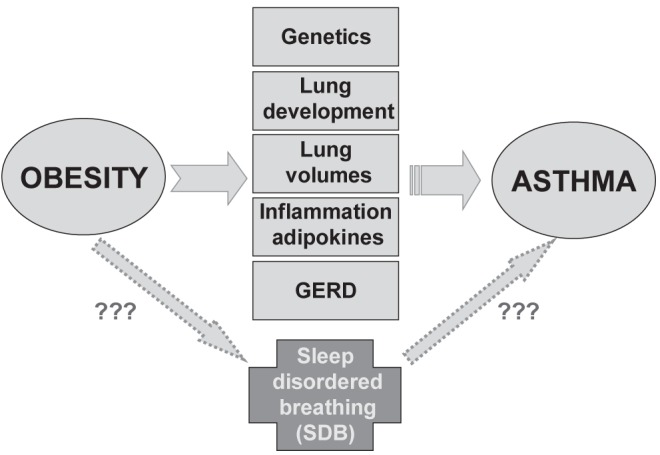
Asthma and obesity are two complex traits that afflict patients of all ages, cause important morbidity and mortality, and consequently have critical public health impacts (1). These conditions are increasing in terms of prevalence, and their etiology is under the influence of both genetic and environmental factors. A literature review (1) of the genetic component of these traits reports that many genes are involved, the effects of which may vary in different populations based on genetic background and environmental exposures. In addition, it is important to take into account gene-gene and gene-environment interactions (epigenetics) when evaluating the severity of the clinical manifestations of the diseases. Asthma and obesity pathophysiology involves several cell types, and their respective molecules are involved in different metabolic pathways that affect the disease status. Both asthma and obesity have an important inflammatory component implying a large number of cytokines and chemokines. It will be important to document the common and the specific inflammatory markers to gain a better knowledge of their molecular basis and also to improve our comprehension of these two diseases. Although some of the underlying mechanisms have been elucidated in recent years, much work is needed to gain a clear understanding of the genetic factors that contribute to the development of different forms of asthma and obesity, which may help to clarify the biological link between these two traits.
One can then hypothesize that the development of these traits is under the influence of common inflammatory genes. Based on the literature, an inventory of potential candidate genes for asthma and obesity has been made. For asthma, 12 complete genome scans have been published, identifying a total of 20 chromosomal regions linked to asthma (2). Recent reviews (3,4) report more than 100 genes associated with asthma. Among these, an elite group of 10 genes were replicated in more than 10 independent studies. For obesity, a total of 52 genomic regions harbour quantitative trait loci supported by two or more studies (obesity gene map). Numerous studies report associations between genetic variants and obesity phenotypes with 426 associations within 127 candidate genes (5). A promising observation is that 22 genes are each supported by at least five positive studies.
Expression studies using microarrays identified 79 genes differentially expressed in bronchial biopsies of four asthmatic subjects compared with four control subjects. These included 21 genes previously implicated in asthma (NOS2A and GPX3), as well as new candidates (ALOX15, CTSC, SERPINB2 and CX3CR1) (6). Similar experiments have been performed on human adipose tissue to compare the gene expression profiles of subcutaneous and visceral adipose tissues of 10 nondiabetic, normolipidemic obese men (7). A total of 409 transcripts were differentially expressed, including genes involved in lipolytic stimuli and cytokine secretion, genes of the Wnt signalling pathway, as well as the CEPBA and HOX genes. Another study compared the gene expression profiles of obese individuals with and without metabolic complications (8). A total of 489 genes were differentially expressed between the two groups. Of those, 80 were located within a previously identified region of linkage.
The combination results from linkage and expression studies to identify candidate genes for both asthma and obesity in Quebec studies (data unpublished) indicate that the genes for the human leukocyte antigen cluster, tumour necrosis factor-alpha, peroxisome proliferator-activated receptors, signal transducer and activator of transcription 6, and interleukins, as well as beta-2-adrenergic receptor and high affinity immunoglobulin E receptor (membrane-spanning four domains, subfamily A, member 2), appear to be involved in both conditions, and thus, should be further explored. In perspective, it would be relevant to test for association considering body mass index and waist circumference in the asthma sample. Another interesting avenue could be microarray studies to compare expression profiles of lung tissue of obese asthmatic subjects.
REFERENCES
-
1.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174:112–9. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
2.Blumenthal MN. The role of genetics in the development of asthma and atopy. Curr Opin Allergy Clin Immunol. 2005;5:141–5. doi: 10.1097/01.all.0000162306.12728.c2. [DOI] [PubMed] [Google Scholar]
-
3.Hoffjan S, Nicolae D, Ober C. Association studies for asthma and atopic diseases: A comprehensive review of the literature. Respir Res. 2003;4:14. doi: 10.1186/1465-9921-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
4.Ober C, Hoffjan S. Asthma genetics 2006: The long and winding road to gene discovery. Genes Immun. 2006;7:95–100. doi: 10.1038/sj.gene.6364284. [DOI] [PubMed] [Google Scholar]
-
5.Rankinen T, Zuberi A, Chagnon YC, et al. The human obesity gene map: The 2005 update. Obesity (Silver Spring) 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
-
6.Laprise C, Sladek R, Ponton A, Bernier MC, Hudson TJ, Laviolette M. Functional classes of bronchial mucosa genes that are differentially expressed in asthma. BMC Genomics. 2004;5:21. doi: 10.1186/1471-2164-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
7.Vohl MC, Sladek R, Robitaille J, et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12:1217–22. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
-
8.Bouchard L, Tchernof A, Deshaies Y, et al. ZFP36: A promising candidate gene for obesity-related metabolic complications identified by converging genomics. Obes Surg. 2007;17:372–82. doi: 10.1007/s11695-007-9067-5. [DOI] [PubMed] [Google Scholar]