Abstract
The cleavage of unactivated C—H bonds is one of the most challenging reactions in chemical biology. Metalloenzymes have evolved that efficiently perform these transformations with exquisite control of selectivity; however, a proposed requirement is the generation of highly reactive intermediates that could be lethal. A thermodynamic argument involving the putative reactive species is outlined, whereby the interplay between two tunable parameters, redox potential and pKa, may be the key to sustainable function. In addition, factors that control these parameters are also described, including hydrogen-bonding networks found within protein active sites. Synthetic examples are used to corroborate these ideas.
Introduction
This Opinion discusses current ideas of how the active sites of proteins can modulate highly reactive intermediates to perform chemically challenging reactions. The concepts described are rooted in inorganic and physical organic chemistry, but clearly pertain to chemical biology. The reaction in question is the cleavage of C—H bonds, one of the most difficult chemical transformations [i, ii], yet an essential biological process for survival. The thermodynamic stability of most C—H bonds is often cited as a reason for their low reactivity: many biologically relevant substrates have C—H bond dissociation energies of greater than 90 kcal/mol. To overcome this thermodynamic barrier an arsenal of enzymes have evolved that catalyze the cleavage, most having active sites containing at least one metal center. The paradigm system is the cytochrome P450s, whose hydroxylase components have active sites composed of a heme center with a single Fe-thiolate linkage [iii, iv]. There is also a growing family of non-heme iron enzymes that catalyze C—H bond cleavage—these systems usually contain active sites with either a single iron complex [v] or, in the case of soluble methane monooxygenase, a dinuclear iron center [vi, vii]. In the last 50 years an enormous amount of effort has gone into determining the mechanistic details of their function; while great progress has been made there are still many unanswered questions. A central challenge is determining the structure of competent oxidants responsible for the cleavage events, and elucidating their properties that lead to efficient, and in most cases selective, cleavage of C—H bonds.
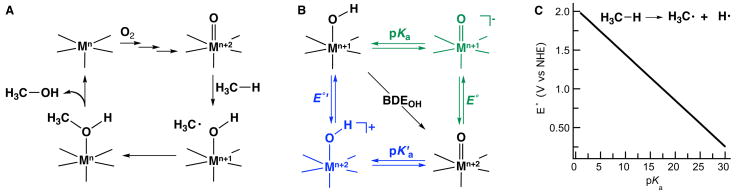
A common pattern among these metalloenzymes is that dioxygen binding and activation must precede C—H bond cleavage. The mechanistic implication is that a metal-O2 derived species is involved in the oxidation of substrates. However, the activation of dioxygen is a complicated process, with the identity of intermediates along the reaction paths still in dispute. In many protein systems, it is proposed that O2 activation leads to high valent metal-oxo species [4, 5], which serve as the key species in the oxidation of substrates. A possible reaction sequence that follows this concept is summarized in Figure 1, in which a radical species and a M—OH compound are initially formed.
Figure 1.
A possible mechanism for metal-oxo mediated C—H bond cleavage (A), thermodynamic cycle describing the BDEOH (B), and the relationship between redox potential and pKa for a metal-oxo species in the cleavage of a C—H bond in methane with BDEC-H = 104 kcal/mol (C).
Generation of Strong Oxidants within a Protein Active Site
There are obvious evolutionary benefits in coupling O2 activation with C—H bond cleavage, leading to the success of aerobic life. However, an underlying problem is how a highly reactive form of the active site can exist without destroying the protein [viii]. The dioxygen-derived metal species proposed in Figure 1A exist long enough to cause irreversible damage to essential components of the protein. These deleterious effects are often found in synthetic systems and are the major obstacles for the development of useful synthetic oxidation catalysts using O2 as a substrate. It is well known that active sites impose structural constraints around metal centers to promote efficient catalysis, yet the details into how proteins can withstand the production of powerful oxidants are still lacking. We describe two approaches that could work in synergy to combat oxidative destruction by metal-oxo centers: tuning the basicity of the metal-oxo unit and hydrogen-bonding networks that are proximal to the active site metals. These approaches are illustrated with synthetic systems, in which structure-function relationships have been established.
