Abstract
Objective: In the present study, we have examined the safety and efficacy of recombinant adenovirus encoding human p53 tumor suppressor gene (rAd-p53) injection in patients with advanced non-small-cell lung cancer (NSCLC) in the combination with the therapy of bronchial arterial infusion (BAI). Methods: A total of 58 patients with advanced NSCLC were enrolled in a non-randomized, two-armed clinical trial. Of which, 19 received a combination treatment of BAI and rAd-p53 (the combo group), while the remaining 39 were treated with only BAI (the control group). Patients were followed up for 12 months, with safety and local response evaluated by the National Cancer Institute’s Common Toxicity Criteria and response evaluation criteria in solid tumor (RECIST), respectively. Time to progression (TTP) and survival rates were also analyzed by Kaplan-Meier method. Results: In the combo group, 19 patients received a total of 49 injections of rAd-p53 and 46 times of BAI, respectively, while 39 patients in the control group received a total of 113 times of BAI. The combination treatment was found to have less adverse events such as anorexia, nausea and emesis, pain, and leucopenia (P<0.05) but more arthralgia, fever, influenza-like symptom, and myalgia (P<0.05), compared with the control group. The overall response rates (complete response (CR)+partial response (PR)) were 47.3% and 38.4% for the combo group and the control group, respectively (P>0.05). Patients in the combo group had a longer TTP than those in the control group (a median 7.75 vs 5.5 months, P=0.018). However, the combination treatment did not lead to better survival, with survival rates at 3, 6, and 12 months in the combo group being 94.74%, 89.47%, and 52.63%, respectively, compared with 92.31%, 69.23%, and 38.83% in the control group (P=0.224). Conclusion: Our results show that the combination of rAd-p53 and BAI was well tolerated in patients with NSCLC and may have improved the quality of life and delayed the disease progression. A further study to better determine the efficacy of this combination therapy is warranted.
Keywords: RAd-p53 gene therapy, Clinical trial, Non-small-cell lung cancer (NSCLC), Bronchial arterial infusion (BAI)
INTRODUCTION
Lung cancer is one of the leading causes of cancer deaths worldwide, and non-small-cell lung cancer (NSCLC) accounts for 75% to 80% of all the lung cancer cases (Parker et al., 1997; Frasci et al., 1999). Conventional treatments such as surgery, chemotherapy, and radiation are not very effective for the majority of the patients with lung cancer and have not significantly changed overall 5-year survival rate in last years (Daniel and Smythe, 2003). Current standard care has resulted in 15%~20% of cases for local control (Le Chevalier et al., 1991; Schaake-koning et al., 1992), with approximately 20% of advanced patients surviving for 2 years. Therefore, a new treatment modality for NSCLC patients is desirable.
Over the past decade, many gene therapy approaches have been tested in vitro, in animal models or clinical trials (Osaki et al., 2000; Inoue et al., 2000; Kawabe et al., 2001). At the beginning of 2005, there were a total of 1020 clinical trials for gene therapy world wide (http://www.wiley.co.uk/genetherapy/clinical), while over 60% of them were conducted in cancer patients. Of these cancer gene therapies, 58 patients used recombinant adenovirus encoding human p53 tumor suppressor gene (rAd-p53). In these trials, rAd-p53 has been demonstrated to be safe either as monotherapy or in combination with conventional therapies, but the efficacy of rAd-p53 in NSCLC patients has been inconclusive (Junker et al., 2000; Schuler et al., 2001; Nishizaki et al., 2001; Swisher et al., 2003). rAd-p53 (Gendicine®) was approved in 2003 by the State Food and Drug Administration (SFDA) of China for the treatment of head and neck squamous cell carcinoma (HNSCC) (Peng, 2005). In this article, we have summarized the safety and efficacy of rAd-p53 (Gendicine®) in patients with (NSCLC), when combined with the therapy of bronchial arterial infusion (BAI).
MATERIALS AND METHODS
rAd-p53 handling
rAd-p53 is a replication-defective adenovirus vector expressing the wild-type tumor suppressor gene p53. It is grown, tittered on the human embryonic kidney cell line HEK293, manufactured by Shenzhen SiBionoGenTech Co. Ltd. (Shenzhen, China), and marketed under the trade name of Gendcine (Peng, 2005). rAd-p53 is provided as a sterile viral solution at −20 °C in single-use, plastic screwcap vials. Each vial contains 2 ml virus solution at the specified viral titer of 1×1012 viral particles (VPs). Vials of virus solution were warmed and diluted to the appropriate titer of dosing before administration.
Enrollment criteria
Patients enrolled in this study had histologically proven NSCLC (stages 3~4) and were ineligible for surgical resection. Patients had to be 18 years of age or older and were required to have Karnofsky performance status of 70 or more. All patients signed a consent form approved by the Institutional Review Board Committee before the enrollment. The exclusion criteria included: having received chemotherapy, radiotherapy, or other anti-tumor therapy before study treatments, or with a life expectancy shorter than 3 months. Also, pregnant or nursing patients, patients with uncontrolled serious infections, and patients with acute or chronic respiratory distress were excluded for the enrollment. The p53 gene mutation was not required specially for enrolling because studies in vitro had proven that the p53 gene increased anti-tumor effect regardless of the cellular p53 status (Inoue et al., 2000; Kawabe et al., 2001).
Study design
The enrolled patients were assigned to two groups: the combo group (rAd-p53 gene injection in combination with BAI) and the control group (solo BAI). Patients assigned to the combo group were those who not only met the criteria described above but also were willing to receive rAd-p53 gene therapy, while those who met the inclusion criteria but were not willing to take gene therapy were assigned to the control group. The patients in the combo group received rAd-p53 gene transfer via intratumoral or bronchial arterial access on Day 1, 8, 15, or 22. A maximum of four times of gene transfer was planed. BAI was carried out on Day 5, with at least 1 month interval between two BAIs. Patients in the control group received BAI with the same interval as those in the combo group. Vector administration was performed in patients with easily accessible lesions by percutaneous injection under computed tomography (CT) guidance as previously described (Kauczor et al., 1999). Other patients received vector transfer through bronchial artery via a catheter. After the tip of a catheter was confirmed within the bronchical artery, the rAd-p53 solution was injected into this artery through the catheter. The vector dose ranged from 1 to 4×1012 VPs according to the tumor size. The BAI procedure has been expounded in detail (Wang et al., 1996) and the anti-tumor drug regime consisted of 0.8~1.0 g fluorouracil, 30~40 mg nevelbine, and/or 80~100 mg cisplatin.
