a cells induced Ras activation (36). The extremely low level of endogenous MIP-2 and KC secretion in the parental melan-a cells was paralleled by little, if any, endogenous Ras activity in these cells (Table II). To directly evaluate whether Ras mediates MGSA/GRO-induced NF-κB activation, we examined the effect of dominant negative M-Ras (S27N) on MGSA/GRO-induced NF-κB transactivation. The NF-κB transactivation enhanced by expression of MGSA/GROα in Mel-a-6 was reduced by the dominant negative M-Ras (S27N) in a plasmid concentration-dependent manner. With a dominant negative M-Ras (S27N) plasmid concentration of 0.4, 0.8, or 1.2 μg in the transfection reaction, the basal NF-κB activation in V1 cells was decreased to 83, 65, or 41% of the control in V1 cells transfected with pBK-CMV vector only, respectively (Fig. 2A). Similarly, the expression of the dominant negative M-Ras directly blocked NF-κB activation stimulated by exogenous MGSA/GROα treatment after transfection in V1 cells. (Fig. 2B). In parallel, the expression of the dominant negative M-Ras in parental melan-a cells inhibited the exogenous MGSA/GROα-induced NF-κB activation (data not shown). The expression of dominant negative M-Ras in Mel-a-6 cells did not affect the activation of the IP-10 CAT reporter (data not shown), demonstrating that the effects of dominant negative M-Ras on NF-κB activation are not nonspecific. Taken together, these data demonstrate that Ras mediates MGSA/GRO-enhanced NF-κB activation.
Fig. 4. MEKK1 mediates MGSA/GROα-enhanced NF-κB activation.
A, Dominant active MEKK1 increases NF-κB transactivation in V1, whereas dominant negative MEKK1 blocks NF-κB transactivation enhanced by expression of MGSA/GROα. In the left panel, V1 cells were cotransfected with 0.2 μg of NF-κB luciferase reporter and 0.4 μg of the indicated construct (either pCMV5Myc vector or dominant active MEKK1), together with 0.4 μg of pSV-β-galactosidase expression construct. In the right panel, V1 cells or MGSA/GROα-expressing cells (Mel-a-6) were cotransfected with 0.2 μg of NF-κB luciferase reporter and indicated amount of constructs (either pCMV5Myc vector or/and the dominant negative MEKK1), together with 0.2 μg of pSV-β-galactosidase expression construct. The luciferase activity and β-galactosidase activity were measured. The results are reported as the means ± S.E. of the relative luciferase activity (the absolute luciferase activity of sample was normalized by β-galactosidae activity) from three independent experiments. B, dominant negative MEKK1 blocks exogenous MGSA/GROα-induced NF-κB transactivation. Parental melan-a cells were cotransfected as described for A. After transfection, cells were either untreated or treated with 50 ng/ml MGSA/GROα for 48 h. The results are reported as the means ± S.E. of the relative luciferase activity (the absolute luciferase activity of sample was normalized by β-galactosidase activity) from three independent experiments. C, dominant negative MEKK1 blocks Ras-induced NF-κB g of NF-κB transactivation. V1 cells were cotransfected with 0.2 μluciferase reporter and 0.4 μg of the indicated constructs, together with 0.4 μg of pSV-β-galactosidase expression construct. The luciferase activity and β-galactosidase activity were measured. The relative luciferase activity represents the luciferase activity normalized by β-galactosidase activity. The results are reported as a the means ± S.E. of fold induction considering 1 as the relative luciferase activity of the cells transfected with the corresponding empty vectors from three independent experiments.
Fig. 2. Ras mediates MGSA/GROα-enhanced NF-κB activation.
A, Dominant negative M-Ras blocks NF-κB transactivation enhanced by expression of MGSA/GRO. V1 cells (empty bars) or MGSA/GROα-expressing cells (Mel-a-6; solid bar) were cotransfected with 0.2 μg NF-κB luciferase reporter and indicated amount of pBK-CMV vector or/and the dominant negative M-Ras, together with 0.2 μg of pBL-CAT3 construct. Luciferase and CAT activity were measured 48 h later. The relative luciferase activity represents the luciferase activity normalized by CAT activity. The results are reported as a mean (± S.E.) of relative luciferase activity (the luciferase activity normalized by CAT activity) from three independent experiments. B, dominant negative M-Ras blocks MGSA/GROα-induced NF-κB transactivation. V1 cells were cotransfected as described for A. After transfection, cells were either untreated (control) or treated with 50 ng/ml MGSA/GROα for 48 h. The results are reported as a mean (± S.E.) of the relative luciferase activity (the luciferase activity normalized by CAT activity) from three independent experiments.
