Abstract
Ectopic expression of LIM-only protein 2 (LMO2) in T-cells, as a result of chromosomal translocations or retroviral insertion, plays an important role in the onset of T-cell leukemias. Two transcripts of LMO2 gene (LMO2-a and LMO2-b) have been reported to encode a same 158-amino-acid protein. We have previously reported a novel transcript of human LMO2 gene (LMO2-c) encoding a 151-amino-acid protein, and defined its promoter region. In the present study, we investigated the regulation of the LMO2-c expression and the functions of LMO2-c. We found that LMO2-c expression is regulated by the cooperation of two essential hematopoietic transcription factors GATA-1 and PU.1 in various hematopoietic cell lines, suggesting an important functional role for LMO2-c in the hematopoietic system. More importantly, we demonstrated that LMO2-c acts as an antagonist of LMO2-a/b binding to its partners, therefore blocking the transactivation of LMO2-a/b on its target genes. These findings provide novel evidence to the functions of LMO2 gene in the hematopoietic system and leukemia.
Keywords: leukemia, LIM-only protein 2 (LMO2), GATA-1, PU.1, antagonist
Introduction
The T-cell oncogene LIM domain only 2 (LMO2, also named RBTN2 or TTG2) was first identified in patients suffering from T-lineage acute lymphoblastic leukemias (T-ALL) who carried chromosomal translocations t(11;14)(p13;q11) or t(7;11)(q35;p13) and is aberrantly expressed in several other leukemias.1–4 Normally, LMO2 is expressed in blood and endothelial progenitors, which is highly conserved across vertebrate species. Mice with targeted disruption of LMO2 are severely anemic and are embryonic lethal at E9.5–10.5 due to a complete failure of embryonic erythropoiesis.5 Furthermore, studies with embryonic stem cells and chimeric mice indicate that LMO2 is crucial for the development of all hematopoietic lineages with the exception of T-cells.6–8
LMO2 encodes a cysteine-rich protein with two tandem LIM-motifs, cysteine- and histidine-rich protein motifs that are structurally similar to zinc finger DNA-binding domains. However, no evidence of direct DNA binding by LMO proteins has been found. Rather, the function of LMO2 appears to be a mediator of protein-protein interactions in the nucleus.9 LMO2 has been shown to physically associate with a number of nuclear factors, such as GATA-1, TAL1/SCL, LDB and E2A, to form a pentameric complex in erythroid cells.10 Such regulatory complex has been found on the promoter regions of several essential hematopoietic specific genes, such as retinaldehyde dehydrogenase 2,11 erythroid Krüppel-like factor gene,12 c-kit gene,13 protein 4.2 (P4.2) gene,14 α-globin gene15 and glycophorin A (GPA) gene.16
LMO2 gene encodes three alternative transcripts (LMO2-a/b/c), LMO2-a initiated from a distal promoter (P1), LMO2-b from a proximal promoter (P2) and LMO2-c from a novel promoter. LMO2-a and LMO2-b transcripts differ in the length of their 5′-untranslated regions (UTR), but encode the same 158-amino acid protein (GenBank accession number: NM_005574.2).2 We have previously reported a novel transcript of LMO2, LMO2-c, (GenBank accession number: AF257211) which encodes a 151 amino acid protein and is controlled by a novel promoter (see Supplementary Information).17 It has been reported that the P1 promoter of LMO2 is regulated by the erythroid specific transcription factor GATA-1,18 whereas the regulation of the P2 promoter of LMO2 is dependent on three conserved Ets sites that are bound by E74-like factor 1, Friend leukemia integration 1, and erythroblastosis virus oncogene homolog E twenty-six-1 in vivo.19 However, the transcriptional regulation mechanism and the function of LMO2-c are not clear.
The development of the hematopoietic system involves a progressive restriction of differentiation potential and the establishment of lineage-specific gene expression profiles. The establishment of these expression profiles relies on lineage specific transcription factors, such as LMO2, GATA-1 and PU.1, to modulate the expression of their target genes. GATA1 (also named NF-E1, NF-1, Ery-1 and GF-1) is the founding member of the GATA family of zinc-finger factors implicated in the development and differentiation of erythroid, megakaryocytic, eosinophilic and mast cell lineages. The oncogene PU.1 (also named Spi-1) is a member of the Ets family and is essential in the development of monocytic, granulocytic and lymphoid lineages. GATA-1 and PU.1 are known to inhibit the transactivation activities of each other.20–23 In particular, GATA-1 inhibits PU.1 by preventing it from interacting with its essential coactivator c-Jun,21 whereas PU.1 represses GATA-1 function by directly disrupting its ability to bind to DNA22 or recruiting a repression complex, which leads to the methylation of lysine 9 in histone H3 in the vicinity of the GATA-1 binding site.23
We report here the mechanism of the transcriptional regulation and the function of the LMO2-c transcript. We characterize one GATA-1 binding site and identify several PU.1 binding sites at the LMO2-c promoter. We show that both GATA-1 and PU.1 mediate LMO2-c transcription through binding to its promoter region. Furthermore, PU.1 suppresses GATA-1-mediated stimulation of LMO2-c promoter through interacting with GATA-1 on the GATA-1 binding site. Interestingly, we demonstrate that LMO2-c can directly interact with the GATA-1/TAL1/LDB1 complex to antagonize LMO2-a/b binding, which results in repression of LMO2-a/b target genes.
Materials and methods
Cell culture and transfection
COS7 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100U/ml penicillin and 100 μg/ml streptomycin. Different constructs of the LMO2-c promoter were cotransfected with different transcription factors (GATA-1, PU.1, NFκB, C/EBPβ, JunD, TCF4 and β-Catenin) or control expression plasmids into COS7 cells in 6-cm culture dishes using the Lipofectamine 2000 reagents (Invitrogen, Carlsbad, CA, USA). Luciferase assays were performed 24 h after transfection. K562 cells were maintained in RPMI medium 1640 supplemented with 10% FBS. Reporter constructs of LMO2-c promoter, expression plasmids and siRNA constructs of GATA-1 and PU.1, or control expression plasmids were transfected into K562 cells through electroporation. Electroporation was performed on 2×106 cells, with 960 μF and 300V in a Bio-Rad gene-pulser. After a 10 min incubation on ice the cells were seeded in RPMI medium 1640 with stabilized L-glutamine supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. Luciferase assays, real-time RT-PCR, coimmunoprecipitation (co-IP), Western blot and ChIP or qChIP assays were performed 24 h after electroporation. The total amount of DNA transfected was constant in the experiments.
PCR based site-directed mutagenesis
LMO2-c promoter construct, −394/+332 with mutation at the GATA-1-binding site was constructed using Stratagene Quik-Change Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) and cloned into pGL4 vector (Promega, Madison, WI, USA). The mutation site was confirmed by sequencing the entire promoter fragment.