Tumor assessments
1. Toxicity and Follow-up protocol
Before and after treatment, the patients’ clinical features were observed and documented in detail. The toxic effects of therapy were evaluated according to the National Cancer Institute’s Common Toxicity Criteria (Ajani et al., 1990). All patients were followed up for local response (tumor size), time to progression (TTP), and survival rate by CT scan and telephone enquiry. The start time was defined as the time of diagnosis. Assessments of therapeutic effect were made of local response according to the response evaluation criteria in solid tumor (RECIST) (Therasse et al., 2000), TTP, and survival rate. TTP for metastatic (pleural effusion, pulmonary nodules, lymph nodes, systemic metastasis) and locoregional diseases (primary tumor becoming 20% larger or more) was defined as the time from the start point to document progression. Survival duration was measured from the start point to the date of the last follow-up or death.
2. Statistical considerations
The aim of this nonrandomized clinical study was to evaluate the efficacy of rAd-p53 (Gendicine®) as an adjunct to arterial infusion chemotherapy in the treatment of patients with advanced NSCLC. The statistics between the two groups in baseline, toxicity, local response, TTP and survival rate were assessed by the SPSS 11.0. Values with P<0.05 were considered as statistically significant.
RESULTS
Patients and tumor characteristics
Fifty-eight patients with advanced NSCLC, who had viable NSCLC histologically determined by bronchoscopy, biopsy, and sputum examinations for tumor cells before treatment and were not eligible for or did not previously receive surgical resection, systemic chemotherapy, local radiation, or other anti-tumor therapies, were recruited in this trial. There was no statistical difference between baselines of the two groups (Table 1). Nineteen patients (15 males, 4 females, median 56 years and age range 39~80 years) were enrolled into the combo group, while the remaining 39 (25 males, 14 females, median 59 years and age range 36~78 years) were enrolled in the control group of this nonrandomized clinical study from January 2004 through May 2005.
Table 1.
Characteristics of enrolled patients with advanced NSCLC
| Characteristics | Value |
Statistic analysis | |
| Combo (n=19) | Control (n=39) | ||
| Age (year) | 39~80 | 36~78 | NS |
| Sex, M/F | 15/4 | 25/14 | NS |
| Histology | NS | ||
| Squamous | 9 | 18 | |
| Adenocarcinoma | 7 | 16 | |
| NSCLC (large cell, adenosquamous) | 2 | 5 | |
| UICC TNM classification | NS | ||
| Stages I & II | 0 | 0 | |
| Stage IIIa | 7 (36.8%) | 13 (33.3%) | |
| Stage IIIb | 5 (26.3%) | 10 (25.6%) | |
| Stage IV | 7 (36.8%) | 16 (41.0%) | |
M: male; F: female; NSCLC: non-small-cell lung cancer; UICC: International Union Against Cancer; TNM: T, size of tumor; N, regional lymph nodes that are involved; M, presence of metastasis; NS: no statistical difference by χ 2 test
The treatment regimen of the combo group was summarized in Table 2. Patients in the control group received repeated BAI with an interval of minimum 30 d for a total of 113 times, while in the combo group the patients received totally 46 times of BAI and a total of 49 times of rAd-p53 gene transfer, of which 9 patients received 26 times of rAd-p53 treatment via percutaneous intratumoral injection, another 9 patients received 19 times of rAd-p53 gene transfer via bronchial artery, and 1 patient (patient No. 5) was treated with rAd-p53 through both delivery approaches (1 time of intratumoral injection and 3 times of bronchial artery access) since his tumor was completely regressed after the 1 time of intratumoral injection. Additionally, patient Nos. 6 and 8 in the combo group received rAd-p53 transfer into the right subclavicular lymph nodes and the right thoracic cavity, respectively, in the course of percutaneous gene transfer. We were able to assess toxic effects on all patients, and in all the 58 patients, we evaluated disease response to the treatment. The date of the last follow-up was Nov. 15, 2005, with at least 12 months of follow-up from the start point of each patient except the censored patients.
Table 2.