Tuning the Reactivity of the Metal-Oxo Moiety: The pKa Effect
The oxidative power of compounds is often discussed within the context of redox potentials, whereby stronger oxidants have higher reduction potentials. This metric is important but is not the only parameter needed to evaluate the oxidative ability of metal-oxo complexes. Mayer has elegantly described the use of thermodynamic cycles to evaluate the ability of metal-oxo complexes to cleave C—H bonds [ix, x]: these analyses examine the O—H bond dissociation energies (BDEOH) of metal-hydroxo (MOH) complexes formed after the initial cleavage event (Figure 1A). The relevance to C—H bond cleavage is readily apparent: for the scheme in Figure 1 to be thermodynamically viable, the energy required for cleaving the C—H bond must be similar to that produced on forming the MO—H bond.
A thermodynamic cycle for the treatment of this reaction is shown in Figure 1B, from which the BDEOH equation can be derived: BDEOH = 23.06 E° + 1.37 pKa + C [xi]. The constant (C) is included in the analysis to account for the solvation of the hydrogen atom and is dependent on solvent and reference for the redox potential. One can see that both a one-electron reduction potential and a pKa value are needed to evaluate the BDEOH. The inclusion of the pKa (that is, a gauge of the basicity of an oxo ligand) is an important outcome because it is another tunable parameter that affects the reactivity of metal-oxo complexes. The effect is illustrated graphically (Figure 1C) for the cleavage of a C—H bond in methane [xii], which has a bond dissociation energy of 104 kcal/mol. Note that at low pKa values the redox potentials required are prohibitory to maintain function: for instance, the metal-oxo unit would irreversibly oxidize key amino acids (e.g. tryptophan). However, as the basicity of the oxo ligand increases there is a decrease in the redox potential to values compatible with protein systems.
Thermodynamic cycles similar to the one in Figure 1B have been utilized to understand the interplay between basicity and redox potential of a metal-oxo species in the cleavage of a C—H bond. Note that two key metal-oxo complexes are presented in the cycle and the basicities of oxo ligands in each species could make important contributions. For instance, some have suggested that the basicity of the oxo ligand in the one-electron reduced species (green path in Figure 1B) plays an important role in this process. The most relevant example is from the work of Green, who argues that the redox potentials in P450s are much lower than previously thought, preventing oxidative damage of the active site [xiii]. Specifically, he proposes that the moderate redox potentials in compound I, the iron-oxo radical species thought to cleave C—H bonds, are offset by the relatively high basicity of the oxo ligand of compound II (the one-electron reduced form of compound I). He further contends that ligation of the cysteine thiolates to the iron centers is partly responsible for this effect [xiv]. Support for the basicity of a thiolate-ligated compound II species was revealed recently by resonance Raman spectroscopy [xv].
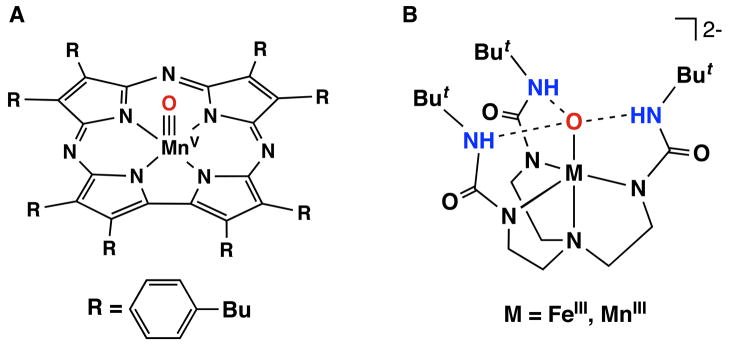
Recent reports on well-characterized synthetic manganese-oxo complexes support this idea. Examples developed by Goldberg using MnV-oxo species containing the porphyrinoid-like ligands, corralozines, illustrate this point [xvi]. [MnV(TBP8Cz)(O)] (Figure 2A) can cleave the X—H (X=C or O) bonds in a variety of substrates, a reactivity attributed to the basicity of the oxo ligand in the one-electron reduced species [MnIV(TBP8Cz)(O)]−. A pKa value of 15 in acetonitrile has been estimated for [MnIV(TBP8Cz)(OH)]+, the conjugate acid of [MnIV(TBP8Cz)(O)]−, which compensates the modest one-electron reduction potential of +0.24 vs NHE measured for [MnV(TBP8Cz)(O)].
Figure 2.