Luciferase assay
COS7 cells were cotransfected with wild-type (WT) or deletion constructs of the LMO2-c promoter −1904/+332-Luc, −735/+332-Luc −294/+332-Luc, and NFκB, C/EBPβ, JunD, TCF4, β-catenin, GATA-1, PU.1. or control expression plasmids. In the CheckMate Mammalian Two-Hybrid System (Promega), pG5Luc vector, pBind-LMO2-a/b, pBind-LMO2-c, pACT-GATA- 1, pACT-TAL1, pACT-LDB1 or control expression plasmids were transfected into COS7 cells. K562 cells were transfected with −1904/+332-Luc, −735/+332-Luc, −294/+332-Luc (both WT and GATA-1 binding site mutant) and GATA-1, PU.1, GATA-1- or PU.1-targetted siRNAs, or control expression plasmids. Cell lysates were extracted 24 h after transfection or electroporation and luciferase activity was measured using a Promega’s dual luciferase reporter assay kit (Promega). The efficiency of transfection was monitored by co transfection with phRL-null vector (0.5 μg/well). The luciferase assay results were reported as relative light units of firefly luciferase activity normalized with respect to the Renilla luciferase activity.
RNA isolation, RT-PCR and real-time RT-PCR
Total RNA of cells was isolated using TRIzol reagent (Life Technologies Inc., Grand Island, NY, USA) according to the manufacturer’s instructions. After reverse transcription reaction, synthesized cDNAs were amplified by PCR and real-time PCR. Real-time PCR was performed by an ABI PRISM 7000 (ABI, USA) sequence detection system according to the manufacturer’s instructions. The reaction mixture contained 1×EvaGreen dye, 0.5pM of each primer, 2.5mM MgCl2 and 0.5 μl cDNA from 20 μl of reverse transcription reaction. The conditions of real-time PCR were as follows: 94°C for 4 min followed by 40 cycles at 94°C for 30 s, 59°C for LMO2-c/LMO2-a/b or 61°C for GATA-1/PU.1 or 60°C for GPA/GAPDH for 1.5 min. There is no non-specific amplification determined by dissolve curve. There are four independent repeat tubes per cDNA specimen, three cDNA specimens independently for each data point. The real-time RT-PCR result was reported as the percentage of relative light units for GATA-1, PU.1 or LMO2-c after normalization to GAPDH expression in cells. The primers used for PCR and real-time PCR are listed in the Supplementary Information.
Chromatin Immunoprecipitation (ChIP) and Quantitative ChIP
ChIP assays were performed using a kit from Upstate Corp. (Lake Placid, NY, USA). The following antibodies (10 μg) were used: anti-GATA-1 antibody (sc265; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-PU.1 antibody (sc-352; Santa Cruz Biotechnology), anti-Myc antibody (clone 9E10; Sigma, St Louis, MO, USA), and anti-FLAG M2 antibody (Sigma) with 100 μg of chromatin per ChIP. The amounts of each specific DNA fragment in immunoprecipitates were determined by PCR or real-time PCR reactions. The primers used for this analysis were described in the Supplementary Information. The conditions of PCR for the detection of each specific DNA fragment were as follows: 94°C for 4 min followed by 28 cycles at 94°C for 30 s 62°C for 30 s, and 72°C for 60 s. For the quantitative ChIP (qChIP), a standard curve was generated using primer set A for LMO2-c promoter or primer set D for GPA promoter. Copy numbers for the DNA fragments −123/+142 of LMO2-c gene in each anti-GATA-1- or anti-PU.1-immunoprecipitated sample and −138/+57 of GPA gene in anti-Myc-IP samples were determined and compared to copy numbers of the DNA fragment without IP (input DNA). Anti-FLAG antibody was used as controls for IP reactions. The percentage of the input was then calculated. The final value was the percentage of the DNA input obtained from specific antibody-IP samples subtracting the percentage of DNA input obtained from control antibody-IP samples. The dissociation curve was determined for each real-time PCR reaction to assure that a single band was produced. Each data point represents four independent samples.
Co-Immunoprecipitation and Western blot analysis
COS7 cells were transiently transfected with expression plasmids using Lipofectamine 2000 reagents (Invitrogen) and incubated for 24 h before analysis. After transfection, cells were washed twice with phosphate-buffered saline, lysed for 30 min in lysis buffer (50mM Tris-HCl, pH 7.5, 150mM NaCl, 1% Nonidet P-40) containing protease inhibitors (10 μg/ml leupeptin, 10 μg/ml pepstatin A and 10 μg/ml aprotinin) and phosphatase inhibitors (1mM NaF and 1mM Na3VO4) and clarified by centrifugation at 4°C for 30 min. Supernatants were precleared with protein G plus agarose (Santa Cruz Biotechnology) for 2 h at 4°C. Then 5 μg of antibodies specific for each target protein were added in each sample. Immune complexes were precipitated by protein G plus agarose beads overnight at 4°C, washed five times with the lysis buffer. The immune complexes were boiled for 10 min in SDS sample buffer (100mM Tris-HCl, pH 8.8, 0.01% bromphenol blue, 20% glycerol, 4% SDS) containing 10mM dithiothreitol and analyzed by 10% SDS-PAGE. Western blot analysis was performed after immunoprecipitation (IP) or using the cell lysates. After the fractionation by SDS-PAGE, proteins were transferred to a nitrocellulose membrane, and detected using the following antibodies: anti-V5 (Invitrogen), anti-Myc, anti-Flag, anti-GATA-1, anti-PU.1, anti-GPA (Sigma) and anti-β-Actin (Sigma) antibodies. Immunostaining was detected using an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Buckinghamshire, UK).
siRNA constructs
DNA templates for siRNAs targeting human GATA-1 or PU.1. mRNA and scrambled siRNAs were synthesized and cloned into pSilencer 4.1-CMV neo vector (Ambion, CA, USA). The target sequences of the siRNAs are 5′-TCAGGGGTTTTCTTCCCCT-3′ for human GATA-1,24 5′-GAAGCTCACCTACCAGTTC-3′ for human PU.1.25
Results
LMO2-c expression is dependent on the levels of GATA-1 and PU. 1
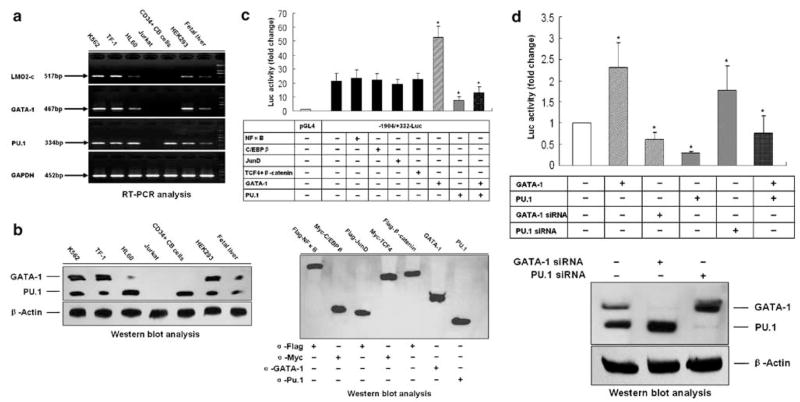
LMO2-c mRNA expression was analyzed in human erythroleukemia cell line K562 and TF-1, promyelocytic cell line HL60, Human T cell lymphoblast-like cell line Jurkat, CD34+ umbilical cord blood (CB) cells, fetal liver cells, as well as the HEK293 cells, where the LMO2-c transcript was first identified. LMO2-c was detected in K562, TF-1, HL60, HEK293 and fetal liver cells but not in Jurkat or CD34+ CB cells (Figure 1a). We then analyzed the mRNA and protein levels of two erythroid specific transcription factors GATA-1 and PU.1 and found that GATA-1 expression was also absent in Jurkat and CD34+ CB cells. In contrast, PU.1 was detected in CD34+ CB cells but not in Jurkat cells (Figure 1a and b). These results suggest that LMO2-c expression may be regulated by GATA-1 and PU.1.