MGSA/GROα Increases Activation of MEKK1
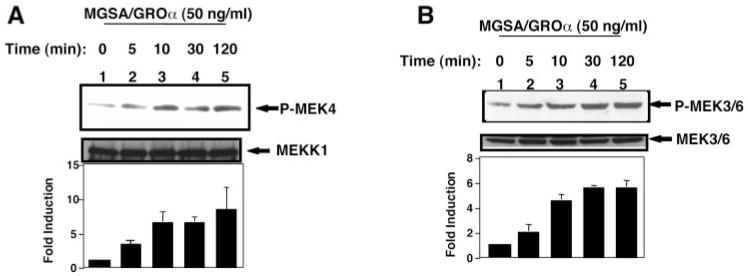
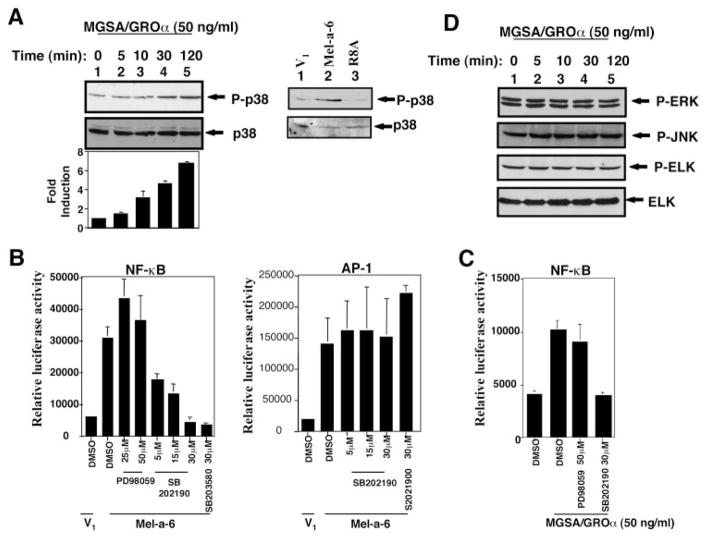
Because MGSA/GRO triggered Ras activation and Ras regulates the MEKK1 pathway, it was of interest to determine whether MGSA/GROα could activate the MEKK1 pathway in mouse melanocytes (parental melan-a cells). To address the question of whether MGSA/GROα increases the endogenous MEKK1 kinase activity, immune complex kinase assays were performed. The results of these assays showed that MGSA/GROα increased the phosphorylation of MEK4, which is one of the substrates of MEKK1 (Fig. 3A). The time course of MGSA/GROα induction of MEKK1 activation is comparable with that of MGSA/GROα activation of Ras. The maximal Ras activation occurs at 10 min and lasts 120 min (36).
Fig. 3. MGSA/GROα increases MEKK1 activity.
A, MEKK1 kinase activity. Parental melan-a cells were either untreated or stimulated with 50 ng/ml MGSA/GROα for the indicated times after serum starvation for 4 h. Endogenous MEKK1 was immunoprecipitated from whole cell extracts. MEKK1 activity was determined by an immunocomplex kinase assay using inactive MEK4 as a substrate as described under “Experimental Procedures.” Phosphorylated proteins were resolved on 10% SDS-PAGE and transferred to a nitrocellulose membrane. The phosphorylated MEK4 bands were visualized by autoradiography (top panel). The blot was probed with MEKK1 antibody to monitor equal loading of MEKK1 (middle panel). This figure is representative of three different experiments. The bar graph (bottom panel) represents the mean fold-induction (± S.E.) of the relative band density of MEK4 (the band density of MEK4 were normalized by the MEKK1 band density) quantitated by densitometer from three independent experiments. B, MEK3/6 phosphorylation. Parental melan-a cells were treated as in A. Whole cell extracts were subjected to 10% SDS-PAGE and immunoblotting with anti-p-MEK3/6 (top panel). The blot was stripped and reprobed with anti-MEK3/6 to assess equal loading of lysates (middle panel). This figure is representative of three different experiments. The bar graph (bottom panel) represents the mean fold-induction (± S.E.) of the relative band density of phosphorylated MEK3/6 (the band density of phosphorylated MEK3/6 were normalized by the MEK3/6 band density) quantitated by densitometer from three independent experiments.
MEKs Are Downstream Targets of MEKKs
Previous studies have identified MEK3, MEK4, or MEK6 as a cellular kinase directly regulating p38 activity. To determine whether MGSA/GROα could activate endogenous MEK3/6 activity, Western blots were performed to detect Ser-189 and Ser-207 phosphorylated MEK3/6 using a specific anti-phosphorylated MEK3/6 antibody (B-9) (Santa Cruz). We observed that MGSA/GROα enhanced the phosphorylation of endogenous MEK3/6 (Fig. 3B). MGSA/GROα activation of MEK3/6 follows the activation profiles of MEKK1. These data indicate that MEKK1 and its substrates MEK3/4/6 could be involved in MGSA/GROα signal pathways.
MEKK1 Mediates MGSA/GROα-enhanced NF-κB Activation
The above observation indicated that MEKK1 is involved in MGSA/GROα signal pathways. To determine whether MEKK1 could induce NF-κB activation in melanocytes, V1 cells were cotransfected with an expression vector containing the constitutively active MEKK1 construct together with the NF-κB luciferase reporter and pSV-β-galactosidase expression construct. The expression of a constitutively active MEKK1 (dominant active) increased NF-κB activity by 6-fold (p < 0.01) (Fig. 4A, left panel). To examine whether MEKK1 mediates MGSA/GROα-enhanced NF-κB activation, we examined the effect of dominant negative MEKK1 (dN) on MGSA/GROα-induced NF-κB transactivation. The NF-κB transactivation enhanced by the expression of MGSA/GROα in Mel-a-6 cells was reduced by the dominant negative MEKK1 (dN) to 74, 46, or 27% at MEKK1 (dN) plasmid concentration of 0.4, 0.8, or 1.2 μg in the transfection reaction, respectively, whereas the basal NF-κB activation in V1 cells was reduced by the dominant negative MEKK1 (dN) to 37% or 30% at MEKK1 (dN) plasmid concentration of 0.8 or 1.2 μg in the transfection reaction, respectively (Fig. 4A, right panel). Similarly, the expression of dominant negative MEKK1 (dN) can directly block NF-κB activation stimulated by exogenous MGSA/GROα treatment after the transfection in parental melan-a cells by 78% (p < 0.01) (Fig. 4B). These results are similar to those obtained in MGSA/GROα-expressing clones. These data demonstrate that MEKK1 mediates MGSA/GROα-enhanced NF-κB activation.