Structure of [MnV(TBP8Cz)(O)] (A) and [MnIIIH3buea(O)]2− (B).
Synthetic examples have also been reported showing that the basicity of the higher valent metal-oxo species can dictate reactivity (blue path in Figure 1B). A striking example of this is found in the chemistry of [MnIIIH3buea(O)]2− (Figure 2B, M = MnIII), the rare example of a terminal oxo ligand bonded to a manganese(III) ion [xvii]. This complex has an extremely low reduction potential of less than −1.3 V vs NHE. However, [MnIIIH3buea(O)]2− behaves as an oxidant, cleaving C—H bonds in a variety of substrates. The mechanism leading to this reactivity is still under investigation, but preliminary indicators point toward the basicity of the oxo ligand in [MnIIIH3buea(O)]2− because of its unusually high pKa of 28.3 measured in DMSO.
Tuning the Reactivity of the Metal-Oxo Moiety: Non-Covalent Interactions
The above discussion illustrates how adjusting redox potential and pKa can affect the reactivity and durability of metal-based oxygenases. From a broader viewpoint, metalloproteins accomplish chemical transformations with efficiencies, selectivities, and speeds that exceed what is currently possible in synthetic systems. The causes for these differences are just emerging, but clearly factors that govern basic physical properties, such as redox properties and basicity, are crucial aspects. One factor is hydrogen bond (H-bond) networks within protein active sites. Information derived from structural biology has indicated that many metalloproteins, especially those involved in oxidative processes, have (H-bond) networks within their active sites that directly interact with the oxo (or hydroxo) ligands. For example, the structure of compound I of cytochrome C peroxidase determined by X-ray diffraction methods shows a H-bond network involving a structural water molecule and active site amino acid residues that directly interact with the iron-oxo unit (Figure 3A) [xviii]. We have advocated that these types of non-covalent interactions arising from protein-induced microenvironments can have profound impact on function [xix, xx].
Figure 3.
Active site structures of cytochrome C peroxidase (A, 1ZBZ) and human MnSOD (B, 2ADQ) highlighting the H-bonding networks surrounding the metal centers. Gln69 refers to FeSOD.
Evidence for these microenvironmental effects is found in chemical biology and synthetic chemistry. The iron and manganese superoxide dismutases (SODs) dramatically illustrate this effect: these proteins have nearly identical active site structures (Figure 3B) with extensive H-bonding networks surrounding coordinated water molecules [xxi, xxii, xxiii]. It has been shown that interruption of the networks in both SODs causes major changes in function. For instance, substitution of His30 in human MnSOD with a series of amino acids results in a ten-fold diminution in kcat, even though this residue is more than 5 Å from the manganese center [xxiv]. In another set of detailed structure-function studies, Miller has found that H-bonding networks regulate the protonation state of the bound water molecule (H2O or OH−), which in turn affects the redox potential. Moreover, she showed that a series of single amino acid substitutions at Gln69 in FeSOD produces changes in redox potentials that span 0.660 V [xxv, xxvi]. Substantial alterations in redox potential have also been observed in synthetic systems, whereby changes in the number of H-bonds to copper-chloride units alter reduction potentials by nearly 0.200 V (Figure 5A) [xxvii]. Correspondingly, a series of zinc-hydroxo complexes with varied H-bond networks can change the pKa values over two units. These pKa changes correlate with functional changes of approximately 104 in the rate of hydrolysis of phosphate esters [xxviii, xxix].
To investigate these effects in synthetic compounds, we introduced systems that create intramolecular H-bond networks proximal to metal centers [19, xx]. This design has allowed isolation of new types of metal-oxo and their corresponding metal-hydroxo analogs, as discussed above for the manganese-oxo complex (see Figure 2B, M = Mn). In another case, we have prepared the iron-oxo complex, [FeIIIH3buea(O)]2−, in which the oxo ligand is derived from the cleavage of dioxygen (Figure 2B, M = Fe). Numerous studies with this complex show that there are three intramolecular H-bonds involving the oxo ligand and the NH group of the [H3buea]3− ligand [xvii, xxx, xxxi]. Our findings on [FeIIIH3buea(O)]2− and related compounds indicate that H-bonds can regulate key properties of metal-oxo complexes, such as the metal-oxo bond strength, oxo basicity, and redox potentials.