Figure 1.
GATA-1 and PU.1 regulate LMO2-c expression. (a) Total RNA of K562, TF-1, HL60, Jurkat cells, CD34+ CB cells, HEK293 and fetal liver cells were isolated and reverse transcribed. To detect mRNA expression of each gene, PCR assays were performed using the primers listed in the Supplementary Information. (b) Protein expression of GATA-1 and PU.1 in these cells was detected by Western blot using anti-GATA-1 and anti-PU.1 antibodies. (c) COS7 cells were cotransfected with the LMO2-c promoter construct (−1904/+332) with NFκB, C/EBPβ, JunD, TCF4, β-catenin, GATA-1, PU.1 or control expression plasmids. Cell lysates were collected 24 h after transfection and luciferase assay was performed (upper panel). The protein expression of these transcription factors was detected by Western blot using the anti-Flag, anti-Myc anti-GATA-1 and anti-PU.1 antibodies (lower panel). *P<0.05, unpaired Student’s t-test, compared with vector alone. (d) K562 cells were transfected with the GATA-1, GATA-1, siRNA, PU.1, PU.1 siRNA or control expression plasmids. Twenty-four hours after transfection, LMO2-c mRNA levels were detected by real-time RT-PCR (upper panel). Silencing of GATA-1 and PU.1 by siRNAs was examined in K562 cells by Western blot using the anti-GATA-1 and anti-PU.1 antibodies (lower panel). *P<0.05, unpaired Student’s t-test, compared with vector alone.
To determine the regulatory mechanism of LMO2-c transcription, we amplified the human LMO2-c promoter −1904/+332 by PCR using human genomic DNA as a template and cloned this DNA fragment into pGL4 vector in front of the luciferase gene. The activity of the reporter −1904/+332-Luc in COS7 cells was 20-folds higher than that of the control vector. Putative binding sites for several transcription factors essential for hematopoiesis (NFκB, C/EBPβ, JunD, β-catenin/TCF4, GATA-1 and PU.1) were identified in the promoter region −1904/+332 using the Transcription Element Search System (URL: http://www.cbil.upenn.edu/tess). Expression plasmids encoding transcription factors (Flag-NFκB, Myc-C/EBPβ, Flag-JunD, Myc-TCF4, Flag-β-catenin, GATA-1 and PU.1.) were constructed and were shown to be expressed at similar levels in COS7 cells through Western blot analysis (Figure 1c). We then tested the response of LMO2-c promoter to each transcription factor by cotransfecting expression plasmids of different transcription factors with human LMO2-c promoter construct −1904/+332-Luc in heterologous nonerythroid COS7 cells. Through luciferase assay, we found that GATA-1 activated the LMO2-c promoter activity (~2.5 fold increase), whereas a significant reduction (~70%) in the promoter activity was observed when PU.1 was overexpressed (Figure 1c). These findings indicate that both GATA-1 and PU.1 can regulate LMO2-c gene transcription. Both factors combined showed a reduced activity when compared to GATA-1 alone (Figure 1c), suggesting that PU.1 inhibits the positive effect of GATA-1 on the LMO2-c transcription.
To determine if LMO2-c expression depends on GATA-1 and PU.1 levels, we monitored LMO2-c mRNA expression by real-time RT-PCR in erythroid/myeloid K562 cells, which express LMO2-c, GATA-1 and PU.1. Alteration of LMO2-c expression occurred at the transcriptional level, since LMO2-c mRNA levels were twofold higher following ectopic GATA-1 expression and were reduced by 60% following PU.1 overexpression (Figure 1d). Conversely, transfection of siRNA constructs targeting GATA-1 or PU.1 reversed the effect of these two factors on LMO2-c expression respectively (Figure 1d). Silencing of gene expressions using the siRNA constructs was verified through Western blot analysis and protein levels were reduced to less than 10% (Figure 1d). Furthermore, the increase of LMO2-c mRNA expression induced by GATA-1 was completely knocked down when PU.1 was cotransfected (Figure 1d). These results indicate that the LMO2-c expression is controlled by GATA-1 and PU.1.
GATA-1 and PU.1 regulate the LMO2-c promoter through GATA-1-and PU.1-binding sites
As described above, GATA-1 and PU.1 mediate the promoter activity of LMO2-c (−1904/+332-Luc) in COS7 cells (Figure 1c) and mRNA expression in K562 cells (Figure 1d). These findings demonstrate that LMO2-c is a direct target of both GATA-1 and PU.1. We next sought to identify the cis elements that are required to recruit GATA-1 and PU.1 to the LMO2-c promoter. We generated two deletion constructs of the LMO2-c promoter (−735/+332-Luc, −294/+332-Luc). As shown in Figure 2a, all three promoter constructs showed similar activity in K562 cells. GATA-1 stimulated the promoter activity of the three reporters with similar potency (2.5-fold), whereas PU.1 repressed the promoter activity of these constructs with similar potency (50%). Transactivation of GATA-1 was completely blocked by PU.1 throughout the three reporters (Figure 2a). Meanwhile, we also found that GATA-1 and PU.1 regulated the promoter activity of −294/+332-Luc in a dose-dependent manner, whereas transfection with GATA-1 or PU.1 siRNA blocked the activities of the two factors on −294/+332- Luc reporter, respectively (Figure 2b). These results demonstrate that the minimal responsive region exists within the −294/+332 DNA fragment of the LMO2-c promoter, which contains the cis-regulatory elements necessary to recapitulate endogenous LMO2-c expression in hematopoietic cells. In this region of the LMO2-c promoter, we identified one putative GATA-1-binding site located at −17/−12, and five putative PU.1-binding sites located at −214/−209, −41/−36, +126/+134, +211/+216, +324/+329 regions by sequence analysis (Figure 2c).
Figure 2.
GATA-1 and PU.1 regulate LMO2-c promoter through their binding elements. (a) K562 cells were cotransfected with the LMO2-c promoter (−1904/+332-Luc) or its two deletion constructs (−735/+332-Luc and −294/+332-Luc) with GATA-1 or PU.1 expression plasmid. Cell lysates were collected 24 h after transfection and luciferase assay was performed. *P<0.05, unpaired student’s t-test, compared with vector alone. (b) LMO2-c promoter −294/+332-Luc was cotransfected with different amounts of GATA-1 (0.2, 0.6 and 1.8 μg/dish) or PU.1 (0.2, 0.6 and 1.8 μg/dish) expression plasmid and siRNA constructs targeting these factors into K562 cells. Cell lysates were collected 24 h after transfection and luciferase assay was performed. unpaired Student’s t-test, *P<0.05, compared with vector alone; **P<0.05, one-way analysis of variance (ANOVA) followed by Dunnett’s test, compared with vector alone. (c) The diagram shows the structure of the human LMO2-c promoter −294/+332-Luc construct. The putative GATA-1-binding site (−17/−12), PU.1 binding sites (−214/−209, −41/−36, +126/+134, +211/+216, +324/+329), transcription and translation start sites are indicated.