Characteristics, treatment times, and local responses of the gene-receiving patients
| Patient No. | Gender | Age (year) | Histology | Stage | Gene transfer access | rAd-p53 |
BAI times | Local response | TTP (month) | Survival duration (month) | |
| Time | Dose (×1012 VP) | ||||||||||
| 1 | M | 40 | Adeno | IIIa | TA | 2 | 2 | 3 | SD | 9 | 12 |
| 2 | M | 56 | Squam | IIIb | TA | 2 | 1 | 2 | SD | 6 | 8 |
| 3 | M | 39 | Adeno | IIIa | PI | 3 | 2 | 3 | PD | 1 | 2 |
| 4 | M | 51 | Large cell | IIIb | PI | 2 | 2 | 2 | PR | 10 | 12 |
| 5 | M | 53 | Squam | IIIa | PI/TA | 4 | 3 | 4 | CR | 13 | 12 |
| 6 | M | 71 | Adeno | IV | PI & TC | 2 | 2 | 1 | SD | 5 | 9 |
| 7 | M | 80 | Adeno | IV | PI | 2 | 1 | 2 | SD | 6 | 7 |
| 8 | M | 48 | Adeno | IIIb | PI & LN | 2 | 3 | 3 | PR | 7 | 12 |
| 9 | F | 56 | Squam | IV | TA | 2 | 4 | 4 | SD | 4 | 7 |
| 10 | F | 72 | Large cell | IV | PI | 3 | 3 | 2 | PD | 2 | 3 |
| 11 | M | 74 | Squam | IV | PI | 1 | 1 | 2 | SD | 5 | 6 |
| 12 | M | 61 | Adeno Squam | IIIb | PI | 4 | 1 | 2 | PR | 7 | 11 |
| 13 | F | 54 | Adeno | IIIa | TA | 2 | 3 | 3 | PR | 9 | 12 |
| 14 | F | 53 | Adeno | IV | TA | 3 | 2 | 3 | PR | 6 | 8 |
| 15 | M | 54 | Squam | IIIb | TA | 4 | 2 | 2 | CR | 13 | 12 |
| 16 | M | 59 | Squam | IIIa | PI | 4 | 2 | 2 | PR | 11 | 12 |
| 17 | M | 79 | Squam | IV | TA | 2 | 1 | 2 | PR | 7 | 12 |
| 18 | M | 46 | Squam | IIIa | TA | 3 | 2 | 3 | PR | 8 | 12 |
| 19 | M | 68 | Squam | IIIa | TA | 2 | 2 | 1 | PR | 10 | 12 |
M: male; F: female; Adeno: adenocarcinoma; Squam: squamous; TA: transarterial infusion; TC: thoracic cavity; PI: percutaneous injection; LN: lymph nodes; BAI: bronchial arterial infusion; SD: stable disease; PD: progressive disease; PR: partial response; CR: complete response; TTP: time to progression
Adverse events
Within the two groups, grades of treatment related toxicities were summarized in Table 3 according to the World Health Organization (WHO) criteria (Ajani et al., 1990). No treatment-related deaths occurred. Patients reported grade 3 (severe) adverse events and no grade 4 (life-threatening) events. All the patients did not have major complications such as spinal marrow infarction or thoracic skin injury except for hematoma in the puncture sites after receiving bronchial artery injection of rAd-p53 or anti-tumor drugs.
Table 3.
The highest grade adverse events observed in the combo regiment and the control regiment during treatment period
| Adverse events | Patient number |
P | |||||||
| Combo regiment |
Control regiment |
||||||||
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 0 | Grade 1 | Grade 2 | Grade 3 | ||
| Anorexia | 10 | 6 | 3 | 0 | 9 | 19 | 9 | 2 | 0.038 |
| Arthralgia | 6 | 9 | 4 | 0 | 32 | 6 | 1 | 0 | 0.000 |
| Constipation | 15 | 3 | 1 | 0 | 32 | 5 | 2 | 0 | 0.789 |
| Diarrhea | 13 | 4 | 2 | 0 | 30 | 8 | 1 | 0 | 0.416 |
| Fever | 3 | 6 | 10 | 0 | 26 | 10 | 3 | 0 | 0.000 |
| Influenza-like symptom | 6 | 8 | 5 | 0 | 32 | 5 | 2 | 0 | 0.000 |
| Myalgia | 5 | 10 | 4 | 0 | 33 | 4 | 2 | 0 | 0.000 |
| Nausea and emesis | 7 | 10 | 2 | 0 | 6 | 11 | 12 | 10 | 0.001 |
| Pain | 10 | 5 | 4 | 0 | 9 | 13 | 10 | 7 | 0.016 |
| HGB | 7 | 9 | 3 | 0 | 14 | 15 | 10 | 0 | 0.644 |
| WBC | 15 | 3 | 1 | 0 | 16 | 13 | 8 | 2 | 0.006 |
| PLT | 16 | 2 | 1 | 0 | 16 | 13 | 8 | 2 | 0.085 |
| TB | 14 | 4 | 1 | 0 | 21 | 11 | 5 | 2 | 0.115 |
| ALT | 14 | 3 | 2 | 0 | 23 | 13 | 2 | 1 | 0.359 |
| Crea | 17 | 1 | 1 | 0 | 31 | 4 | 2 | 2 | 0.338 |
| Albuminuria | 17 | 1 | 1 | 0 | 28 | 7 | 3 | 1 | 0.142 |
| Hematuria | 18 | 1 | 0 | 0 | 35 | 3 | 1 | 0 | 0.518 |
Wilcoxon Rank test involves comparisons of differences between measurements of the two groups. HGB: hemoglobin; WBC: white blood cell; PLT: platelets; TB: total bilirubin; ALT: alanine aminotransferase; Crea: creatine
In the combo group, patients had higher grades of adverse events including fever (patient Nos. 10 and 6 with WHO grades 2 and 1, respectively), influenza-like symptoms (patient Nos. 5 and 8 with WHO grades 2 and 1, respectively), arthralgia (patient Nos. 4 and 9 with WHO grades 2 and 1, respectively), and myalgia (patient Nos. 4 and 10 with WHO grades 2 and 1, respectively), whereas patients in the control group had higher grades of anorexia (patient Nos. 2, 9, and 19 with WHO grades 3, 2, and 1, respectively), nausea and emesis (patient Nos. 10, 12, and 11 with WHO grades 3, 2, and 1, respectively), leukopenia (patient Nos. 12, 8, and 13 with WHO grades 3, 2 and 1, respectively) and pain (patient Nos. 7, 10, and 13 with WHO grades 3, 2, and 1, respectively). In the remaining items such as constipation, diarrhea, and hemoglobin (HGB), there were no statistical differences, with no patient in the combo group suffering from adverse events in grades higher than WHO grade 2, and only two patients in the control group experiencing WHO grade 3 adverse events. A substantial majority of patients in the combo group (68.4%) reported some flu-like symptoms, including asthenia and/or chills. No patients discontinued the treatment due to the flu-like symptoms. The combination of rAd-p53 and BAI therapies did not appear to increase toxicity according to data in Table 3.