We have elaborated on our initial system to develop a series of compounds, whereby the H-bonding networks can be varied as shown in Figure 4. This design accommodates site-directed modifications of the microenvironment around the metal centers without altering the metal coordination geometry. Therefore, we can systematically address how physical and functional properties change as a function of increasing H-bond donors. In one study, we showed that a decrease in H-bond donors leads to incremental increases in Fe—O bond lengths for a series of FeIII—OH complexes; these structural changes are correlated to decreases in the strength of the O—H bond [xxxii]. A series of cobalt complexes illustrated the effects of H-bonds on metal-mediated O2 activation/C—H bond cleavage [xxxiii]. The complexes with H-bond donors are able to bind and activate dioxygen, albeit with different efficiencies. However, the compound without H-bonding capabilities does not bind O2, even though it has vacant coordination sites and redox properties sufficient for binding/activation.
Figure 4.
Example of a modular synthetic system containing varied H-bonding networks.
Summary
Advances in understanding the cleavage of C—H bonds have been achieved through interdisciplinary studies on a variety of systems. The crucial question of how proteins can maintain function while producing highly reactive intermediates can be understood within a thermodynamic framework that relies on the interplay between basicity and redox potential. Tuning of these two parameters could lead to reactive species with depressed redox potentials that can co-exist with other elements present in active sites. Proximal H-bonds control these basic properties in proteins and are now being used in synthetic systems to discover new functions.
Acknowledgments
We thank the National Institutes of Health (GM 050781) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- i.Labinger JA, Bercaw JE. Understanding and Exploiting C—H Bond Activation . Nature. 2002;417:507–514. doi: 10.1038/417507a. [DOI] [PubMed] [Google Scholar]
- ii.Shilov AE, Shteinman AA. Oxygen Atom Transfer into C—H Bond in Biological and Model Chemical Systems. Mechanistic Aspects. Acc Chem Res. 1999;32:763–771. [Google Scholar]
- iii.Sono M, Roach MP, Coulter ED, Dawson JH. Heme Containing Oxygenases . Chem Rev. 1996;96:2841–2887. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- iv.Groves JT. High-Valent Iron in Chemical and Biological Oxidations. J Inorg Biochem. 2006;100:434–447. doi: 10.1016/j.jinorgbio.2006.01.012. [DOI] [PubMed] [Google Scholar]
- v.Krebs C, Fujimori DG, Walsh CT, Bollinger JM. Non-Heme Fe(IV)–Oxo Intermediates. Acc Chem Res. 2007;40:484–492. doi: 10.1021/ar700066p. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vi.Sazinsky MH, Lippard SJ. Correlating Structure and Function in Bacterial Multicomponent Monooxygenase and Related Diiron Proteins. Acc Chem Res. 2006;39:558–566. doi: 10.1021/ar030204v. [DOI] [PubMed] [Google Scholar]
- vii.Que L, Jr, Tolman WB. Bis-(μ-oxo)dimetal “Diamond” Cores in Copper and Iron Complexes Relevant to Biocatalysis. Angew Chem Int Ed. 2002;41:1114–1137. doi: 10.1002/1521-3773(20020402)41:7<1114::aid-anie1114>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- viii.Klinman JP. How Do Enzymes Activate Oxygen without Inactivating Themselves? Acc Chem Res. 2007;40:325–333. doi: 10.1021/ar6000507. [DOI] [PubMed] [Google Scholar]
- •ix.Gardner KA, Mayer JM. Understanding C—H Bond Oxidations: H. and H−Transfer in the Oxidation of Toluene by Permanganate. Science. 1995;269:1849–1851. doi: 10.1126/science.7569922. A classic paper on the mechanism of C—H bond cleavage by a metal-oxo complex that provides insight into the underlying causes for the transformations. [DOI] [PubMed] [Google Scholar]