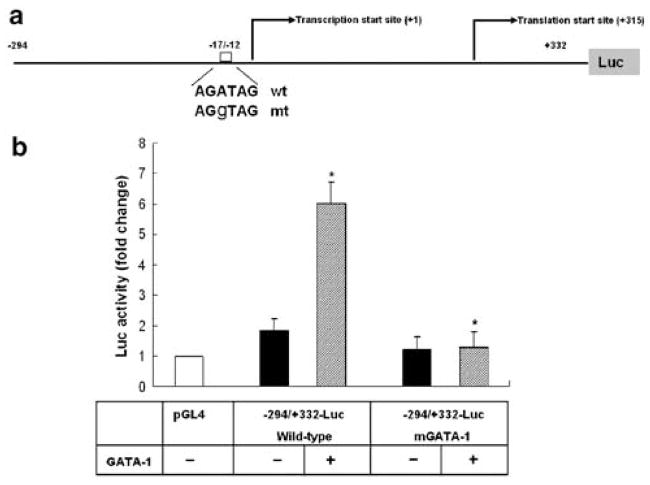
To determine whether the GATA-1-binding site is functional, we performed site-direct mutagenesis (Figure 3a). Mutation of the GATA-1-binding site caused a significant reduction in the basal promoter activity and completely abolished GATA-1- induced promoter activity of the −294/+332-Luc reporter in K562 cells (Figure 3b), demonstrating that the binding element located at −17/−12 is an important functional binding site for GATA-1 at the LMO2-c promoter.
Figure 3.
GATA-1 activates the LMO2-c promoter through GATA-1 binding site. (a) The diagram shows the construct harboring the mutant (mt) GATA-1 binding site derived from −294/+332-Luc reporter. The wt and mt sequences of GATA-1 binding site are indicated. (b) K562 cells were transfected with GATA-1 expression plasmid with wt and mt −294/+332-Luc reporter harboring GATA-1 binding site mutation. Cell lysates were collected 24 h after transfection and luciferase assay was performed. *P<0.05, unpaired Student’s t-test, compared with vector alone.
To investigate the repressive effect of PU.1 on the LMO2-c promoter, we generated four deletion reporters covering different parts of −294/+332 region (Figure 4a) and cloned them in pGL4 vector upstream of the SV40 basal promoter. Transfection of GATA-1 upregulated the promoter activity of both −294/-6-SV40-Luc and −26/+332-SV40-Luc reporters with similar potency, but not −294/-21-SV40-Luc, and −10/+332-SV40-Luc which excluded the GATA-1 binding site (Figure 4b). However, stimulatory effect of GATA-1 on −294/-6-SV40-Luc and −26/+332-SV40-Luc was repressed by cotransfection of PU.1 expression plasmid, but much weaker than that of the full length −294/+332-Luc construct (Figure 4b and c). PU.1 did not repress the activity of all four deletion constructs. Instead, it upregulated the activity of the constructs relatively modestly (Figure 4b), which was consistent with its transactivator character. These results indicate that the repressive effect of PU.1 on GATA-1-induced LMO2-c promoter relies on the PU.1-binding sites both upstream and downstream of the GATA-1-binding site. PU.1 has been demonstrated to interact physically with GATA-1, thus block the binding of GATA-1 to DNA,22 or induce the methylation of histone H3 in the vicinity of the GATA-1-binding site.23 Our findings indicate that PU.1 is sufficient to confer transcriptional repression activity to LMO2-c through both directly binding to DNA thus reducing the promoter activity of LMO2-c in COS7 cells which do not express GATA-1 (Figure 1c), and physically interacting with GATA-1 thereby suppressing the activation of GATA-1 on both −294/-6-SV40-Luc and −26/+332-SV40-Luc reporters in COS7 cells, where no repressive effect induced by PU.1 was found when GATA-1 expression plasmid was not cotransfected (Figure 4b).
Figure 4.
PU.1 represses the LMO2-c promoter through direct binding to DNA and interaction with GATA-1. (a) The diagram shows the four deletion constructs (−294/−21, −294/−6, −26/+332, −10/+332) covering different parts of −294/+332 promoter region. (b) COS7 cells were transfected with the four fragments of LMO2-c promoter which were cloned in front of a SV40 basal promoter in pGL4 vector with GATA-1 or PU.1 expression plasmids. Cell lysates were collected 24 h after transfection and luciferase assay was performed. *P<0.05, unpaired Student’s t-test, compared with vector alone.
To confirm further these results, we performed ChIP assay in K562 cells. IP was performed using the anti-GATA-1 or anti-PU.1 antibody. Anti-FLAG antibody was used as a control in IP experiment. A segment of the LMO2-c promoter (−123/+142) containing the GATA-1-binding site and two PU.1-binding sites was amplified by PCR using primer set A (Supplementary Information) and DNA templates extracted from protein/DNA crosslinked samples. Primer sets recognizing 5′ and 3′-distal regions (Supplementary Information, primer sets B and C) were used as negative controls in the PCR reactions. The ChIP assay showed that both GATA-1 and PU.1 bound specifically to −123/+142 region of the LMO2-c promoter (Figure 5a). Such binding was enhanced with the ectopic expression of both factors and reduced when endogenous GATA-1 and PU.1 were silenced by their respective siRNA, which was demonstrated by qChIP assay (Figure 5b).
Figure 5.
GATA-1 and PU.1 bind to the LMO2-c promoter. (a) For ChIP assay, IP was performed using the anti-PU.1 (lanes 1–3), anti- GATA-1 (lanes 4–6) or control anti-FLAG (lane 7) antibody. The PCR was performed using primer set A (lanes 1, 4, 7 and 8) to detect GATA-1 or PU.1 binding and the PCR products (265-bp) of the proximal region (−123/+142, lanes 1 and 4) of LMO2-c promoter were amplified using crosslinked IP products as templates. Primer set B (−735/−441, lanes 2 and 5) and primer set C (+531/+881, lanes 3 and 6) were used as negative controls for PCR. PCRs with primer sets A, B and C using human genomic DNA as the template were performed as the positive control of PCR reaction (lanes 8–10). (b) For qChIP assay, K562 cells were transfected with GATA-1, PU.1 or vector control expression plasmid or their siRNA expression plasmids. Cell lysates were collected 24 h later. IP was performed using the anti-GATA-1 or anti-PU.1 antibody and anti-FLAG antibody was used as a negative control. Copy numbers of the DNA fragment containing LMO2-c promoter region (−123/+142) in anti-GATA-1- or anti-PU.1-IP samples before (Input DNA) and after IP were quantified by real-time PCR using the primer set A. The bars indicate final values of the percentage of the DNA input obtained from specific antibody-IP samples subtracting the percentage of DNA input obtained from control antibody-IP samples. unpaired student’s t-test, *P<0.05, n=4, compared with vector group.