To determine whether MEKK1 is a downstream effector of Ras in the signal pathway of MGSA/GROα-enhanced NF-κB activation, we analyzed the effect of inhibition of MEKK1 on Ras induction of NF-κB activation by using dominant negative MEKK1 (dN). V1 cells were cotransfected with the dominant negative MEKK1 expression construct and either the wild type M-Ras or the dominant active M-Ras (G22V) expression construct, together with the NF-κB luciferase reporter and pSV-β-galactosidase expression constructs. The expression of dominant negative MEKK1 (dN) blocks both wild type and dominant active M-Ras activation of NF-κB by 67% (p < 0.02) and 95% (p < 0.03), respectively (the luciferase activity in cells cotransfected with M-Ras and MEKK1 constructs was compared with the cells cotransfected with M-Ras and pCMV5Myc empty vector) (Fig. 4C). This observation was reproduced in parental melan-a cells (data not shown). These data demonstrate that MEKK1 is a downstream target of Ras in MGSA/GROα activation of NF-κB.
p38 MAP Kinase Is Involved in MGSA/GROα-enhanced NF-κB Activation
MEKK1 phosphorylates and activates MEK3, MEK4, and MEK6 kinases in the p38 pathway in vitro (24–27). Activation of p38 requires phosphorylation on Thr-180 and Tyr-182 (41, 42) and can be assayed directly using specific antibodies to the phosphorylated form of p38. To examine whether MGSA/GROα induces p38 phosphorylation, Western blots were performed by using whole cell extracts from parental melan-a cells treated with MGSA/GROα for the indicated times. As seen in Fig. 5A, MGSA/GROα enhanced p38 phosphorylation at 30 min (Fig. 5A, left panel). The results revealed that p38 is involved in MGSA/GRO signal pathways and that the profile of p38 activation is consistent with that of MEK3/6 activation. In parallel, Western blots analysis of phosphorylated p38 from V1, MGSA/GROα-expressing clones (Mel-a-6), and ELR motif mutant MGSA/GROα-expressing clones (R8A) revealed that expression of MGSA/GROα increased the p38 phosphorylation, whereas the ELR motif mutant form failed to enhance this phosphorylation (Fig. 5A, right panel).
Fig. 5. p38, but not ERK and JNK, is involved in MGSA/GROα-enhanced NF-κB activation.
A, MGSA/GROα increases p38 phosphorylation. Left panel, parental melan-a cells were treated as in Fig. 3A. Whole cell extracts were subjected to 10% SDS-PAGE and immunoblotting with anti-p-p38 (top panel). The blot was stripped and reprobed with anti-p-38 to assess equal loading of lysates (middle panel). This figure is representative of three different experiments. The bar graph (bottom panel) represents the mean fold induction (± S.E.) of the relative band density of phosphorylated p38 (the band density of phosphorylated p38 were normalized by the p38 band density) quantitated by densitometer from three independent experiments. Right panel, whole cell extracts from V1, Mel-a-6, and R8A clones were subjected to 10% SDS-PAGE and immunoblotting with anti-p-p38 (top panel). The blot was stripped and reprobed with anti-p38 to assess equal loading of lysates (bottom panel). This figure is representative of two different experiments. B, p38 inhibitors, but not MEK1 inhibitor, block NF-κB transactivation enhanced by expression of MGSA/GROα. Left panel, V1 or MGSA/GROα-expressing cells (Mel-a-6) were cotransfected as in Fig. 1C. After transfection, the cells were treated with either the solvent control (Me2SO) or the indicated concentrations of PD98059, SB202190, or SB203580 for 24 h. Right panel, V1 or Mel-a-6 cells were transiently cotransfected with 0.2 μg of AP-1 luciferase reporter gene and pSV-β-galactosidase expression construct. After transfection, the cells were treated as in the above panel. C, p38 inhibitors, but not MEK1 inhibitor, block exogenous MGSA/GROα-enhanced NF-κB transactivation. Parental melan-a cells were transiently cotransfected with 0.2 μg of NF-κB luciferase reporter gene and 0.4 μg of pSV-β-galactosidase expression construct. The cells were pretreated with either the solvent control (Me2SO) or the indicated concentrations of PD98059 or SB202190 for 2 h and then stimulated with MGSA/GROα for 24 h. Luciferase activity and β-galactosidase activity were measured. The results are reported as the means (± S.E.) of the relative luciferase activity (the absolute luciferase activity of sample was normalized to β-galactosidase activity) from three independent experiments. D, MGSA/GROα failed to increase ERK, JNK, and ELK phosphorylation. Parental melan-a cells were treated as in A. Whole cell extracts were subjected to 10% SDS-PAGE and immunoblotting with anti-P-ERK (top panel), anti-P-JNK (second panel), or anti-P-ELK (third panel). The blot was stripped and reprobed with anti-ELK to assess equal loading of lysates (bottom panel). This figure is representative of three different experiments.