For the 10 patients receiving gene transfer via percutaneous injection, the puncture-associated complication was pneumothorax, occurring 6 times in 27 sessions of puncture procedures. All were satisfactorily managed with such symptom treatments as sedation, inhalation of oxygen, antibiotics, and percutaneous catheter was rarely needed. Of the 10 patients receiving intratumoral injection of rAd-p53, 40% reported mild injection site pain, which in most of the cases lasted for 5 h or less.
Overall anti-tumor efficacy
Within the treatment course, CT scan was repeated with intervals of at least 1 month and the lesions were evaluated according to RECIST (Therasse et al., 2000). For each patient, the maximum efficacy by CT follow-up was documented and evaluated referring to data of the baseline. Treatment shrank the tumors in 12 of the 19 cases evaluated in the combo group (Figs.1~3). We documented objective response (decrease of 50% or more) of the tumors in 47.3% (9/19) of patients in the combo group and 38.4% (15/39) in the control group. The local responses included 2 complete responses (CRs), 7 partial responses (PRs), 8 stable diseases (SDs), and 2 progressive diseases (PDs) in the combo group and 0 CR, 15 PRs, 18 SDs, and 6 PDs in the control group, respectively (P=0.333, Wilcoxon rank test, Table 4). Of the 9 patients receiving gene therapy via arterial infusion, 2 had CR, 4 had PR, 3 had SD and none had PD, while in the other 11 patients receiving intratumoral injection (patient No. 5 received both transfer methods), there were 1 CR, 3 PRs, 5 SDs, and 2 PDs. In the combo group, patients at stage IIIa or IIIb did not have better efficacy than those at stage IV (P=0.187, Kruskal-Wallis rank sum test, Table 5).
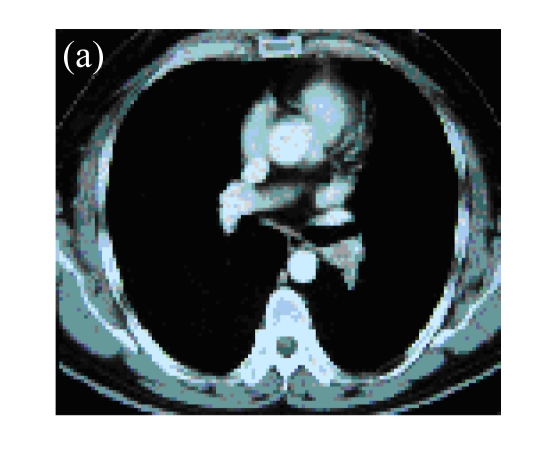
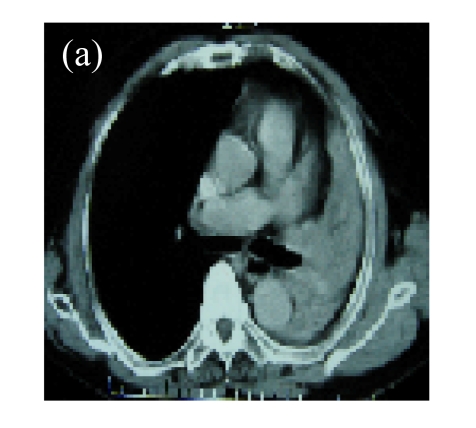
Fig. 1.
(a) A 45-year-old male with squamous cancer in the left lung confirmed by bronchoscopy. The tumor lesion was behind the left main bronchus; (b) Percutaneous puncture under guidance of CT. A total of 3×1012 VPs (Gendicine®) were injected by a fine needle in a fashion of multiple sites; (c) One month later, CT image of the same slice showed disappearance of the lesion
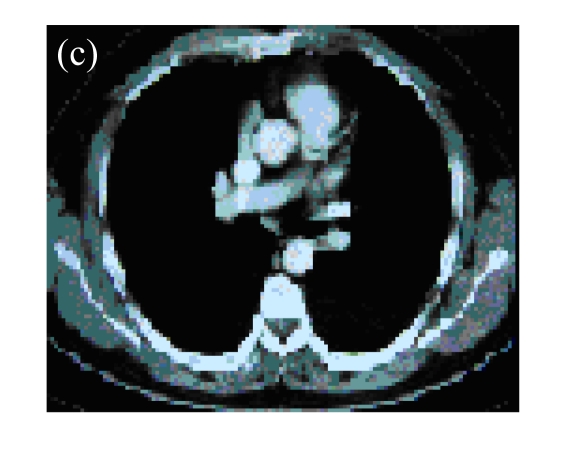
Fig. 3.