Taken together, these findings showed that −17/−12 is a functional binding site for GATA-1 in the LMO2-c promoter, and that PU.1 binds specifically to the −123/+142 region of the LMO2-c promoter. The bindings of GATA-1 and PU.1 were closely correlated with the levels of both factors in cells.
GATA-1 and PU.1 cooperate to regulate LMO2-c expression in different hematopoietic lineages
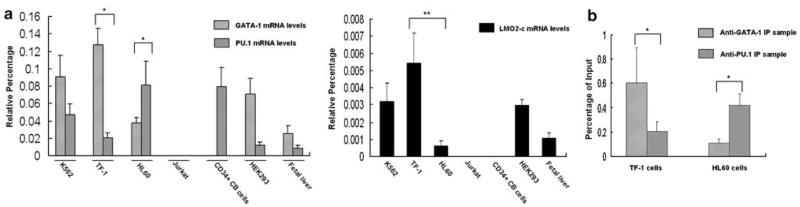
To determine if GATA-1 and PU.1 mediate LMO2-c expression in both multipotential progenitor cells, and erythroleukemia cells, we performed real-time RT-PCR and qChIP assays. We found that LMO2-c is highly expressed in human erythroid cells, but not human myeloid cells (Figure 6a). As shown in Figure 6a, LMO2-c and GATA-1 but not PU.1 was highly expressed in K562, TF-1, HEK293 and fetal liver cells. In contrast, PU.1 but not GATA-1 is highly expressed in HL60 and CD34+ CB cells. These results demonstrate that the expression levels of LMO2-c are closely correlated with the levels of GATA-1 and PU.1 in K562, TF-1, HL60 and HEK293 cells as well as CD34+ umbilical CB cells and fetal liver cells. Jurkat cells did not express GATA-1, PU.1 or LMO2-c (Figure 1a and b, 6a). We then performed the qChIP assay for in vivo bindings of GATA-1 and PU.1 on LMO2-c promoter in TF-1 and HL60 cells. As expected, both GATA-1 and PU.1 bound to the −123/+142 region of LMO2-c promoter. More importantly, these bindings were closely correlated with the endogenous levels of GATA-1 and PU.1 in cells (Figure 6b). These observations demonstrate that the expression pattern of the two factors (GATA-1 and PU.1) in multipotential progenitor cells and erythroleukemic cells is a key determinant in LMO2-c gene expression.
Figure 6.
LMO2-c expression is regulated by GATA-1 and PU.1 in different cells. (a) real-time RT-PCR assay. LMO2-c, GATA-1 and PU.1 mRNA expressions in different cells were detected by real-time RT-PCR assay. The real-time RT-PCR result was reported as the percentage of relative light units for GATA-1, PU.1 or LMO2-c after normalization to GAPDH expression in cells. unpaired student’s t-test *P<0.05, (GATA-1 vs PU.1); **P<0.05, unpaired Student’s t-test (TF-1 vs HL60). (b) For qChIP assay, IP was performed in TF-1 and HL60 cells using the anti-GATA-1 or anti-PU.1 antibody. Copy numbers of the DNA fragment containing LMO2-c promoter region (−123/+142) in anti-GATA-1- or anti-PU.1-IP samples before (Input DNA) and after IP were quantified by real-time PCR using the primer set A. The bars indicate final values of the percentage of the DNA input obtained from specific antibody-IP samples subtracting the percentage of DNA input obtained from control antibody-IP samples. *P<0.05, unpaired student’s t-test (GATA-1 vs PU.1).
LMO2-c interacts with GATA-1, TAL1 and LDB1
LMO2-a/b and LMO2-c proteins share the same functional domains (LIM1 and LIM2). Expression of both genes is regulated by the same transcription factor GATA-1. LMO2-a/b directly interacts with GATA-1, LDB1 and TAL1 and forms a pentameric complex on the promoter of its target genes. In order to evaluate the transcriptional activity and functions of LMO2-c, we employed the CheckMate Mammalian Two-Hybrid System. LMO2-a/b and LMO2-c were cloned respectively into the pBIND vector encoding a GAL4 DNA-binding domain, and cofactors of interest (GATA-1, LDB1 and TAL1) were cloned into the pACT vector encoding the VP16 transactivating domain. COS7 cells were cotransfected with different expression plasmids along with the pG5Luc vector containing 5×Gal4-binding elements in front of the luciferase gene. Interactions between different factors were evaluated by luciferase assay. As shown in Figure 7a, we found that (i) LMO2-c had a much lower (~85% decrease) transcriptional activity when compared to that of LMO2-a/b; (ii) GATA-1 and TAL1 showed similar binding affinities to both LMO2-a/b and LMO2-c; and (iii) the interaction between LDB1 with LMO2-c was much weaker than that with LMO2-a/b (10-fold for LMO2-a/b; 3-fold for LMO2-c). These results demonstrate that LMO2-c has a much weaker transcriptional activity than LMO2-a/b and that LMO2-c physically interacts with GATA-1, TAL and LDB1, although with much weaker affinity to LDB1.
Figure 7.
LMO2-c interacts with GATA-1, TAL-1 and LDB1 with different affinities from that of LMO2-a/b. (a) COS7 cells were cotransfected with pG5Luc vector and pBind, pBind-LMO2-c, pBind-LMO2-a/b, with pACT, pACT-GATA-1, pACT-TAL1, pACT-LDB1 expression plasmids. Cell lysates were collected and luciferase assay was performed 24 h after transfection. unpaired student’s t-test, *P<0.05, compared with vector alone. (b–d) LMO2-c interacts with GATA-1 (b), TAL1 (c) and LDB1 (d). GATA-1, Flag-TAL1, or Flag-LDB1 expression plasmid and V5-LMO2-a/b or Myc-LMO2-c expression plasmid were cotransfected into COS7 cells. GATA-1, Flag-TAL1, or Flag-LDB1 proteins were detected by Western blot after V5-LMO2-a/b or Myc-LMO2-c proteins were immunoprecipitated by the anti-V5 or anti-Myc antibody (upper panels). V5-LMO2-a/b or Myc-LMO2-c proteins were detected by Western blot after GATA-1, Flag-TAL1 or Flag-LDB1 proteins were immunoprecipitated by the anti-GATA-1 (b) or anti-Flag (c, d) antibody (middle panels). Protein levels of GATA-1 (b), Flag-TAL1 (c), Flag-LDB1 (d), V5-LMO2-a/b and Myc-LMO2-c in total cell lysates were detected by Western blot and shown in lower panels. (e) LMO2-c competes with LMO2-a/b binding to GATA-1. GATA-1 (0.4 μg/dish), V5-LMO2-a/b (0.4 μg/dish) and different amounts of Myc-LMO2-c (0.2, 0.4, 0.8 and 2.0 μg/dish) expression plasmids were cotransfected into COS7 cells. V5-LMO2-a/b or Myc-LMO2-c proteins were detected by Western blot using the anti-V5 (upper panel, lanes 1, 3, 5, 7, 9) or anti-Myc (upper panel, lanes 2, 4, 6, 8, 10) antibody after GATA-1 proteins were immunoprecipitated by the anti-Flag antibody. GATA-1 proteins were detected by Western blot after V5-LMO2-a/b or Myc-LMO2-c proteins were immunoprecipitated by the anti-V5 (lower panel, lanes 1, 3, 5, 7, 9) or anti-Myc (lower panel, lanes 2, 4, 6, 8, 10) antibody.
