Abstract
Current guidelines suggest that patients with a pretransplant carbon monoxide diffusion capacity (DLCO) ≤60% are not ideal candidates for hematopoietic cell transplantation (HCT). However, recent studies suggest this criterion may exclude patients who will benefit from the procedure. We conducted a study of all adult patients who received an autologous or allogeneic HCT from 1990 to 2005 and had a DLCO ≤ 60% of predicted normal to examine whether there is a lower limit for the DLCO threshold in the context of respiratory failure and nonrelapse related mortality risk, and whether a comprehensive risk scoring system such as the Pretransplant Assessment of Mortality (PAM) risk score can more effectively risk stratify these patients with a very low pretransplant DLCO. Regardless of how low the pretransplant DLCO was below 60%, there was no significant association with the risk for respiratory failure or NRM. However, the PAM score effectively risk stratified the allogeneic HCT patients for NRM risk. There was a stepwise relationship between PAM score category and NRM risk; the highest PAM score category had a 4.38-fold increase in risk for NRM (p<0.001). These data suggest that the pretransplant DLCO should not be considered as a sole eligibility criteria for HCT.
INTRODUCTION
It is well accepted that pretransplant lung function assessment provides critical information for the management of hematopoietic cell transplant (HCT) patients and that pulmonary function tests (PFT) should be obtained prior to both autologous and allogeneic transplantation as part of standard practice protocols for evaluating lung function as an eligibility criteria for transplantation [1–3]. Many studies have examined the predictive value of pretransplant PFTs for post transplant outcomes, such as pulmonary complications and mortality [2–7]. While these studies were not definitive, a seminal paper published by Crawford et al demonstrated in a large cohort that patients with a pretransplant carbon monoxide diffusion capacity (DLCO) <60% had a significant 1.5 fold higher risk for mortality after transplant [8]. This observation was extended in a follow-up study that found patients with a pretransplant DLCO <70% of the predicted normal had a significant 2.4 fold increased risk for severe hepatic veno-occulsive disease after marrow transplantation [9]. Based upon these findings, current National Marrow Donor Program transplant eligibility guidelines suggest that in the absence of other comorbid conditions, a DLCO threshold of 60% should be considered as an eligibility criteria for stem cell transplantation (i.e. patients with a DLCO ≤ 60% should not undergo stem cell transplantation) [10].
However, several recent studies indicate that transplant outcome (e.g. mortality) is likely dependent upon multiple baseline risk factors and comorbidities, which includes but is not exclusive to DLCO [11–15]. Sorror et al have conducted multiple studies that indicate a comprehensive assessment of 18 pretransplant comorbid conditions is informative and predictive with regards to a patient’s risk for nonrelapse related mortality and survival [13–15]. We have taken a parsimonious approach, demonstrating that eight commonly available pretransplant clinical variables can accurately predict the risk of all-cause mortality within the first two years after allogeneic HCT [11]. Based upon these findings, the authors have urged the transplant community to consider not using any single variable, such as the DLCO, as a sole eligibility criterion for transplantation.
In light of these recent publications, the issue of whether a DLCO threshold should be used as an eligibility criterion need to be reassessed. Therefore, we conducted an analysis of patients whose pretransplant DLCO was ≤ 60% to determine whether there is a lower limit for the DLCO threshold in the context of post transplant outcomes, and evaluated whether a comprehensive risk scoring system can more effectively risk stratify this subgroup of patients with a very low pretransplant DLCO, and identify patients who are more likely to benefit from this potentially dangerous procedure.
METHODS
Patient Selection
This study was conducted using clinical and laboratory data collected prospectively at the Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance (the “Center”). Patients who had a first autologous or allogeneic HCT between January 1, 1990 and December 31, 2005, had a carbon monoxide diffusion capacity (DLCO) ≤ 60% of predicted normal, and were ≥15 years of age, were included in this study.