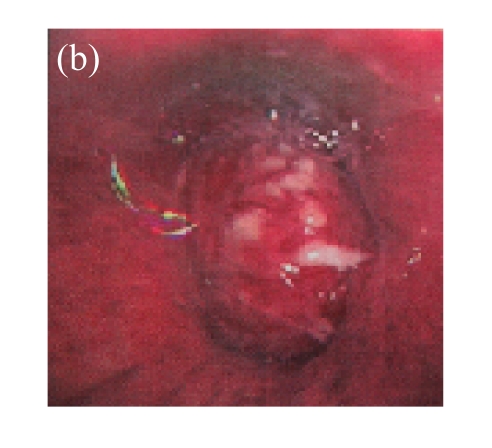
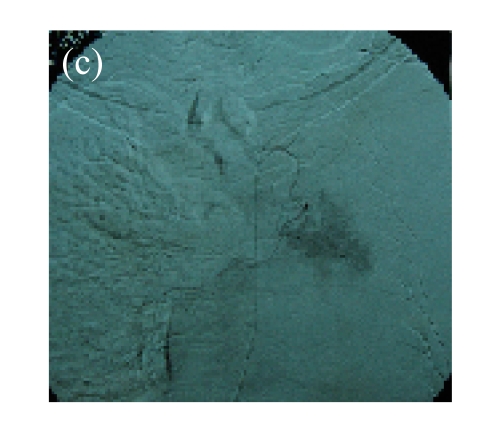
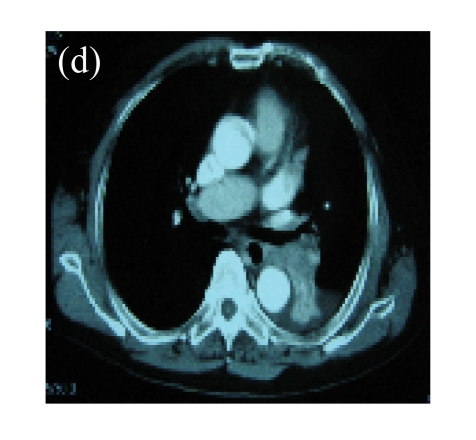
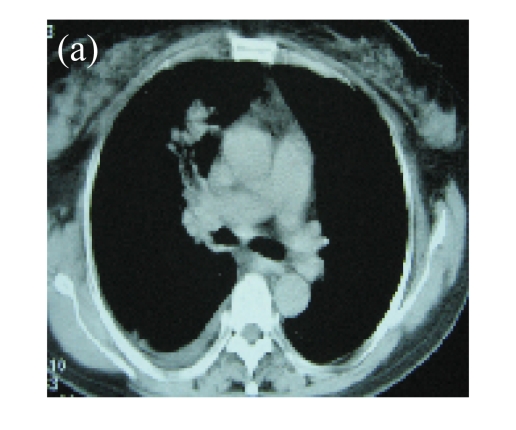
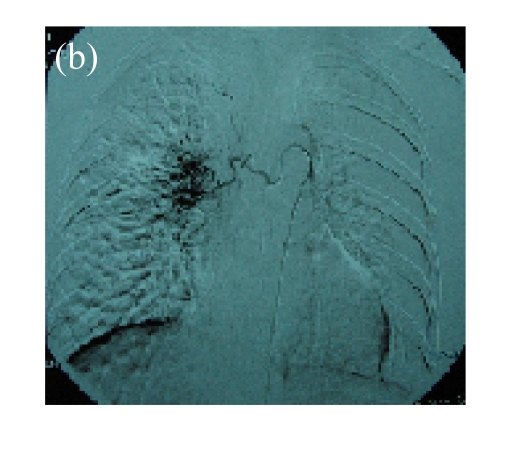
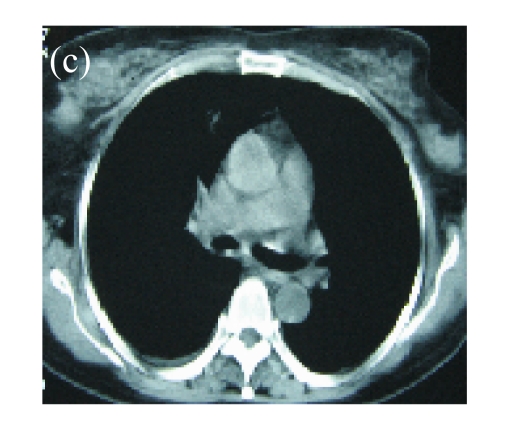
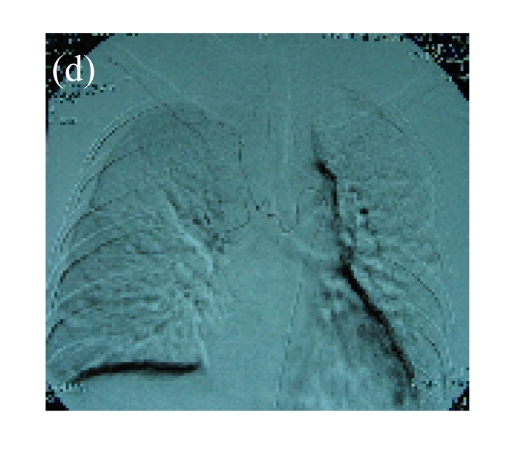
(a) A 68-year-old male with the left central-type lung squamous cancer confirmed by bronchoscopy. CT scan showed complete atelectasis of the left lung with obvious shift of mediastinum into the left chest; (b) Bronchoscopy identified a neoplasm obstructing left bronchial cavity; (c) BAI demonstrated the abnormal tumoral vascularity; (d) After one session of rAd-p53 gene arterial infusion in conjunction with BAI, enhancement CT showed restoration of mediastinum and the left lung recruitment except for superior segment of the left lower lung. Also, a mass behind the left main bronchus was identified
Table 4.
Local responses evaluated by RECIST criteria after treatment
| Group | Patient number |
Effective rate (%)* | ||||
| Total | CR | PR | SD | PD | ||
| Combo | 19 | 2 | 7 | 8 | 2 | 47.3 |
| Control | 39 | 0 | 15 | 18 | 6 | 38.4 |
P=0.333, Wilcoxon rank test;
Effective rate=(patient numbers of CR and PR)/(total patient number)
Table 5.
Local responses by different tumor stages in the combo group
| Patients’ response | Patient number |
||
| Stage IIIa | Stage IIIb | Stage IV | |
| CR+PR | 5 | 4 | 2 |
| PD | 1 | 1 | 4 |
| SD | 1 | 0 | 1 |
P=0.187, Kruskal-Wallis rank sum test
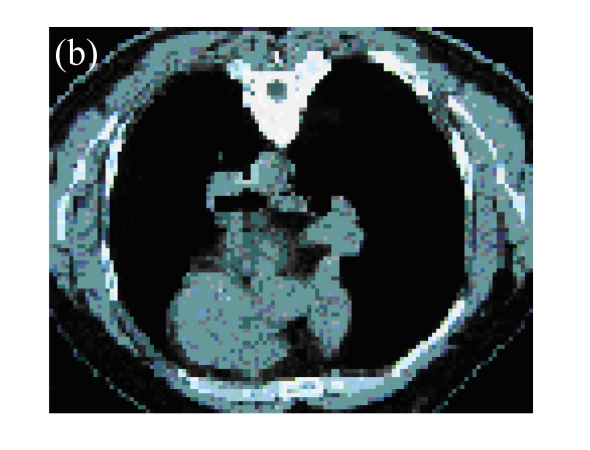
Fig. 2.