In order to clarify the different interactions between LMO2-a/b and LMO2-c with their partners, co-IP was performed in COS7 cells transfected with GATA-1, Flag-LDB1 or Flag-TAL1 and V5-LMO2-a/b or Myc-LMO2-c. As shown in Figure 7b–d, both LMO2-a/b and LMO2-c bound to GATA-1, TAL1 and LDB1 in COS7 cells. Consistent with the two-hybrid luciferase assay results, no significant difference was observed for the interaction of LMO2-c and LMO2-a/b with their interacting proteins, GATA-1 and TAL1 (Figure 7b and c), but a significant reduction in the binding affinity was observed between LDB1 and LMO2-c compared to LMO2-a/b (Figure 7d).
As both LMO2-c and LMO2-a/b bind to GATA-1 with similar potency, we further studied whether LMO2-c and LMO2-a/b compete for binding. We cotransfected different amounts of Myc-LMO2-c expression plasmid with the same amount of V5-LMO2-a/b expression plasmid into COS7 cells. Through co-IP assay, we found that both LMO2-c and LMO2-a/b bound to GATA-1 with similar affinities (Figure 7e, lanes 5, 6). Co-expression of LMO2-c almost completely blocked the interaction between LMO2-a/b and GATA-1 (Figure 7e, lanes 9, 10). Similar results were also obtained from the co-IP of LMO2-c and TAL1 (data not shown). These observations suggest that LMO2-c interacts with GATA-1 and TAL1 with similar affinities to that of LMO2-a/b, suggesting that it might act as an antagonist and compete LMO2-a/b binding to GATA-1 and TAL1.
Taken together, these results suggest that LMO2-c competes the binding of LMO2-a/b to GATA-1 and TAL1, but has much weaker binding affinity to LDB1 than that of LMO2-a/b. It has been reported that LIM2 domain mutated LMO2-a/b retains its ability to inhibit MEL cell differentiation. However, such function is completely absent when its LIM1 domain is mutated or deleted.26 As LDB1 interacts with LMO2-a/b through directly binding to the LIM1 domain and such binding is crucial for the activity of LMO2-a/b,26 we speculated that the amino acid sequence at the N-terminus (close to the LIM1 domain) of LMO2-a/b as well as LMO2-c is important for their transcriptional activity. Our findings demonstrate for the first time that LMO2-c serves as a competitor of LMO2-a/b through direct interaction with its partners, GATA-1 and TAL1, suggesting that LMO2-c is a novel antagonist of LMO2-a/b.
LMO2-c is an antagonist of LMO2-a/b
As described above, LMO2-c is closely regulated by GATA-1 and PU.1, two essential factors playing critical roles in lineage commitment of normal hematopoietic system and leukemia development. LMO2-c competed with LMO2-a/b to bind to GATA-1. These findings indicate that LMO2-c is a functional regulator in hematopoiesis or leukemia. We then sought to investigate the biological functions of LMO2-c. The synergistic transactivation by LMO2-a/b and its partners on their target genes has been reported.16,27 Here we investigated the response of a direct target gene of LMO2-a/b complex, GPA,16 to LMO2-c. The mRNA expressions of LMO2-a/b, LMO2-c and GPA, as well as the protein levels of GPA in K562, TF-1 and HL60 leukemia cells, Jurkat T-lymphoma cells, CD34+ umbilical CB cells, fetal liver cells, and HEK293 cells were examined through RT-PCR and Western blot analysis (Figure 8a). GPA was detected in K562, TF-1, Jurkat cells, CD34+ CB cells, and fetal liver cells. Meanwhile, LMO2-a/b was detected in K562, TF-1, HL60, CD34+ CB cells, and fetal liver cells. These results indicate the positive correlation of LMO2-a/b and GPA in most cell lines. In contrast, LMO2-c was detected in HL60 and HEK293 cells, which do not express GPA, suggesting a negative correlation of LMO2-c on GPA expression. We then transfected LMO2-c expression plasmids into K562 cells and examined the GPA expression. Results of real-time RT-PCR and Western blot showed that LMO2-c downregulated GPA, mRNA and protein expression in a dose-dependent manner in K562 cells (Figure 8b). To assess the correlation between the levels of DNA binding of LMO2-c and GPA expression, we performed qChIP assay. The qChIP was performed using anti-Myc antibody and the PCR primer set D listed in the Supplementary Information.16 LMO2-c overexpression reduced GPA mRNA and protein levels, which closely correlated with the highlighted binding of LMO2-c to GPA promoter in a dose dependent manner (Figure 8b). These observations demonstrate that LMO2-c competes with LMO2-a/b and disturbs the interaction of LMO2-a/b with its partners, thus inhibits the synergistic effect of LMO2-a/b and its cofactors on the target gene, resulting in a significant reduction in the expression levels of LMO2-a/b target gene.
Figure 8.
LMO2-c represses LMO2-a/b target gene expression through direct binding to its promoter. (a) The mRNA and protein expression of LMO2-a/b, LMO2-c and GPA in different cells. Total RNA was extracted from K562, TF-1, HL60, Jurkat, CD34+ CB cells and fetal liver cells and reversely transcribed. Expression of each gene was detected by PCR using the primer listed in the Supplementary Information (upper panel). GPA protein was detected through Western blot using the anti-GPA antibody (lower panel). (b) GPA protein and mRNA expression, as well as qChIP assay for LMO2-c binding to GPA promoter. Cells (K562) were transfected with different amounts of Myc-LMO2-c (0.2, 0.4, 0.8, 2.0 μg/dish) or control expression plasmid. Twenty-four hours after transfection, GPA protein expression was detected by Western blot using the anti-GPA antibody (upper panel). GPA mRNA expression was detected by real-time RT-PCR (middle panel). For the qChIP assay, IP was performed using the anti-Myc antibody and anti-FLAG antibody was used as a negative control. Copy numbers of the DNA fragment containing human GPA promoter region (−138/+57) in anti-Myc-IP samples before (Input DNA) and after IP were quantified by real-time PCR using the primer set D. The bars indicate final values of the percentage of the DNA input obtained from anti-Myc-IP samples subtracting the percentage of DNA input obtained from control antibody-IP samples (lower panel). one-way ANOVA followed by Dunnett’s test, *P<0.05, n=4, compared with vector alone.
Discussion
In the present study, we investigated the regulation mechanism of LMO2-c transcription and identified it as a functional antagonist for LMO2-a/b. We identified and characterized one GATA-1 and five PU.1-binding sites at the LMO2-c promoter, and demonstrated the cooperation between GATA-1 and PU.1 on regulating LMO2-c expression in different hematopoietic cell lines. In addition, we also discovered that LMO2-c directly interacts with GATA-1, TAL1 and LDB1 with different affinities from that of LMO2-a/b and inhibits expression of GPA, a LMO2-a/b target gene. LMO2-c and LMO2-a/b appear to compete with each other through direct binding with their partners. Altogether, these results provide a novel evidence for the regulation of LMO2-c expression during hematopoietic system development, and the regulation of LMO2-a/b activity by its antagonist LMO2-c.