Clinical Variables
The Pretransplant Assessment of Mortality (PAM) score is a comprehensive scoring system that can be used to accurately estimate a patient’s risk for all cause mortality within the first two years after allogeneic HCT [11]. This scoring system has been validated in multiple patient cohorts, including patients who also received a reduced-intensity conditioning regimen. Components of the PAM score, age at transplant, disease risk, donor type, conditioning regimen, liver function, kidney function, and lung function, were collected and scored for all patients according to previously described methods [11]. To simplify the disease categories for the analysis, we ranked them as low, intermediate, or high risk according to disease type, stage, and our experience with their outcomes. Matching between the donor and recipient was determined according to donor-recipient ABO compatibility and HLA-A, HLA-B, and HLA-DR compatibility. Conditioning regimens were first grouped according to myeloablative or nonmyeloablative categories. Myeloablative regimens were categorized according to the use of total body irradiation and amount of total body irradiation: ≤12 Gy or >12 Gy. All patients in the nonmyeloablative group received 2 Gy total body irradiation. A lung shielding protocol for TBI was initiated at our Center in 1999. To account for potential changes in clinical practice over time, we considered the year of transplant as a categorical variable in the analysis.
All pulmonary function tests were performed at our Center according to American Thoracic Society guidelines [16–18]. Pulmonary function assessments included the percent of predicted one-second forced expiratory volume (FEV1) and percent of predicted DLCO, which was adjusted for hemoglobin level. As part of usual protocol, pretransplant and day 100 (day 80 ± 20 days) PFTs were obtained regardless of the presence or absence of symptoms. After discharge from our institution by day 120, patients were encouraged to follow-up at the Center one year (365 days ± 100 days) after transplant. Those who could and elected to return for follow-up at that time had a PFT as per protocol. All pulmonary function values were expressed as a percent of the predicted values calculated according to published equations [16, 19]. Absolute change in FEV1 or DLCO was calculated by subtracting the day 100 or one year value from the pretransplant value. Serum creatinine and glutamic pyruvic transaminase (SGPT) concentrations obtained most recently before the beginning of the conditioning regimen were categorized as normal or abnormal according to our center’s laboratory standards (abnormal values: creatinine >1.2 mg/dL; total bilirubin >1.3 mg/dL; SGPT >49 U/L).
Two outcomes were considered: early respiratory failure and nonrelapse related mortality. Patients were defined as having developed early respiratory failure if they required mechanical ventilation for a non-elective reason within 120 days after transplant. Respiratory failure occurring after 120 days was not assessed because patients are routinely discharged from our Center after the first 120 days post transplant. Nonrelapse related mortality was defined as death occurring prior to clinical evidence of disease relapse.
Statistical analysis
All statistical analyses were performed using STATA 8.0 (StataCorp, College Station, TX 2003). Pearson χ2 test was used to compare categorical variables. Unpaired T-test and one-way analysis of variance were used to compare continuous variables. Paired T-test and Wilcoxon Rank-sum test were used to compare changes in DLCO. The Pretransplant Assessment of Mortality (PAM) Risk Score, including the survival probability and 95% confidence intervals, were calculated for all allogeneic HCT patients according to previously described methods [11]. Cox proportional hazards regression models were used to conduct a time to event analysis for respiratory failure and nonrelapse related mortality. Disease relapse was considered as a competing event for nonrelapse related mortality. The proportional hazard assumption was tested using Schoenfeld residuals. The rates of developing early respiratory failure and mortality according to pretransplant DLCO were estimated using Kaplan-Meier curves and assessed using the log-rank test. Two-sided P-values <0.05 were considered statistically significant.
RESULTS
Between January 1, 1990 and December 31, 2005, we identified 56 autologous and 165 allogeneic HCT patients who had a pretransplant DLCO ≤ 60% (Table 1). The median DLCO was 55% (range 35% to 60%) and 54% (18% to 60%) among autologous and allogeneic patients respectively. The majority of patients had a pretransplant DLCO between 40% and 60%. Among autologous patients, only one (2%) had a DLCO <40%. Among allogeneic patients 13 (8%) had a DLCO <40%. To assess whether lower DLCO was associated with demographic and physiologic differences, autologous and allogeneic patients were combined and divided into three groups according to pretransplant DLCO: 50%–60%, 40%–49%, and <40%. There were no significant differences in age at transplant, conditioning regimen, donor type, disease risk, pretransplant renal and liver function, and pretransplant FEV1 between the DLCO groups (Table 2). Only 24 (15%) of the 165 allogeneic patients received a nonmyeloablative conditioning regimen. Among these patients, 6/24 (25%) and 20/24 (83%) developed respiratory failure and NRM respectively. Among patients who received a myeloablative conditioning regimen, 50/141 (35%) and 84/141 (60%) developed respiratory failure and NRM respectively.