(a) A 56-year-old female with squamous cancer in the right lobe. Lesion was located in anterior segment of the right lung around the right hilus; (b) Bronchial arterial angiography showed the abnormal vascularity of tumor; (c) One month after two circles of rAd-p53 gene arterial infusion (3×1012 VPs per time) in combination with BAI, the CT scan found that the lesions in anterior segment and around the hilus were significant regression; (d) The third BAI showed the significant reduction of tumor vascularity
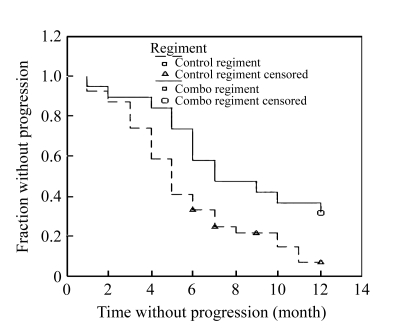
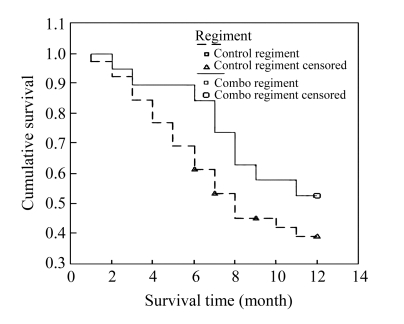
Each patient was followed up for at least 12 months and the TTP and survival time were documented. When the follow-up ended on Nov. 15, 2005, no patients in the combo group quit the follow-up, except three in the control group. The median TTP in the combo group and the control group was 7.75 and 5.5 months, respectively. Moreover, the TTP curves of the two groups demonstrated that the patients in the combo group had a longer TTP than those in the control group (P=0.018, Log-Rank test, Fig.4). The 1-year survival analysis showed that there was no statistical difference between the two groups (P=0.224, Log-Rank test, Fig.5), although the survival rates of 3, 6, and 12 months were 94.74%, 89.47%, and 52.63% in the combo group and 92.31%, 69.23%, and 38.83% in the control group, respectively.
Fig. 4.
Time to progression (TTP) analysis showed that the patients in the combo regiment had a longer time without progression (P=0.018, Log-Rank test)
Fig. 5.
Survival curve showed that there was no statistical difference between survival rates in the two regiments (P=0.224, Log-Rank test)
DISCUSSION AND CONCLUSION
The loss of normal p53 function is found in the majority of human cancers, and about 50% of this gene is mutated somatically or deleted in various human cancers (Hollstein et al., 1994). As a tumor suppressor, p53 acts to restrict proliferation in response to DNA damage or deregulation of mitogenic oncogenes by regulation of the expression of p53 target genes responsible for cellular responses such as growth arrest, senescence, or apoptosis. On the other hand, loss of this tumor suppressor function increases cell proliferation and survival, and in some circumstances promotes genomic instability and resistance to certain chemotherapies (Lowe, 1995; Vogelstein and Kinzler, 1995; Levine, 1997).
p53 mutation was demonstrated in rates from 45.40% to 50% (Takahashi et al., 1989; Junker et al., 2000) of investigated lung cancer. Gene therapy in which wild-type p53 gene is transferred into tumors that are deficient in functional p53 has shown a significant tumor suppressing efficacy both in lung cancer cell line and in animal systems (Inoue et al., 2000; Osaki et al., 2000; Kawabe et al., 2001). Roth et al.(1996) firstly reported that in a clinical setting, the replacement of a tumor suppressor gene was shown to mediate tumor regression and, more importantly, vector-related toxicity was minimal. After which, many clinical trials of rAd-p53 gene in combination with systemic chemotherapy or ionizing radiation for NSCLC were completed, and encouraging outcomes were reported (Swisher et al., 1999; 2003; Nemunaitis et al., 2000; Nishizaki et al., 2001), while still studies manifested negative results (Junker et al., 2000; Schuler et al., 2001).
In these studies, the transfer of rAd-p53 was usually performed by transthoracic CT-guided injection or bronchoscopy, and combined anticancer modalities were involved, such as systemic chemotherapy or radiation. Gene transfer and p53 transgene expression in the injected tumors as well as the safety and feasibility of this type of gene therapy have been documented. However, it was also reported that rAd-p53 gene transfer in conjunction with chemotherapy provided no additional benefit in patients with advanced NSCLC (Schuler et al., 2001).
rAd-p53 (Gendicine®) has been approved in China for the treatment of patients with HNSCC. In combination with chemo- and radiotherapy, it improved treatment efficacy more than 3-fold (Chen et al., 2003). Although its recommended indications are limited in HNSCC according to the specification, we have preliminarily applied this drug in a patient with hepatocellular carcinoma (HCC) and good efficacy was achieved in a previous study (Guan et al., 2005). In the current study, rAd-p53 (Gendicine®) was used in NSCLC to evaluate the effect and to provide some evidence for its off-label use in NSCLC.
In the present study, all patients were in stage III or IV NSCLC and could not be surgically cured. They all preferred BAI to other therapies such as irradiation or chemotherapy. They were all informed of what is about rAd-p53 gene therapy, and 19 of them were willing to receive the gene therapy and enrolled into the combo group.
In the present study, we did not examine the expression of p53 gene in the targeted tumor tissue and performed the gene transfer access via bronchial artery, because many previous studies had confirmed conclusively the forced overexpression of p53 by both retroviral and adenoviral vectors, and confirmed that the expression of p53 is associated with marked increase in the apoptotic fraction of NSCLC cells in biopsies 72 h after injection of rAd-p53 (Roth et al., 1996; Swisher et al., 1999; Nemunaitis et al., 2000). These studies also confirmed that a marked increase of p53 in the induction of expression of several genes (MDM2, p21, and BAK) closely attributed to p53 gene transfer (Swisher et al., 2003).