LMO2-c in normal hematopoietic system
The function of LMO2-a/b in normal hematopoiesis has been determined by the genetic knockout approach.7,9 Together with similar findings in Xenopus and zebrafish embryos,27,28 a pivotal role for the LMO2-a/b protein in lineage specification during mammalian development, especially for early stages of hematopoiesis, has been demonstrated. In the present studies, we have shown that LMO2-c functions as an endogenous antagonist of LMO2-a/b. Therefore, it is of much interest to determine how LMO2-c functions in the normal hematopoietic system.
GATA-1 and PU.1 are two essential transcription factors in the hematopoietic system, determining the cell lineage commitment by controlling the expression of many critical hematopoietic marker genes, promoting common myelo-erythroid progenitors (CMPs) to become megakaryocytic-erythroid progenitors (MEPs) or myeloid progenitors, respectively.29 Furthermore, the mutually antagonistic relationship between GATA-1 and PU.1 is very important in determining the outcome of the differentiation decision from the short-term repopulating hematopoietic stem cells (STR-HSCs) via the CMPs into the granulocyte-macrophage progenitors (GMPs) or MEPs.30 We found that LMO2-c expression was closely regulated by both GATA-1 and PU.1, emphasizing its important functional role during hematopoiesis. GATA-1, the LMO2-c transcriptional activator, is essential for development of erythorid cells and megakaryocytes, whereas PU.1, the LMO2-c transcriptional repressor, is important for the development of monocytes, granulocytes and lymphoid cells. On the basis of these findings, LMO2-c might play a potential role in promoting erythorid development or inhibiting myeloid development.
LMO2-c in T-ALL
To date, about 25% of T-ALL cases are associated with specific chromosomal translocations,31 which repeatedly occurs at the region encoding hematopoietic transcription factors, including LMO2,1 HOX11,32 TAL1/SCL,33 TAL2,34 LMO135 and LYL1.36 Such translocations lead to unregulated expression of the gene. In addition to chromosomal rearrangements, three cases of a T cell lymphoproliferative disorder resulted from aberrant activation of the LMO2-a/b gene via integration of the retroviral vector used for gene therapy of X-linked severe combined immunodeficiency (X-SCID).37,38 Microarray analysis demonstrate that ectopic expression of LMO2-a/b occurs in ~45% of T-ALL, even in the absence of chromosomal changes.4 Consequently, LMO2-a/b is a critical gene for the leukemogenesis of T-ALL.
In our findings, LMO2-c and LMO2-a/b are expressed in many human leukemia cell lines. The expression levels of LMO2-c are closely correlated with the stoichiometry of GATA-1 and PU.1 in different leukemia cells. Overexpression of PU.1 significantly reduced LMO2-c expression. Considering that leukemogenesis is likely to result, at least in part, from the ectopic expression of PU.1,39 PU.1 mediated downregulation of LMO2-c may be a requirement for LMO2-a/b induced leukemogenesis. Such hypothesis is further supported by the finding that LMO2-c blocks the transactivation activity of LMO2-a/b. Altogether, current findings support the hypothesis that LMO2-c plays an important role in leukemia, in particular in the repression of the leukemogenesis induced by ectopic expression of LMO2-a/b. LMO2-c may be a potential molecular target for the treatment of leukemia. The mutual antagonism of the LMO2-a/b and LMO2-c may be involved in shutting down the tumor-related transcriptional programs. Our findings might open new approaches to blocking LMO2-a/b in the development of T-ALL. The potential role of LMO2-c in tumor inhibition needs to be further explored.
Supplementary Material
Acknowledgments
This work was supported by a grant from The Natural Sciences Foundation of Tianjin City (No. 05YFJMJC01800).
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Royer-Pokora B, Loos U, Ludwig W-D. TTG-2, a new gene encoding a cysteine-rich protein with the LIM motif, is over-expressed in acute T-cell leukaemia with the t(11:14)(p13:q11) Oncogene. 1991;6:1887–1893. [PubMed] [Google Scholar]
- 2.Royer-Pokora B, Rogers M, Zhu TH, Schneider S, Loos U, Bolitz U. The TTG-2/RBTN2 T cell oncogene encodes two alternative transcripts from two promoters: the distal promoter is removed by most 11p13 translocations in acute T cell leukaemia’s (T-ALL) Oncogene. 1995;10:1353–1360. [PubMed] [Google Scholar]
- 3.Nam CH, Rabbitts TH. The role of LMO2 in development and in T cell leukemia after chromosomal translocation or retroviral insertion. Mol Ther. 2006;13:15–25. doi: 10.1016/j.ymthe.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1:75–87. doi: 10.1016/s1535-6108(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 5.Warren AJ, Colledge WH, Carlton MB, Evans MJ, Smith AJ, Rabbitts TH. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell. 1994;78:45–57. doi: 10.1016/0092-8674(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 6.Visvader JE, Mao X, Fujiwara Y, Hahm K, Orkin SH. The LIM-domain binding protein Ldb1 and its partner LMO2 act as negative regulators of erythroid differentiation. Proc Natl Acad Sci USA. 1997;94:13707–13712. doi: 10.1073/pnas.94.25.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada Y, Warren AJ, Dobson C, Forster A, Pannell R, Rabbitts TH. The T cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis. Proc Natl Acad Sci USA. 1998;95:3890–3895. doi: 10.1073/pnas.95.7.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormack MP, Forster A, Drynan L, Pannell R, Rabbitts TH. The LMO2 T-cell oncogene is activated via chromosomal translocations or retroviral insertion during gene therapy but has no mandatory role in normal T-cell development. Mol Cell Biol. 2003;23:9003–9013. doi: 10.1128/MCB.23.24.9003-9013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bach I. The LIM domain: regulation by association. Mech Dev. 2000;91:5–17. doi: 10.1016/s0925-4773(99)00314-7. [DOI] [PubMed] [Google Scholar]
- 10.Wadman IA, Osada H, Grutz GG, Agulnick AD, Westphal H, Forster A, et al. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NL1 proteins. EMBO. 1997;16:3145–3157. doi: 10.1093/emboj/16.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ono Y, Fukuhara N, Yoshie O. TAL1 and LIM-only proteins synergistically induce retinaldehyde dehydrogenase 2 expression in T-cell acute lymphoblastic leukemia by acting as cofactors for GATA3. Mol Cell Biol. 1998;18:6939–6950. doi: 10.1128/mcb.18.12.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson KP, Crable SC, Lingrel JB. Multiple proteins binding to a GATA-E box-GATA motif regulate the erythroid Krüppel-like factor (EKLF) gene. J Biol Chem. 1998;273:14347–14354. doi: 10.1074/jbc.273.23.14347. [DOI] [PubMed] [Google Scholar]