Table 1.
Patient characteristics and outcomes according to autologous versus allogeneic transplant
| Characteristic | Autologous (N=56) | Allogeneic (N=165) |
|---|---|---|
| Age (years) | 41.3 ± 15.3 | 39.3 ± 13.2 |
| Female patients | 22 (39) | 58 (35) |
| Donor type | ||
| Autologous | 56 (100) | - |
| Related, matched | - | 83 (50) |
| Related, mismatched | - | 23 (14) |
| Unrelated | - | 59 (36) |
| Diagnosis | ||
| Acute leukemia | 5 (8) | 83 (50) |
| Chronic leukemia | 0 (0) | 28(17) |
| Hodgkin’s lymphoma | 14 (22) | 7 (4) |
| Non-Hodgkins lymphoma | 14 (22) | 10 (6) |
| Multiple myeloma | 13 (20) | 8 (5) |
| Myelodysplastic syndrome | 0 (0) | 20 (12) |
| Other | 10 (18) | 9 (5) |
| Disease status | ||
| Accelerated phase | 0 (0) | 8 (6) |
| Blast crisis | 0 (0) | 9 (6) |
| Chronic phase | 0 (0) | 8 (6) |
| De novo | 1 (2) | 5 (3) |
| Relapse | 42 (75) | 78 (55) |
| Remission | 12 (21) | 33 (23) |
| Unknown | 1 (2) | 2 (1) |
| Percent of predicted DLCO | 55.5% (34%–60%) | 54% (19%–60%) |
| Percent of predicted DLCO category | ||
| 50–60% | ||
| 40–50% | 49 (87) | 118 (71) |
| <40% | 6 (11) | 34 (21) |
| 1 (2) | 13 (8) | |
| Respiratory failure | 10 (18) | 56 (34) |
| Nonrelapse mortality | 27 (48) | 104 (63) |
Numbers represent either mean ± standard deviation, median (range), or count (%).
Table 2.
Baseline clinical characteristics and Pretransplant Assessment of Mortality (PAM) score according to DLCO category
| Pretransplant percent of predicted DLCO category |
||||
|---|---|---|---|---|
| Characteristic | 50–60% (N=167) | 40–49% (N=40) | <40% (N=14) | P-value |
| Age (years) | 41.7 (16.1–70.7) | 40.5 (21.4–67.9) | 35.3 (15.7–54.15) | 0.677 |
| Conditioning regimen | ||||
| Nonmyeloablative | 12 (7) | 7 (17) | 5 (36) | 0.066 |
| Non-total body irradiation | 67 (40) | 13 (33) | 3 (21) | |
| Total-body irradiation with ≤12Gy | 42 (25) | 9 (22) | 3 (21) | |
| Total-body irradiation with >12Gy | 46 (28) | 11 (28) | 3 (21) | |
| Donor type | ||||
| Autologous | 49 (29) | 6 (15) | 1 (7) | 0.294 |
| Matched related | 58 (35) | 18 (45) | 7 (50) | |
| Mismatched related | 42 (25) | 12 (30) | 5 (36) | |
| Unrelated | 18 (11) | 4 (10) | 1 (7) | |
| Disease risk | ||||
| Low | 9 (5) | 2 (5) | 0 (0) | 0.384 |
| Moderate | 47 (28) | 6 (15) | 5 (36) | |
| High | 111 (66) | 32 (80) | 9 (64) | |
| Serum alanine aminotransferase (U/L) | 26.0 (6–476) | 26 (5–217) | 31 (2–100) | 0.6 |
| Serum creatinine (mg/dL) | 0.8 (0.3–5.1) | 0.85 (0.4–8.7) | 0.95 (0.4–2.4) | 0.750 |
| Percent of predicted FEV1 | 74% (39%–111%) | 66% (35%–90%) | 52% (26%–100%) | 0.06 |
| PAM score* | 30.69 (15–42) | 31.74 (15–43) | 32.0 (26–43) | 0.997 |
Numbers represent either median (range) or count (%).