- ••x.Mayer JM. Proton Coupled Electron Transfer: A Reaction Chemists View. Annu Rev Phys Chem. 2004;55:363–390. doi: 10.1146/annurev.physchem.55.091602.094446. The key concepts of metal assisted C—H bond activation are outlined eloquently in this review. Although most of the examples are non-biological, the ideas are expressed clearly and written in an understandable manner that can be transferred to understand biological systems. This review is certainly required reading for anyone in the field. [DOI] [PubMed] [Google Scholar]
- xi.Bordwell FG, Cheng JP, Ji G-Z, Satish AV, Zhang X. Bond Dissociation Energies in DMSO Related to the Gas Phase. J Am Chem Soc. 1991;113:9790–9795. [Google Scholar]
- xii.Behan RK, Green MT. On the Status of Ferryl Protonation. J Inorg Biochem. 2006;100:448–459. doi: 10.1016/j.jinorgbio.2005.12.019. [DOI] [PubMed] [Google Scholar]
- ••xiii.Green MT, Dawson JH, Gray HB. Oxoiron(IV) in Chloroperoxidase Compound II Is Basic: Implications for P450 Chemistry. Science. 2004;304:1653–1656. doi: 10.1126/science.1096897. The connection between pKa and C—H bond activation in cytochrome P450 is discussed. [DOI] [PubMed] [Google Scholar]
- xiv.Behan RK, Hoffart LM, Stone KL, Krebs C, Green MT. Evidence for Basic Ferryls in Cytochrome P450. J Am Chem Soc. 2006;128:11471–11474. doi: 10.1021/ja062428p. [DOI] [PubMed] [Google Scholar]
- xv.Stone KL, Behan RK, Green MT. Resonance Raman Spectroscopy of Chloroperoxidase Compound II Provides Direct Evidence for the Existence of an Iron(IV)-Hydroxide. Proc Nat Acad Sci. 2006;103:12307–12310. doi: 10.1073/pnas.0603159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •xvi.Lansky DE, Goldberg DP. Hydrogen Atom Abstraction by a High-Valent Manganese(V)-OxoCorrolazine. Inorg Chem. 2006;45:5119–5125. doi: 10.1021/ic060491+. This paper describes a synthetic system, in which the basicity of the oxo ligand is a key contributor to function. The approach and concepts are clearly presented and show that oxo basicity is a factor in the reactivity of synthetic metal-oxo complexes. [DOI] [PubMed] [Google Scholar]
- xvii.MacBeth CE, Gupta R, Mitchell-Koch KR, Young VG, Jr, Lushington GH, Thompson WH, Hendrich MP, Borovik AS. Utilization of Hydrogen Bonds to Stabilize M—O(H) Units: Synthesis and Properties of Monomeric Iron and Manganese Complexes with Terminal Oxo and Hydroxo Ligands. J Am Chem Soc. 2004;126:2556–2567. doi: 10.1021/ja0305151. [DOI] [PubMed] [Google Scholar]
- xviii.Bonagura CA, Bhaskar B, Shimizu H, Li H, Sundaramoorthy M, McRee DE, Goodin DB, Poulos TL. High-Resolution Crystal Structures and Spectroscopy of Native and Compound I Cytochrome C Peroxidase. Biochemistry. 2003;42:5600–5608. doi: 10.1021/bi034058c. [DOI] [PubMed] [Google Scholar]
- xix.Borovik AS. Bio-Inspired Hydrogen Bond Motifs in Ligand Design. The Role of Non-Covalent Interactions in Metal Ion Mediated Activation of Dioxygen. Acc Chem Res. 2005;38:54–61. doi: 10.1021/ar030160q. [DOI] [PubMed] [Google Scholar]
- xx.Shook RL, Borovik AS. The Effects of Hydrogen Bonds on Metal Mediated Processes. Chem Commun. 2008:6095–6107. doi: 10.1039/b810957e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxi.Quint P, Reutzel R, Mikulski R, McKenna R, Silverman DN. Crystal structure of nitrated human manganese superoxide dismutase: mechanism of inactivation. Free Radic Biol Med. 2006;40:453–458. doi: 10.1016/j.freeradbiomed.2005.08.045. [DOI] [PubMed] [Google Scholar]
- xxii.Lah MS, Dixon MM, Pattridge KA, Stallings WC, Fee JA, Ludwig ML. Structure-Function in Escherichia coli Iron Superoxide Dismutase: Comparisons with the Manganese Enzyme from Thermus Thermophilus. Biochemistry. 1995;34:1646–1660. doi: 10.1021/bi00005a021. [DOI] [PubMed] [Google Scholar]