To evaluate the role of p38 in regulating MGSA/GROα-enhanced NF-κB activation in vivo, MGSA/GROα-expressing cells (Mel-a-6) were cotransfected with an NF-κB-dependent luciferase reporter and the pSV-β-galactosidase expression construct and then treated with highly specific chemical inhibitors for p38 (SB202190 and SB203580) (41) at the indicated concentrations. The results showed that NF-κB transactivation enhanced by expression of MGSA/GROα is blocked 53% (p < 0.02), 70% (p < 0.01), or 100% (p < 0.01) by these inhibitors at 5, 15, or 30 μM, respectively (Fig. 5B, left panel). In contrast, these inhibitors have no effect on AP-1 activation enhanced by expression of MGSA/GROα (p > 0.2) (Fig. 5B, right panel). Similarly, parental melan-a cells were cotransfected with an NF-κB-dependent luciferase reporter and the pSV-β-galactosidase expression construct, pretreated with SB202190 or PD98059, and then stimulated by exogenous MGSA/GROα. The inhibition of the p38 kinase with SB202190 reduced NF-κB transactivation to near control levels at 30 μM (p < 0.01) (Fig. 5C). The concentration of inhibitors blocking activation of NF-κB is similar to that needed for inhibition of p38 kinase activity induced by G-CSF in BaF3-WT cells (43). The observation that p38 inhibitors abrogate MGSA/GROα stimulation of NF-κB suggests that p38 is involved in MGSA/GROα activation of NF-κB.
The MEK1/ERK Kinase Cascade Is Not Essential for NF-κB Activation by MGSA/GROα
When overexpressed, MEKK1 also activates the ERK and JNK pathways (44, 45). MEKK1 phosphorylates and activates MEK1, which leads to activation of ERK1/2 (23). We therefore asked whether NF-κB activation by MGSA/GROα is involved in this MEK1-ERK cascade. First, we examined whether MGSA/GROα regulates ERK activity in parental melan-a cells. Phosphorylation of ERK at Tyr-204 is involved in activation of ERK. MGSA/GROα failed to enhance the phosphorylation of ERK in parental melan-a cells (Fig. 5D). A similar approach, using an antibody that only recognizes phosporylated (activated) ELK on Western blots showed that MGSA/GROα was unable to increase the phosphorylation of ELK, a known target of the MEK1-ERK pathway (Fig. 5D). MGSA/GROα also failed to enhance JNK activation (Fig. 5D). These results suggest that the MEK1-ERK cascade is not critical for MGSA/GROα-enhanced NF-κB activation in melan-a cells.
To further explore this question, we tested the effect of the MEK1 inhibitor, PD98059. The inhibition of MEK1 by PD98059 selectively blocks ERK activation at 10–50 μM in many systems (46). PD98059 at 25 or 50 μM was added to MGSA/GROα expressing cells (Mel-a-6) cotransfected with an NF-κB-dependent luciferase reporter and pSV-β-galactosidase expression construct, and the luciferase and β-galactosidase activity were analyzed. Addition of PD98059 at 50 μM did not decrease basal NF-κB transactivation (Fig. 5B). Likewise, the PD98059 at 50 μM did not reduce MGSA/GROα-stimulated NF-κB-dependent reporter activity in parental melan-a cells (Fig. 5C). Taken together, these data suggest that MGSA/GROα-enhanced NF-κB activation is independent of the MEK1-ERK and JNK cascades.
Inhibition of NF-κB Activation Blocks MGSA/GROα-induced Melanocyte Transformation
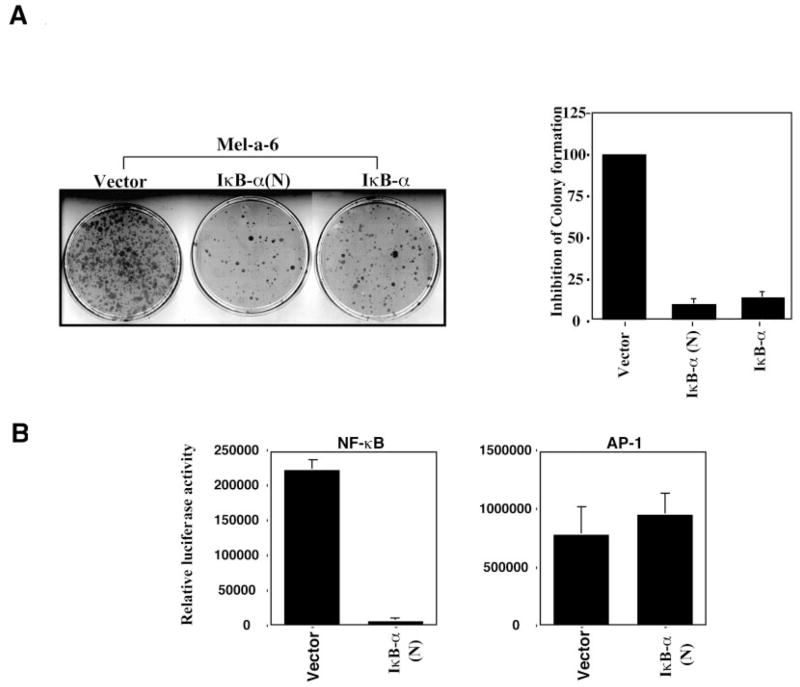
The observation that MGSA/GROα can enhance NF-κB activation prompted us to analyze whether NF-κB activation is critical for cell transformation by MGSA/GROα. We inhibited NF-κB activity with a super-repressor form of IκB-α (N-terminal deletion) that cannot be phosphorylated or degraded and therefore blocks the nuclear translocation of the p65/p50 complex and subsequent transactivation of NF-κB-responsive genes (47). MGSA/GROα-expressing Mel-a-6 cells transfected with the IκB-α expression construct (wild type or N-terminal deletion) exhibited less focus formation than cells transfected with the vector in an in vitro transformation assay (p < 0.001) (Fig. 6A). Because IκB-α (N-terminal deletion) inhibits NF-κB activity (p < 0.001) but not AP-1 activation (Fig. 6B), we conclude that NF-κB activity is involved in MGSA/GROα-induced melanocyte transformation.