PAM score calculated for only allogeneic transplant patients
Low pretransplant DLCO and change in DLCO after transplantation
To assess whether patients with lower DLCO prior to transplantation were at risk for a lower DLCO after transplantation, we assessed the change in DLCO by day 100 (N=99) and one year (N=33) after transplantation. Although there was a borderline trend of more patients with lower pretransplant DLCO experiencing an increase in DLCO at day 80 (p=0.055), there were no significant differences in the number of patients who experienced a decrease in DLCO or the absolute change in DLCO at one year (Table 3).
Table 3.
Change in DLCO by day 100 and one year after transplant according to pretransplant DLCO categories
| Pretransplant percent of predicted DLCO category |
||||
|---|---|---|---|---|
| Time interval | 50–60% | 40–49% | <40% | P-value |
| Pretransplant to day 80 | ||||
| Number experiencing | 39/75 (52) | 12/17 (71) | 5/7 (71) | 0.055 |
| Increase in DLCO | 5.1% | 14.9% | 20.5% | |
| Change in DLCO | (−40.7% − 96%) | (−32.7% − 55.8%) | (−11.1%−415.8%) | |
| Pretransplant to one year | ||||
| Number experiencing | 12/27 (44) | 3/5 (60) | 1/1 (100) | 0.235 |
| Increase in DLCO | −10.3% | 2.3% | 22.3% | |
| Change in DLCO | (−43.3% − 64.4%) | (−33.3% − 77.8%) | ||
Numbers represent patients who experienced an increase in DLCO (%) and median change in DLCO (range) during the designated time intervals.
Early respiratory failure risk and nonrelapse related mortality
To assess whether patients with lower DLCO prior to transplantation were at higher risk for the two outcomes we considered, we examined the time to development of early respiratory failure and nonrelapse related mortality in separate models according to pretransplant DLCO categories. Regardless of the pretransplant DLCO category, there was no significant difference in the risk for developing respiratory failure or nonrelapse mortality (Table 4). The lack of association persisted even after adjustment for use of lung shielding and year of transplant. The results remained similar when the analysis was stratified according to whether allogeneic transplant patients received a nonmyeloablative versus myeloabative conditioning regimen.
Table 4.
Risk of developing respiratory failure and nonrelapse related mortality according to pretransplant DLCO categories and PAM score
| Number of cases (%) | Hazard ratio (95% Confidence interval) | P-value | |
|---|---|---|---|
| Respiratory failure* | 66 | ||
| Percent of predicted DLCO | |||
| 50%–60% | 47/167 (28) | Referent | - |
| 40%–49% | 14/40 (35) | 1.37 (0.76–2.49) | 0.298 |
| <40% | 5/14 (36) | 1.50 (0.60–3.78) | 0.388 |
| Nonrelapse mortality* | 131 | ||
| Percent of predicted DLCO | |||
| 50%–60% | 92/167 (55) | Referent | - |
| 40%–49% | 29/40 (73) | 1.28 (0.88–1.87) | 0.198 |
| <40% | 10/14 (71) | 1.37 (0.77–2.42) | 0.282 |
| PAM score categories† | |||
| Quartile 1 (17–27) | 28/37 (76) | Referent | - |
| Quartile 2 (28–31) | 18/34 (53) | 1.78 (1.08–2.95) | 0.025 |
| Quartile 3 (32–33) | 28/55 (51) | 2.27 (1.42–3.65) | 0.001 |
| Quartile 4 (35–42) | 30/39 (77) | 4.38 (2.69–7.14) | <0.001 |
Analysis includes autologous and allogeneic transplant patients (Total N=221)
Analysis restricted to nonrelapse mortality among allogeneic transplant patients (Total N=165)
To be certain that this lack of association was not due to the way that DLCO categories were determined, we also dichotomized the DLCO categories according to two additional DLCO thresholds. When patients with a pretransplant DLCO ≥45% were compared to patients with a DLCO <45% (N=29), the risk for respiratory failure (hazard ratio [HR] 1.36, 95% confidence interval [CI] 0.69–2.67, p=0.370) and nonrelapse mortality (HR 1.31, 95% CI 0.74–2.30, p=0.354) were still not significantly different. This was also true when the DLCO threshold was set at 40%. Patients with a pretransplant DLCO <40% (N=14) did not have a significantly increased risk for respiratory failure (HR 1.41, 95% CI 0.57–3.5, p=0.3462) or nonrelapse mortality (HR 1.22, 95% CI 0.79–1.87, p=0.37). Adjustment for use of lung shielding did not significantly affect the risk estimates.