- 13.Lecuyer E, Herblot S, Saint-Denis M, Martin R, Begley CG, Porcher C, et al. The SCL complex regulates c-kit expression in hematopoietic cells through functional interaction with Sp1. Blood. 2002;100:2430–2440. doi: 10.1182/blood-2002-02-0568. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z, Huang S, Chang LS, Agulnick AD, Brandt SJ. Identification of a TAL1 target gene reveals a positive role for the LIM domain-binding protein Ldb1 in erythroid gene expression and differentiation. Mol Cell Biol. 2003;23:7585–7599. doi: 10.1128/MCB.23.21.7585-7599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anguita E, Hughes J, Heyworth C, Blobel GA, Wood WG, Higgs DR. Globin gene activation during haemopoiesis is driven by protein complexes nucleated by GATA-1 and GATA-2. EMBO J. 2004;23:2841–2852. doi: 10.1038/sj.emboj.7600274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahlil R, Lecuyer E, Herblot S, Hoang T. SCL assembles a multifactorial complex that determines glycophorin A expression. Mol Cell Biol. 2004;24:1439–1452. doi: 10.1128/MCB.24.4.1439-1452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu TH, Qin G, Royer-Pokora B. A novel post-transcriptional splicing form of the acute T cell leukemia proto-oncogene Lmo2. Sci China (Ser C-Life Sci) 2001;44:561–569. doi: 10.1007/BF02879349. [DOI] [PubMed] [Google Scholar]
- 18.Pruess MM, Drechsler M, Royer-Pokora B. Promoter 1 of LMO2, a master gene for hematopoiesis, is regulated by the erythroid specific transcription factor GATA1. Gene Funct Dis. 2000;2:87–94. [Google Scholar]
- 19.Landry JR, Kinston S, Knezevic K, Donaldson IJ, Green AR, Gottgens B. Fli1, Elf1, and Ets1 regulate the proximal promoter of the LMO2 gene in endothelial cells. Blood. 2005;106:2680–2687. doi: 10.1182/blood-2004-12-4755. [DOI] [PubMed] [Google Scholar]
- 20.Nerlov C, Querfurth E, Kulessa H, Graf T. GATA-1 interacts with the myeloid PU.1 transcription factor and represses PU.1-dependent transcription. Blood. 2000;95:2543–2551. [PubMed] [Google Scholar]
- 21.Zhang P, Behre G, Pan J, Iwama A, Wara-Aswapati N, Rodomska HS, et al. Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proc Natl Acad Sci USA. 1999;96:8705–8710. doi: 10.1073/pnas.96.15.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, Zhang X, Iwama A, Yu C, Smith KA, Mueller BU, et al. PU.1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood. 2000;96:2641–2648. [PubMed] [Google Scholar]
- 23.Stopka T, Amanatullah DF, Papetti M, Skoultchi AI. PU.1 inhibits the erythroid program by binding to GATA-1 on DNA and creating a repressive chromatin structure. EMBO J. 2005;24:3712–3723. doi: 10.1038/sj.emboj.7600834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benchabane H, Wrana JL. GATA- and Smad1-dependent enhancers in the Smad7 gene differentially interpret bone morphogenetic protein concentrations. Mol Cell Biol. 2003;23:6646–6661. doi: 10.1128/MCB.23.18.6646-6661.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo B, Heard AD, Lodish HF. Small interfering RNA production by enzymatic engineering of DNA (SPEED) Proc Natl Acad Sci USA. 2004;101:5494–5499. doi: 10.1073/pnas.0400551101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terano T, Zhong Y, Toyokuni S, Hiai H, Yamada Y. Transcriptional control of fetal liver hematopoiesis: dominant negative effect of the overexpression of the LIM domain mutants of LMO2. Exp Hematol. 2005;33:641–651. doi: 10.1016/j.exphem.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Mead PE, Deconinck AE, Huber TL, Orkin SH, Zon LI. Primitive erythropoiesis in the Xenopus embryo: the synergistic role of LMO-2, SCL and GATA-binding proteins. Development. 2001;128:2301–2308. doi: 10.1242/dev.128.12.2301. [DOI] [PubMed] [Google Scholar]
- 28.Gering M, Yamada Y, Rabbitts TH, Patient RK. Lmo2 and Scl/Tal1 convert non-axial mesoderm into haemangioblasts which differentiate into endothelial cells in the absence of Gata1. Development. 2003;130:6187–6199. doi: 10.1242/dev.00875. [DOI] [PubMed] [Google Scholar]
- 29.Graf T. Differentiation plasticity of hematopoietic cells. Blood. 2002;99:3089–3101. doi: 10.1182/blood.v99.9.3089. [DOI] [PubMed] [Google Scholar]
- 30.Rekhtman N, Choe KS, Matushansky I, Murray S, Stopka T, Skoultchi AI. PU.1 and pRB interact and cooperate to repress GATA-1 and block erythroid differentiation. Mol Cell Biol. 2003;23:7460–7474. doi: 10.1128/MCB.23.21.7460-7474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison CJ, Foroni L. Cytogenetics and molecular genetics of acute lymphoblastic leukemia. Rev Clin Exp Hematol. 2002;62:91–113. doi: 10.1046/j.1468-0734.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- 32.Hatano M, Roberts CWM, Minden M, Crist WM, Korsmeyer SJ. Deregulation of a homeobox gene, HOX11, by the t(10;14) in T-cell leukemia. Science. 1991;253:70–72. doi: 10.1126/science.1676542. [DOI] [PubMed] [Google Scholar]
- 33.Chen Q, Cheng JT, Tsai LH, Schneider N, Buchanan G, Carroll A, et al. The tal gene undergoes chromosome translocation in T cell leukemia and potentially encodes a helix–loop–helix protein. EMBO J. 1990;9:415–424. doi: 10.1002/j.1460-2075.1990.tb08126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia Y, Brown L, Yang CY-C, Tsai JT, Siciliano MJ, Espirosa R, et al. TAL2, a helix–loop–helix gene activated by the (7;9)(q34;q32) translocation in human T-cell leukemia. Proc Natl Acad Sci USA. 1991;88:11416–11420. doi: 10.1073/pnas.88.24.11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGuire EA, Hockett RD, Pollock KM, Bartholdi MF, O’Brien SO, Korsmeyer SJ. The t(11;14)(p15;q11) in a T-cell acute lymphoblastic leukemia cell line activates multiple transcripts, including Ttg-1, a gene encoding a potential zinc finger protein. Mol Cell Biol. 1989;9:2124–2132. doi: 10.1128/mcb.9.5.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellentin JD, Smith SD, Cleary ML. lyl-1, a novel gene altered by chromosomal translocation in T cell leukemia, codes for a protein with a helix–loop–helix DNA binding motif. Cell. 1989;58:77–83. doi: 10.1016/0092-8674(89)90404-2. [DOI] [PubMed] [Google Scholar]
- 37.Hacein-Bey-Abina A, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt M, Hacein-Bey-Abina S, Wissler M, Carlier F, Lim A, Prinz C, et al. Clonal evidence for the transduction of CD34+ cells with lymphomyeloid differentiation potential and self-renewal capacity in the SCID-X1 gene therapy trial. Blood. 2005;105:2699–2706. doi: 10.1182/blood-2004-07-2648. [DOI] [PubMed] [Google Scholar]
- 39.Choe KS, Radparvar F, Matushansky I, Rekhtman N, Han X, Skoultchi AI. Reversal of tumorigenicity and the block to differentiation in erythroleukemia cells by GATA-1. Cancer Res. 2003;63:6363–6369. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.