Fig. 6. NF-κB is involved in MGSA/GROα-induced cellular transformation.
A, left panel, MGSA/GROα-expressing cells (Mel-a-6) were cotransfected with 0.2 μg of a puromycin resistant vector (pBABE) and either 2 μg of pCMV4 (vector) or IκB-α (wild type or N terminus deletion mutant). The cells were cultured in 5% fetal bovine serum/Dulbecco’s modified Eagle’s medium with G418 (0.8 mg/ml) and puromycin (0.5 mg/ml), and the appearance of foci of transformed cells was stained with crystal violet and counted 18 days after transfection. This figure represents one of three independent experiments with similar results each time. Right panel, the bar graph represents the mean (± S.E.) of relative inhibition (the colony numbers from cells transfected with IκB-α (wild type) or IκB-α (ΔN) divided by the colony numbers from cells transfected with vector) from three independent experiments performed in triplicate (n = 9). B, IκB-α inhibits NF-κB activation but not AP-1 activation in Mel-a-6. MGSA/GROα-expressing cells (Mel-a-6) were cotransfected with either 0.2 μg of the NF-κB luciferase reporter (left panel) or the AP-1 luciferase reporter (right panel) and 0.4 μg of the indicated construct (either empty vector or IκB-α expression construct), together with 0.4 μg of pSV-β-galactosidase expression construct. Luciferase and β-galactosidase activity were measured 48 h later. The results are reported as the means (± S.E.) of the relative luciferase activity (the absolute luciferase activity of sample was normalized by β-galactosidase activity) from three independent experiments performed in duplicate (n = 6).
DISCUSSION
Proinflammatory cytokines such as interleukin-1 and tumor necrosis factor-α rapidly activate NF-κB in most cell types through an NIK/MEKK-IKK-IκB signal pathway (48, 49). These cytokines induce NF-κB activation by modulating IκB phosphorylation, ubiquitination, and proteolytic degradation and by releasing functional NF-κB dimers to translocate to the nucleus (50). Chemotactic factors, such as the lipid mediator platelet-activating factor (51) and SDF-1α (18), are also reported to activate NF-κB in leukocytes or murine pre-B cells, respectively. However, the mechanism of chemokine receptor activation of NF-κB is not clear. This is the first report to identify the signaling pathway for MGSA/GROα activation of NF-κB, which is involved in melanocyte transformation. This activation utilizes a signaling pathway different from those employed by tumor necrosis factor-α, interleukin-1, or phorbol 12-myristate 13-acetate.
It is well known that tumors such as melanoma, breast cancer (52), human head and neck carcinoma (53), and T cell lymphoma (54) have elevated constitutive activation of NF-κB. We postulated that the constitutive activation of NF-κB in melanoma cells could be in part due to an MGSA/GROα autocrine loop. In this paper, we have focused on identifying the signaling components involved in MGSA/GROα-enhanced NF-κB activation. We show that either exogenous addition of MGSA/GROα or continuous expression of MGSA/GROα in parental melan-a cells enhances Ras, MEKK1, MEK3/6, p38, and NF-κB activity, but not ERK, JNK, and ELK activity. Using activated and dominant negative forms of kinases or kinase inhibitors, we have demonstrated here that MGSA/GROα activation of NF-κB is through a Ras-MEKK1-MEKs-p38 cascade. Moreover, this MGSA/GROα-enhanced NF-κB activation is required for melanocyte transformation.
In this study, we have used two control cells, V1 cells and parental melan-a cells. The V1 clone is a better control for MGSA/GROα expressing stable clones than parental melan-a cells because the stable clones have been selected to express the neomycin resistance gene based upon survival in culture medium containing with G418. To monitor effects of exogenous MGSA/GROα on downstream signals, the parental melan-a cells are the appropriate control, because parental melan-a cells represent the whole population of the melanocytes. However, in some experiments, such as Figs. 1 (A and C), 2B, and 4C, we used both V1 cells and parental melan-a cells. We found that our observations in these experiments in V1 cells were reproduced by the parental melan-a cells. In general, V1 cells have higher NF-κB basal activity than the parental melan-a cells, although the V1 cells are derived from the parental melan-a cells. Our data suggest that this higher basal NF-κB activity correlates with the constitutive secretion of MIP-2 and KC by these cells.