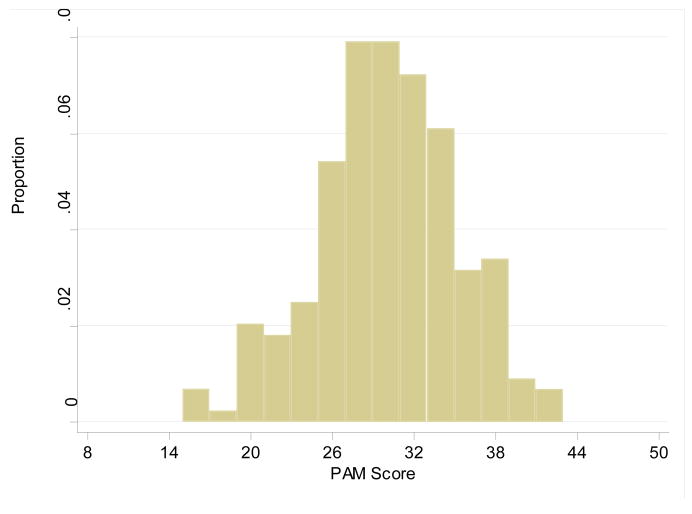
Given previous publications have suggested using a more comprehensive approach for risk stratifying patients, we examined whether an alternative and more comprehensive assessment of mortality risk, the PAM score, can more effectively risk stratify patients than the DLCO alone. Since the PAM score was developed for allogeneic transplant patients, the autologous patients were excluded from this portion of the analysis. The average PAM score was similar for all DLCO categories (Table 2). However, when the PAM score of all 165 allogeneic patients were considered together, it was apparent that the PAM score was somewhat normally distributed with a median of 30 (mean 30.5) and a range from 17 to 43 (Figure 1), which were associated with survival probabilities ranging from 79% (95% CI 75–83%) to 5% (95% CI 3–8%) respectively.
Figure 1.
Histogram for distribution of the PAM scores among allogeneic transplant recipients. Median score of 30 (mean 30.5) with a range from 17 to 43.
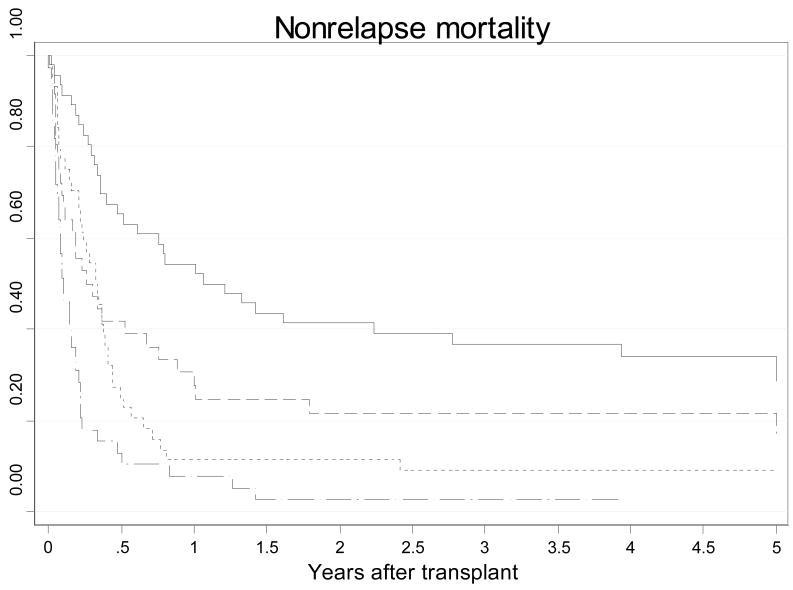
When the patients were subdivided according to PAM score quartiles, patients in the highest PAM score quartile had a four-fold increase in risk for nonrelapse related mortality than the patients in the lowest PAM score quartile (HR 4.38, 95% CI 2.69–7.14). There was also a significant stepwise increase in nonrelapse related mortality risk and lower survival rates as the PAM score increased in quartile (Table 4). Figure 2 provide cumulative incidence curves for nonrelapse mortality according to PAM score quartiles. Adjustment for use of lung shielding did not significantly affect the risk estimates. However, adjustment for year of transplant did influence the risk estimates slightly. In comparison to quartile 1, the HRs were as follows: quartile 2 HR 1.22 (85% CI 0.64–2.33, p=0.542), quartile 3 HR 2.04 (95% CI 1.14–3.65, p=0.017), quartile 4 HR 6.35 (95% CI 3.29–12.28, p<0.001). When this analysis was stratified by myeloablative versus nonmyeloablative status, there was no significant association among the nonmyeloablative patients and the effect sizes did not change significantly among the myeloablative patients. In comparison to quartile 1, the HRs among myeloablative patients were as follows: quartile 2 HR 1.41 (85% CI 0.66–3.03, p=0.377), quartile 3 HR 1.98 (95% CI 0.98–4.03, p=0.059), quartile 4 HR 6.95 (95% CI 3.32–14.57, p<0.001).