As shown in Table 3, patients in the combo group had higher degrees of fever, influenza-like symptom, myalgia, and arthralgia, which were well tolerated by the patients in the present study and also frequently encountered in other studies (Schuler et al., 2001; Swisher et al., 2003). In our study, the fever usually ranged 38~39.5 °C, starting about 3~5 h after rAd-p53 administration and lasting for 5~10 h. This self-limited fever can be well managed with symptom treatment, and steroids were rarely used. It was considered a reflection of the effectiveness of rAd-p53 (Gendicine®) in mobilizing the body’s immune systems (Peng, 2005). Also, it was noticed that the patients in the combo group had lower degrees of adverse events such as anorexia, nausea, emesis, pain, and white blood cell (WBC) count decrease than those in the control group, suggesting that rAd-p53 gene therapy could decrease chemodrugs’ toxicities and improve patients’ life quality. Except for these differences, there was no statistical difference between the two groups in the highest degrees of adverse events within the treatment period. As to pneumothorax in the patients receiving intratumoral injection transthorax, its incidence mainly depended on the operator’s skill and tumor size. If a lesion was not large enough or was far from the thoracic wall, this procedure could take higher risks and should be transferred to other access. It can be concluded that p53 gene transfer did not increase the adverse events when used in combination with BAI and was well tolerated by late-stage NSCLC patients.
Previously, rAd-p53 gene therapy protocols were mainly combined with radiotherapy or systemic chemotherapy, whereas in this study rAd-p53 gene transfer was in conjunction with BAI for the treatment of advanced NSCLC in order to explore another possible management. Although statistical analysis showed no significant difference in local response between the two groups, encouraging outcome was obtained: percentage of objective response (CR and PR accounted for 47.3%, with 2 CRs) in the combo group was higher than that in the control group (38.4%). However, small sample number of this study could be attributed to no statistical difference, which warrants a larger scale study in future to see if gene transfer improves BAI efficacy or not.
In addition, the percentage of cases with objective response (47.3%) can be favorably compared with that in other studies. Schuler et al.(2001) reported that 13 of 25 (52%) patients with advanced NSCLC, experienced objective response after the treatment of intratumoral injection of rAd-p53 in combination with systemic chemotherapy. And Swisher et al.(2003) reported 1 CR, 11 PRs, 3 SDs, and 2 PDs after 19 patients with non-metastatic NSCLC received radiation therapy up to 60 Gy over 6 weeks in conjunction with three intratumoral injection of rAd-p53. The higher objective response (73.7%) in Swisher’s patients could result from the relatively good condition in enrolled patients.
When it came to overall response, there was no statistical difference between the two survival curves (Fig.5), whereas the TTP analysis has demonstrated that patients in the combo group had significantly longer TTP (P=0.018, Log-Rank test), which means that they benefited from the synergistic interactions of rAd-p53 gene and BAI, with life quality improved. The patients in the combo group could have better clinical condition. On the other hand, the 12-month survival rate of 52.3% in the combo group is comparable with those in other clinical trials of gene therapy, in which 1-year survival rates ranged from 44% to 47% (Schuler et al., 2001; Swisher et al., 2003). The negative results could be due to the relatively short follow-up time.
In performance of gene therapy, effective transfer is a key point. Injection of vectors into the bloodstream for treatment of cancer requires not only that the vectors are targeted to enter tumor cells, but also that they are protected from degradation, sequestration, or immune attack for long periods of time so that they can reach their appropriate sites of action and penetrate into the tumor. Thus far, however, no such systemically targeted vectors exist clinically (Moon et al., 2003). Therefore, the most frequently used transfer access in clinical trials is intratumoral injection. However, sometimes this access can become technically impossible or give rise to a large risk especially when the patient is in poor condition or the lesions are too small or sporadically distributed. So, in the current study, we also preliminarily examined the possibility of gene transfer via a novel bronchial artery access, and promising local response (2 CRs, 4 PRs, 3 SDs, and 0 PD in 9 patients) was demonstrated. Still, there is a need to use real-time polymerase chain reaction (PCR) or enzyme-linked immunosorbent assay (ELISA) to confirm the expression of targeted gene in tumor tissue. However, it is clearly required that this kind of gene transfer access be refined further and systemic vector delivery system is still expected to appear (Swisher and Roth, 2002).
It is suggested that there were favorable p53 and unfavorable p53 patients for the p53 gene therapy. Activation of p53 can have beneficial (DNA repair) or detrimental (apoptosis) consequences for individual cells. Although local injection is regarded as the optimal access, there are concerns about systemic use of p53 treatment; that is, it may be harmful to normal cells or it may have senescence effect on the organism (Bauer and Helfand, 2006).
In conclusion, the current clinical study preliminarily demonstrated that the rAd-p53 gene transfer via intratumoral injection or bronchial arterial infusion in combination with BAI can be well tolerated by patients with advanced NSCLC and can provide additional benefits of better life quality and longer TTP. rAd-p53 gene transfer via arterial infusion can be a feasible access and be used clinically. However, further studies for arterial infusion access of gene transfer and for combination of gene transfer with BAI are needed, and other strategies such as oncolytic adenovireses are warranted (Ko et al., 2005).