Ras activation has been shown to contribute to melanoma tumor development (55–57). Our earlier results showed that Ras is required for MGSA/GROα-induced melanocyte transformation (36). Ras has also been shown to interact physically with numbers of downstream targets, such as Raf, Rac, MEKK1, and phosphatidylinositol 3-kinase, and to activate several different signaling pathways. Using Ras effector dominant mutants, which are deficient in specific effector function, Webb et al. (58) reported that Ras-mediated transformation in NIH 3T3 cells can occur independently of the Raf-MEK1-ERK1/2 pathway. However, the downstream components of the Ras-affected pathways in melanoma have not been fully elucidated. In our present study, we show that MEKK1 is a downstream target of Ras in the murine melanoma model used. But we cannot rule out the possibility that Rac is also a component of this pathway, because Ras can activate Rac (59), and Rac can regulate MEKK1 as well (45).
MEKK activates the protein kinase MEK, which activates ERK, JNK, or p38 (23–30). Once activated, MAP kinases translocate to the nucleus and activate transcription factors. ERK and JNK are also involved in activating NF-κB (60, 61), but it is unlikely that ERK and JNK are involved in the MGSA/GROα signal pathway in the melan-a clones studied here based on the following observations: 1) MGSA/GROα was not able to increase ERK/ELK and JNK activation and 2) the inhibition of ERK by the inhibitor of MEK1, PD98059, had no effect on MGSA/GROα-enhanced NF-κB activation. It could be argued that the failure of MGSA/GROα to enhance ERK/ELK and JNK activation in melan-a cells could be in part due to the high basal activity of these kinases. However, the MEK1 inhibitor PD98059 failed to block basal NF-κB activation. This result is in agreement with a recent report showing that Raf-mediated NF-κB activation is independent of the MEK1/ERK cascade (62). Moreover, our findings here correspond well with the observation that constitutively active components of the MAP kinase pathways are involved in oncogenic transformation in NIH 3T3 cells, whereas ERK1/2 activation is not required for this transformation event (63). Thus, we conclude that MGSA/GROα activation of NF-κB in mouse melanocytes involves MEKK1/MEK activation followed by p38 MAP kinase activation.
The p38 kinase is known to regulate various transcription factors, such as ATF-2 and NF-κB, which in turn modulate the transactivation capacity of the transcription enhancers (64–66). It has been reported that phosphorylation of NF-κB p65 strongly increases p65 transcriptional activity (67, 68). Numerous kinases, including IKKα and β, protein kinases C and A, and tyrosine kinases are implicated in the regulation of NF-κB p65 transactivation. Moreover, recent data connect transcriptional activity of NF-κB p65 with the versatile coactivator proteins p300 and CBP, ATF-2, TFIIB, and TBP (69–71). It is possible that p38 kinase may regulate these coactivators to increase NF-κB-dependent gene expression. We have not, however, determined whether NF-κB p65 is a direct target of p38 or is an indirect target of the p38-regulated kinase. Alternatively, p38 may regulate these coactivators to facilitate NF-κB-dependent gene expression. Future studies will address the exact mechanism by which p38 activates NF-κB.
Several lines of evidence suggest that NF-κB also plays an important role in cellular transformation. The Rel family of transcription factors traditionally has been known to be oncogenic (72), and it was recently demonstrated that NF-κB activity protects cells from Ras transformation-associated apoptosis. It has been reported that a low level of NF-κB activation (3–4 fold) is correlated with the anti-apoptotic function. In contrast, a high level of NF-κB activation failed to protect apoptosis (73). MGSA/GROα induces a low level of transactivation of NF-κB (2.5–4 fold) by enhancing p65/p50 heterodimer binding activity in parental melan-a cells or MGSA/GROα-expressing cells without inducing endogenous expression of other NF-κB-regulated chemokines, such as MIP-2 and KC (data not shown). The blocking of this NF-κB activation inhibits MGSA/GROα-induced melanocyte transformation. These results suggest that a low level of NF-κB induction may not be sufficient to up-regulate inflammatory gene expression but may be sufficient to protect cells from Ras transformation-associated apoptosis. In support of this premise is our observation that selective inhibition of NF-κB activation in melanocytes by targeted overexpression of a super-repressor form of IκB-α (ΔN) initiated M-Ras-induced apoptosis (data not shown). In conclusion, MGSA/GROα-induced melanocyte transformation critically depends on a low level of NF-κB activation through the Ras/MEKK1/p38 cascade.
Acknowledgments
We are indebted to Ben Johnston for excellent technical assistance.
Footnotes
This work was supported by a Department of Veterans Affairs Merit Award and a Career Scientist Award (to A. R.), National Institutes of Health Grants CA56704 and CA34590 (to A. R.), Vanderbilt Vascular Biology Training Grant 2T32HL07751-06, and National Institutes of Health Grant CA68485 (to the Vanderbilt Cancer Center). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The abbreviations used are: MGSA/GRO, melanoma growth-stimulatory activity/growth-regulated protein; CMV, cytomegalovirus; EMSA, electrophoresis mobility shift assay(s); ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; MAP, mitogen-activated protein; MEKK, MAP kinase kinase kinase; MEK, MAP kinase kinase; NF-κB, nuclear factor-κB; PAGE, polyacrylamide gel electrophoresis; dN, dominant negative; GST, glutathione S-transferase; PTx, pertussis toxin; CAT, chloramphenicol acetyltransferase.