Figure 2.
Kaplan-Meier curves for nonrelapse related mortality according to PAM score quartiles (p<0.001): solid line=17–28, short dashed line=29–31, dotted line=32–34, long dashed line=35–42.
Although the PAM score was not originally designed for assessment of respiratory failure risk, we also assessed whether a higher PAM score was associated with increased respiratory failure risk. Among patient receiving an allogeneic graft. A PAM score in the highest quartile was associated with a 2.4-fold increase in risk of developing respiratory failure (95% CI 1.14–5.02, p=0.02). The association with lower PAM quartiles was not statistically significant. This association with the highest PAM score quartile increased after adjusting for year of transplant (HR 3.68, 95% CI 1.58–8.54, p=0.002). When this analysis was stratified by myeloablative versus nonmyeloablative status, only the association remained among myeloablative patients (HR 3.52 adjusted for year of transplant 95% CI 1.45–8.56, p=0.005).
DISCUSSION
The diffusion capacity, most commonly assessed by the single-breath carbon monoxide method [17, 20], is a measure of the patient’s ability to absorb alveolar gases into the capillary blood flow, reflecting alveolar membrane thickness, hematocrit level, cardiac output and heterogeneity in the distribution of the diffusion capacity to regional ventilation and perfusion (in patients with pulmonary disease) [21]. Reduction of the diffusion capacity can be due to compromise of any or a combination of these variables, leading to a reduction of the alveolar capillary interface. Unfortunately, the DLCO is the most variable measured parameter in a PFT, particularly when a restrictive or obstructive ventilatory impairment is present [22–26]. Even among normal individuals, there is significant variability in the measured DLCO [27, 28]. Furthermore, selecting reference equations from over nine that are currently available remains a problem [17], resulting in large differences among different reference equations and among different laboratories. For instance, our Center uses a more conservative Crapo reference equation, which can significantly underestimate the DLCO when compared to other reference equations. Thus, despite the initial findings of Crawford et al, using the DLCO in the absence of other comorbidities as an exclusion criterion for stem cell transplantation may not be ideal.