- xxiii.Jackson TA, Brunold TC. Combined Spectroscopic/Computational Studies on Fe- and Mn-Dependent Superoxide Dismutases: Insights into Second-Sphere Tuning of Active Site Properties. Acc Chem Res. 2004;37:461–470. doi: 10.1021/ar030272h. [DOI] [PubMed] [Google Scholar]
- xxiv.Ramilo CA, Leveque V, Guan Y, Lepock JR, Tainer JA, Nick HS, Silverman DN. Interrupting the Hydrogen Bond Network at the Active Site of Human Manganese Superoxide Dismutase. J Biol Chem. 1999;274:27711–27716. doi: 10.1074/jbc.274.39.27711. [DOI] [PubMed] [Google Scholar]
- •xxv.Yikilmaz E, Porta J, Grove LE, Vahedi-Faridi A, Bronshteyn Y, Brunold TC, Borgstahl GEO, Miller AF. How can a Single Secondary Sphere Amino Acid Substitution Cause Midpoint Potential Changes of Hundreds of Millivolts? J Am Chem Soc. 2007;129:9927–9940. doi: 10.1021/ja069224t. The effect of H-bonds on function is dramatically demonstrated in this paper. The authors do a nice job of determining the factors that govern redox control of this enzyme through protein engineering, crystallography, and electrochemical studies. [DOI] [PubMed] [Google Scholar]
- xxvi.Miller AF. Redox Tuning over Almost 1 V in a Structurally Conserved Active Site: Lessons from Fe-Containing Superoxide Dismutase. Acc Chem Res. 2008;41:501–510. doi: 10.1021/ar700237u. [DOI] [PubMed] [Google Scholar]
- xxvii.Mareque-Rivas JC, Hinchley SL, Metteua L, Parson S. The Strength of Hydrogen Bonding to Metal-Bound Ligands can Contribute to Changes in the Redox Behaviour of Metal Centres. Dalton Trans. 2006:2316–2322. doi: 10.1039/b516234c. [DOI] [PubMed] [Google Scholar]
- xxviii.Natale D, Mareques-Rivas JC. The Combinations of Transition Metal Ions and Hydrogen-Bonding Interactions. Chem Commun. 2008:425–437. doi: 10.1039/b709650j. [DOI] [PubMed] [Google Scholar]
- •xxix.Feng G, Mareque-Rivas JC, de Rosales RTM, Williams NH. A Highly Reactive Mononuclear Zn(II) Complex for Phosphodiester Cleavage. J Am Chem Soc. 2005;127:13470–13471. doi: 10.1021/ja054003t. A functional illustration of how H-bonds can affect the rates of metal-mediated reactions is presented. [DOI] [PubMed] [Google Scholar]
- •xxx.MacBeth CE, Golombek AP, Young VG, Jr, Yang C, Kuczera K, Hendrich MP, Borovik AS. O2 Activation by Non-Heme Iron Complexes: A Monomeric Fe(III)—Oxo Complex Derived From O2. Science. 2000;289:938–941. doi: 10.1126/science.289.5481.938. This paper presents a structurally characterized terminal oxo ligand to an iron center via O2 activation. It illustrates how H-bonds can be used to control O2 and C—H bond activation. [DOI] [PubMed] [Google Scholar]
- xxxi.Dey A, Hocking RK, Larsen PL, Borovik AS, Hedman B, Hodgson KO, Solomon EI. X-Ray Absorption and Density Function Theory Studies on Mononuclear FeIII—X Complexes (X = O2−, OH−, and S2−): Effects of Hydrogen Bonds on Iron Species with Terminal Oxo and Sulfido Ligands. J Am Chem Soc. 2006;128:9825–9833. doi: 10.1021/ja061618x. [DOI] [PubMed] [Google Scholar]
- xxxii.Mukherjee J, Lucas RL, Zart MK, Powell DR, Day VW, Borovik AS. Synthesis, Structure, and Physical Properties for a Series of Monomeric Iron(III) Hydroxo Complexes with Varying Hydrogen Bond Networks. Inorg Chem. 2008;47:5780–5786. doi: 10.1021/ic800048e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxxiii.Lucas RL, Muhkerjee J, Zart MK, Sorrell TN, Powell DR, Borovik AS. A Modular Approach Toward Regulating the Secondary Coordination Sphere of Metal Ions: Differential Dioxygen Activation Assisted by Intramolecular Hydrogen Bonds. J Am Chem Soc. 2006;128:15476–15489. doi: 10.1021/ja063935+. [DOI] [PubMed] [Google Scholar]