References
- 1.Richmond A, Shattuck RL. In: Chemoattractant Ligands and Their Receptors. Horuk R, editor. CRC Press; Boca Raton, FL: 1996. pp. 87–124. [Google Scholar]
- 2.Richmond A, Thomas HG. J Cell Physiol. 1986;129:375–384. doi: 10.1002/jcp.1041290316. [DOI] [PubMed] [Google Scholar]
- 3.Haskill S, Peace A, Morris J, Sporn SA, Anisowicz A, Lee SW, Smith T, Martin G, Ralph P, Sager R. Proc Natl Acad Sci USA. 1990;87:7732–7736. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iida N, Grotendorst GR. Mol Cell Biol. 1990;10:5596–5599. doi: 10.1128/mcb.10.10.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tekamp-Olson P, Gallegos C, Bauer D, McClain J, Sherry B, Fabre M, van Deventer S, Cerami A. J Exp Med. 1990;172:911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shattuck-Brandt RL, Wood LD, Richmond A. DNA Sequence. 1997;7:379–386. doi: 10.3109/10425179709034060. [DOI] [PubMed] [Google Scholar]
- 7.Geiser T, Dewald B, Ehrengruber MU, Clark-Lewis I, Baggiolini M. J Biol Chem. 1993;268:15419–15424. [PubMed] [Google Scholar]
- 8.Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. J Leukocyte Biol. 1997;62:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- 9.Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB, Cardwell N, Luan J, Shattuck-Brandt R, Richmond A. J Leukocyte Biol. 2000;67:53–62. doi: 10.1002/jlb.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balentien E, Mufson BE, Shattuck RL, Derynck R, Richmond A. Oncogene. 1991;6:1115–1124. [PubMed] [Google Scholar]
- 11.Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuck-Brandt R, Richmond A. Int J Cancer. 1997;73:94–103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aid-ijc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Norgauer J, Metzner B, Schraufstaetter I. J Immunol. 1996;156:1132–1137. [PubMed] [Google Scholar]
- 13.Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M. Int J Cancer. 1994;56:853–857. doi: 10.1002/ijc.2910560617. [DOI] [PubMed] [Google Scholar]
- 14.Bennett DC, Cooper PJ, Hart IR. Int J Cancer. 1987;39:414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- 15.Hawes BE, Luttrell LM, van Biesen T, Lefkowitz RJ. J Biol Chem. 1996;271:12133–12136. doi: 10.1074/jbc.271.21.12133. [DOI] [PubMed] [Google Scholar]
- 16.Della Rocca GJ, van Biesen T, Daaka Y, Luttrell DK, Luttrell LM, Lefkowitz RJ. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 18.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE. J Biol Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 19.Knall C, Worthen GS, Johnson GL. Proc Natl Acad Sci USA. 1997;94:3052–3057. doi: 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shyamala V, Khoja H. Biochemistry. 1998;37:15918–15924. doi: 10.1021/bi9811415. [DOI] [PubMed] [Google Scholar]
- 21.Russell M, Lange-Carter CA, Johnson GL. J Biol Chem. 1995;270:11757–11760. doi: 10.1074/jbc.270.20.11757. [DOI] [PubMed] [Google Scholar]
- 22.Fanger GR, Johnson NL, Johnson GL. EMBO J. 1997;16:4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng CF, Guan KL. J Biol Chem. 1993;268:11435–11439. [PubMed] [Google Scholar]
- 24.Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 25.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J, Lee JD, Jiang Y, Li Z, Feng L, Ulevitch RJ. J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 27.Lin A, Minden A, Martinetto H, Claret FX, Lange-Carter C, Mercurio F, Johnson GL, Karin M. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 28.Chao TH, Hayashi M, Tapping RI, Kato Y, Lee JD. J Biol Chem. 1999;274:36035–36038. doi: 10.1074/jbc.274.51.36035. [DOI] [PubMed] [Google Scholar]
- 29.Toyoshima F, Moriguchi T, Nishida E. J Cell Biol. 1997;139:1005–1015. doi: 10.1083/jcb.139.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriguchi T, Toyoshima F, Masuyama N, Hanafusa H, Gotoh Y, Nishida E. EMBO J. 1997;16:7045–7053. doi: 10.1093/emboj/16.23.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finco TS, Westwick JK, Norris JL, Beg AA, Der CJ, Baldwin AS. J Biol Chem. 1997;272:24113–24116. doi: 10.1074/jbc.272.39.24113. [DOI] [PubMed] [Google Scholar]
- 32.Mayo MW, Wang CY, Cogswell PC, Rogersgraham KS, Lowe SW, Der CJ, Baldwin AS. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 33.Higgins KA, Perez JR, Coleman TA, Dorshkind K, McComas WA, Sarmiento UM, Rosen CA, Narayanan R. Proc Natl Acad Sci USA. 1993;90:9901–9905. doi: 10.1073/pnas.90.21.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visconti R, Cerutti J, Battista S, Fedele M, Trapasso F, Zeki K, Miano MP, de Nigris F, Casalino L, Curcio F, Santoro M, Fusco A. Oncogene. 1997;15:1987–1994. doi: 10.1038/sj.onc.1201373. [DOI] [PubMed] [Google Scholar]
- 35.Norris JL, Baldwin AS., Jr J Biol Chem. 1999;274:13841–13846. doi: 10.1074/jbc.274.20.13841. [DOI] [PubMed] [Google Scholar]