Based upon the work from Crawford et al, it is clear that a low pretransplant DLCO is associated with an increased risk for nonrelapse mortality after transplant [9]. We have also demonstrated similar findings in a recent analysis of a more contemporaneous cohort [12]. This is further supported by the fact that the prevalence of respiratory failure is 30% in the current study, significantly higher than approximately 14% observed among patients with a pretransplant DLCO >60%. However, despite these data for patients with reasonable DLCOs prior to transplant, the current study revealed two hazards of using DLCO for determining transplant eligibility among patients with low DLCOs. First, we found no evidence of a stepwise relationship between pretransplant DLCO levels ≤60% and the transplant outcomes we evaluated. While this might reflect the ineffectiveness of using 60% as a threshold, our additional analyses examining 40% and 45% as alternative thresholds demonstrated that this is an unlikely explanation. The more likely explanation is that a DLCO, when ≤60%, is simply not a specific enough parameter for risk stratifying patients. Second, when the DLCO threshold of 60% is used to exclude patients from transplantation in the absence of other comorbidities, it is possible that patients who might benefit from this procedure will be rejected. This is clearly demonstrated by our PAM score analysis, where a significant portion of the patients with a DLCO≤ 60% was not only found to have an excellent estimated survival probability, but ultimately actually survived to the 5-year mark.
However, our analysis is not without limitations. First, despite the low pretransplant DLCO, the patients in this analysis were nevertheless selected for transplantation. It is possible that clinical assessment at the time of pretransplant evaluation revealed the patient to be more physiologically fit than was apparent based on their pretransplant DLCO, thereby supporting a decision to proceed. However, it is also possible that these patients had few treatment alternatives, and that transplantation was selected as a last resort despite severe physiologic limitations. Given the normally distributed PAM score among the allogeneic patients, we believe a combination of both explanations is more likely. Second, our study also had very few patients with a pretransplant DLCO in the lowest range, even when we evaluated the patients using two alternative DLCO thresholds. This may have limited our ability to detect an association between the degree of DLCO compromise and the outcomes considered. Unfortunately, given the current practice of excluding patients with a low DLCO from transplantation, it will be difficult to accumulate a group of patients with extremely low DLCOs for such an analysis. Finally, it should be recognized that the lack of association between DLCO and PAM score with these outcomes among nonmyeloablative patients may not be useful because the current study only had a total of 24 patients who received a nonmyeloablative conditioning regimen. As more data are accumulated for nonmyeloablative patients, additional analyses will need to be conducted.
In summary, our analysis suggests that while the pretransplant DLCO may be useful for initial identification of patients at higher risk for poorer stem cell transplant outcomes, this approach should no longer be considered as a sole eligibility criteria for patients whose pretransplant DLCO is below the eligibility threshold. While a low DLCO alone probably does identify patients at risk for a poor outcome, a more comprehensive risk stratification tool should be considered because it can more accurately estimate the mortality risk of allogeneic transplantation, even for patients with the lowest DLCO. Including the pretransplant DLCO as part of such a comprehensive risk assessment tool will allow clinicians to more accurately identify patients who will benefit from transplantation and more effectively counsel patients who are at extremely high risk for poor transplant outcomes.
Acknowledgments
Funded by HL088201
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chien JW, Madtes DK, Clark JG. Pulmonary function testing prior to hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35:429–435. doi: 10.1038/sj.bmt.1704783. [DOI] [PubMed] [Google Scholar]
- 2.Ho VT, Weller E, Lee SJ, Alyea EP, Antin JH, Soiffer RJ. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:223–229. doi: 10.1053/bbmt.2001.v7.pm11349809. [DOI] [PubMed] [Google Scholar]
- 3.Horak DA, Schmidt GM, Zaia JA, Niland JC, Ahn C, Forman SJ. Pretransplant pulmonary function predicts cytomegalovirus-associated interstitial pneumonia following bone marrow transplantation. Chest. 1992;102:1484–1490. doi: 10.1378/chest.102.5.1484. [DOI] [PubMed] [Google Scholar]
- 4.Ghalie R, Szidon JP, Thompson L, Nawas YN, Dolce A, Kaizer H. Evaluation of pulmonary complications after bone marrow transplantation: the role of pretransplant pulmonary function tests. Bone Marrow Transplant. 1992;10:359–365. [PubMed] [Google Scholar]
- 5.Badier M, Guillot C, Delpierre S, Vanuxem P, Blaise D, Maraninchi D. Pulmonary function changes 100 days and one year after bone marrow transplantation. Bone Marrow Transplant. 1993;12:457–461. [PubMed] [Google Scholar]
- 6.Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg SL, Klumpp TR, Magdalinski AJ, Mangan KF. Value of the pretransplant evaluation in predicting toxic day-100 mortality among blood stem-cell and bone marrow transplant recipients. J Clin Oncol. 1998;16:3796–3802. doi: 10.1200/JCO.1998.16.12.3796. [DOI] [PubMed] [Google Scholar]
- 8.Crawford SW, Fisher L. Predictive value of pulmonary function tests before marrow transplantation. Chest. 1992;101:1257–1264. doi: 10.1378/chest.101.5.1257. [DOI] [PubMed] [Google Scholar]
- 9.Matute-Bello G, McDonald GD, Hinds MS, Schoch HG, Crawford SW. Association of pulmonary function testing abnormalities and severe veno-occlusive disease of the liver after marrow transplantation. Bone Marrow Transplant. 1998;21:1125–1130. doi: 10.1038/sj.bmt.1701225. [DOI] [PubMed] [Google Scholar]
- 10.Keller C, Chatchada K. Evaluating Adult Patients Prior to Hematopoietic Cell Transplant. 2008 www.marrow.org.