- 36.Wang DZ, Yang W, Du JG, Devalaraja MN, Liang P, Matsumoto K, Tsubakimoto K, Endo T, Richmond A. Oncogene. 2000;19:4647–4659. doi: 10.1038/sj.onc.1203820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shattuck-Brandt RL, Richmond A. Cancer Res. 1997;57:3032–3039. [PubMed] [Google Scholar]
- 38.Hébert CA, Vitangcol RV, Baker JB. J Biol Chem. 1991;266:18989–18994. [PubMed] [Google Scholar]
- 39.White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Griswold DE, Sarau HM. J Biol Chem. 1998;273:10095–10098. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- 40.Damaj BB, McColl SR, Mahana W, Crouch MF, Naccache PH. J Biol Chem. 1996;271:12783–12789. doi: 10.1074/jbc.271.22.12783. [DOI] [PubMed] [Google Scholar]
- 41.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 42.Han J, Lee JD, Bibbs L, Ulevitch RJ. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 43.Rausch O, Marshall CJ. J Biol Chem. 1999;274:4096–4105. doi: 10.1074/jbc.274.7.4096. [DOI] [PubMed] [Google Scholar]
- 44.Lange-Carter CA, Pleiman CM, Gardner AM, Blumer KJ, Johnson GL. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 45.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis RJ, Johnson GL, Karin M. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 46.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, Ballard DW. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnes PJ, Karin M. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 49.Siebenlist U, Franzoso G, Brown K. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 50.Israel A. Trends Cell Biol. 2000;10:129–133. doi: 10.1016/s0962-8924(00)01729-3. [DOI] [PubMed] [Google Scholar]
- 51.Ye RD, Kravchenko VV, Pan Z, Feng L. Adv Exp Med Biol. 1996;416:143–151. doi: 10.1007/978-1-4899-0179-8_24. [DOI] [PubMed] [Google Scholar]
- 52.Dong G, Chen Z, Kato T, Van Waes C. Cancer Res. 1999;59:3495–3504. [PubMed] [Google Scholar]
- 53.Ondrey FG, Dong G, Sunwoo J, Chen Z, Wolf JS, Crowl-Bancroft CV, Mukaida N, Van Waes C. Mol Carcinog. 1999;26:119–129. doi: 10.1002/(sici)1098-2744(199910)26:2<119::aid-mc6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 54.Giri DK, Aggarwal BB. J Biol Chem. 1998;273:14008–14014. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]
- 55.Powell BM, Gause PR, Hyman P, Gregus J, Lluria-Prevatt M, Nagle R, Bowden GT. Carcinogenesis. 1999;20:1747–1753. doi: 10.1093/carcin/20.9.1747. [DOI] [PubMed] [Google Scholar]
- 56.Jansen B, Schlagbauer-Wadl H, Kahr H, Heere-Ress E, Mayer BX, Eichler H, Pehamberger H, Gana-Weisz M, Ben-David E, Kloog Y, Wolff K. Proc Natl Acad Sci USA. 1999;96:14019–14024. doi: 10.1073/pnas.96.24.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O’Hagan R, Pantginis J, Zhou H, Horner JW, II, Cordon-Cardo C, Yancopoulos GD, DePinho RA. Nature. 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- 58.Webb CP, Van Aelst L, Wigler MH, Woude GF. Proc Natl Acad Sci USA. 1998;95:8773–8778. doi: 10.1073/pnas.95.15.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 60.Tuyt LM, Dokter WH, Birkenkamp K, Koopmans SB, Lummen C, Kruijer W, Vellenga E. J Immunol. 1999;162:4893–4902. [PubMed] [Google Scholar]
- 61.Yang X, Chen Y, Gabuzda D. J Biol Chem. 1999;274:27981–27988. doi: 10.1074/jbc.274.39.27981. [DOI] [PubMed] [Google Scholar]
- 62.Baumann B, Weber CK, Troppmair J, Whiteside S, Israel A, Rapp UR, Wirth T. Proc Natl Acad Sci USA. 2000;97:4615–4620. doi: 10.1073/pnas.080583397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welch DR, Sakamaki T, Pioquinto R, Leonard TO, Goldberg SF, Hon Q, Erikson RL, Rieber M, Rieber MS, Hicks DJ, Bonventre JV, Alessandrini A. Cancer Res. 2000;60:1552–1556. [PubMed] [Google Scholar]
- 64.Brinkman BM, Telliez JB, Schievella AR, Lin LL, Goldfeld AE. J Biol Chem. 1999;274:30882–30886. doi: 10.1074/jbc.274.43.30882. [DOI] [PubMed] [Google Scholar]
- 65.Berghe WV, Plaisance S, Boone E, Bosscher KD, Schmitz ML, Fiers W, Haegeman G. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 66.Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- 67.Naumann M, Scheidereit C. EMBO J. 1994;13:4597–4607. doi: 10.1002/j.1460-2075.1994.tb06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 69.Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du W, Thanos D, Maniatis T. Cell. 1993;74:887–898. doi: 10.1016/0092-8674(93)90468-6. [DOI] [PubMed] [Google Scholar]
- 71.Kaszubska W, Hooft van Huijsduijnen R, Ghersa P, DeRaemy-Schenk AM, Chen BP, Hai T, DeLamarter JF, Whelan J. Mol Cell Biol. 1993;13:7180–7190. doi: 10.1128/mcb.13.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilmore TD, Koedood M, Piffat KA, White DW. Oncogene. 1996;13:1367–1378. [PubMed] [Google Scholar]
- 73.Carlotti F, Chapman R, Dower SK, Qwarnstrom EE. J Biol Chem. 1999;274:37941–37949. doi: 10.1074/jbc.274.53.37941. [DOI] [PubMed] [Google Scholar]