- 11.Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med. 2006;144:407–414. doi: 10.7326/0003-4819-144-6-200603210-00007. [DOI] [PubMed] [Google Scholar]
- 12.Parimon T, Madtes DK, Au DH, Clark JG, Chien JW. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med. 2005;172:384–390. doi: 10.1164/rccm.200502-212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110:4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 16.Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique--1995 update. Am J Respir Crit Care Med. 1995;152:2185–2198. doi: 10.1164/ajrccm.152.6.8520796. [DOI] [PubMed] [Google Scholar]
- 18.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 19.Crapo RO, Morris AH. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123:185–189. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 20.Blakemore WS, Forster RE, Morton JW, Ogilvie CM. A standardized breath holding technique for the clinical measurement of the diffusing capacity of the lung for carbon monoxide. J Clin Invest. 1957;36:1–17. doi: 10.1172/JCI103402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crapo RO, Forster RE., 2nd Carbon monoxide diffusing capacity. Clin Chest Med. 1989;10:187–198. [PubMed] [Google Scholar]
- 22.Cotes JE, Dabbs JM, Elwood PC, Hall AM, McDonald A, Saunders MJ. Iron-deficiency anaemia: its effect on transfer factor for the lung (diffusiong capacity) and ventilation and cardiac frequency during sub-maximal exercise. Clin Sci. 1972;42:325–335. doi: 10.1042/cs0420325. [DOI] [PubMed] [Google Scholar]
- 23.Punjabi NM, Shade D, Patel AM, Wise RA. Measurement variability in single-breath diffusing capacity of the lung. Chest. 2003;123:1082–1089. doi: 10.1378/chest.123.4.1082. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi K, Mori M, Kawai A, Takasugi T, Oyamada Y, Koda E. Inhomogeneities of ventilation and the diffusing capacity to perfusion in various chronic lung diseases. Am J Respir Crit Care Med. 1997;156:86–93. doi: 10.1164/ajrccm.156.1.9607090. [DOI] [PubMed] [Google Scholar]
- 25.Stam H, Splinter TA, Versprille A. Evaluation of diffusing capacity in patients with a restrictive lung disease. Chest. 2000;117:752–757. doi: 10.1378/chest.117.3.752. [DOI] [PubMed] [Google Scholar]
- 26.Johnson DC. Importance of adjusting carbon monoxide diffusing capacity (DLCO) and carbon monoxide transfer coefficient (KCO) for alveolar volume. Respir Med. 2000;94:28–37. doi: 10.1053/rmed.1999.0740. [DOI] [PubMed] [Google Scholar]
- 27.Kangalee KM, Abboud RT. Interlaboratory and intralaboratory variability in pulmonary function testing. A 13-year study using a normal biologic control. Chest. 1992;101:88–92. doi: 10.1378/chest.101.1.88. [DOI] [PubMed] [Google Scholar]
- 28.Mushtaq M, Hayton R, Watts T, Shurvinton J, Gooch R, Perks WH. An audit of pulmonary function laboratories in the West Midlands. Respir Med. 1995;89:263–270. doi: 10.1016/0954-6111(95)90086-1. [DOI] [PubMed] [Google Scholar]