References
- 1.Ajani JA, Welch SR, Raber MN. Comprehensive criteria for assessing therapy-induced toxicity. Cancer Invest. 1990;8(2):147–159. doi: 10.3109/07357909009017560. [DOI] [PubMed] [Google Scholar]
- 2.Bauer JH, Helfand SL. New tricks of an old molecule: lifespan regulation by p53. Aging Cell. 2006;5(5):437–440. doi: 10.1111/j.1474-9726.2006.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CB, Pan JJ, Xu LY. Recombinant adenovirus-p53 agent injection combine with radiotherapy in treatment of nasopharyngeal carcinoma: a phase. II. Clinical trial. Zhonghua Yi Xue Za Zhi. 2003;83(23):2033–2035. (in Chinese) [PubMed] [Google Scholar]
- 4.Daniel JC, Smythe WR. Gene therapy of lung cancer. Semin Surg Oncol. 2003;21(3):196–204. doi: 10.1002/ssu.10038. [DOI] [PubMed] [Google Scholar]
- 5.Frasci G, Panza N, Comella G, Pcillio G. Is there any impact of new drugs on the outcome of advanced NSCLC? An overview of the Southern Italy Cooperative Oncology Group trials. Oncologist. 1999;4(5):379–385. [PubMed] [Google Scholar]
- 6.Guan YS, Liu Y, Zhou XP, Li X, He Q, Sun L. p53 gene (Gendicine) and embolization overcame recurrent hepatocellular carcinoma. Gut. 2005;54(9):1318–1319. doi: 10.1136/gut.2005.069237. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sϕrlie T, Hovig E, Smith-sϕrensen B, Montesano R, Harris CC. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994;22(17):3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue A, Natumi K, Matsubrara N, Sugawara S, Saijo Y, Satoh K, Nukiwa T. Administration of wild-type p53 adenoviral vector synergistically enhances the cytotoxicity of anti-cancer drugs in human lung cancer cells irrespective of the status of p53 gene. Cancer Lett. 2000;157(1):105–112. doi: 10.1016/S0304-3835(00)00480-8. [DOI] [PubMed] [Google Scholar]
- 9.Junker K, Wiethege T, Muller KM, Thomas M. p53 tumour-suppressor gene in non-small-cell lung cancer with neoadjuvant therapy. J Cancer Res Clin Oncol. 2000;126(4):238–245. doi: 10.1007/s004320050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauczor HU, Schuler M, Heussel CP, von Weymarn A, Bongartz G, Rochlitz C, Huber C, Thelen M. CT-guided intratumoral gene therapy in non-small-cell lung cancer. Eur Radiol. 1999;9(2):292–296. doi: 10.1007/s003300050670. [DOI] [PubMed] [Google Scholar]
- 11.Kawabe S, Munshi A, Zumstein LA, Wilson DR, Roth JA, Meyn RE. Adenovirus-mediated wild-type p53 gene expression radiosensitizes non-small cell lung cancer cells but not normal lung fibroblasts. Int J Radiat Biol. 2001;77(2):185–194. doi: 10.1080/09553000010008540. [DOI] [PubMed] [Google Scholar]
- 12.Ko D, Hawkins L, Yu DC. Development of transcriptionally regulated oncolytic adenoviruses. Oncogene. 2005;24(52):7763–7774. doi: 10.1038/sj.onc.1209048. [DOI] [PubMed] [Google Scholar]
- 13.Le Chevalier T, Arriagada R, Quoix E. Radiotherapy alone versus combined chemotherapy and radiotherapy in non-resectable non-small cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991;83(6):417–423. doi: 10.1093/jnci/83.6.417. [DOI] [PubMed] [Google Scholar]
- 14.Levine AJ. p53 the cellular gatekeeper for growth and division. Cell. 1997;88(3):323–331. doi: 10.1016/S0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 15.Lowe SW. Cancer gene therapy and p53. Curr Opin Oncol. 1995;7(6):547–553. doi: 10.1097/00001622-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Moon C, Oh Y, Roth JA. Current status of gene therapy for lung cancer and head and neck cancer. Clin Cancer Res. 2003;9(14):5055–5067. [PubMed] [Google Scholar]
- 17.Nemunaitis J, Swisher SG, Timmons T, Connors D, Mack M, Doerksen L, Weill D, Wait J, Lawrence DD, Kemp BL, et al. Adenovirus-mediated p53 gene transfer in sequence with cisplatin to tumors of patients with non-small-cell lung cancer. J Clin Oncol. 2000;18(3):609–622. doi: 10.1200/JCO.2000.18.3.609. [DOI] [PubMed] [Google Scholar]
- 18.Nishizaki M, Meyn RE, Levy LB, Atkinson EN, White RA, Roth JA, Ji L. Synergistic inhibition of human lung cancer cell growth by adenovirus-mediated wild-type p53 gene transfer in combination with docetaxel and radiation therapeutic in vitro and in vivo. Clin Cancer Res. 2001;7(9):2887–2897. [PubMed] [Google Scholar]
- 19.Osaki S, Nakanishi Y, Takayama K, Pei XH, Ueno H, Hara N. Alteration of drug chemosensitivity caused by the adenovirus-mediated transfer of the wild-type p53 gene in human lung cancer cells. Cancer Gene Ther. 2000;7(2):300–307. doi: 10.1038/sj.cgt.7700096. [DOI] [PubMed] [Google Scholar]
- 20.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin. 1997;47(1):5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 21.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16(9):1016–1027. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 22.Roth JA, Nguyen D, Lawrence DD, Kemp BL, Carrasco CH, Ferson DZ, Hong WK, Komaki R, Lee JJ, Nesbitt JC, et al. Retrovirus-mediated p53 gene therapy. Nat Med. 1996;2(9):985–991. doi: 10.1038/nm0996-985. [DOI] [PubMed] [Google Scholar]
- 23.Schaake-koning C, van den Boggert W, Dalesio O, Festen J, Hoogenhout J, van Houtte P, Kirpatrick A, Koolen M, Maat B, Nijs A. Effects of concomitant cisplatin and radiotherapy on inoperable non-small cell lung cancer. N Engl J Med. 1992;326(8):524–530. doi: 10.1056/NEJM199202203260805. [DOI] [PubMed] [Google Scholar]
- 24.Schuler M, Herrmann R, de Greve JL, Stewart AK, Gatzemeier U, Stewart DJ, Laufman L, Gralla R, Kuball J, Buhl R, et al. Adenovirus-mediated wild-type p53 gene transfer in patients receiving chemotherapy for advanced non-small-cell lung cancer: results of a multicenter phase II study. J Clin Oncol. 2001;19(6):1750–1758. doi: 10.1200/JCO.2001.19.6.1750. [DOI] [PubMed] [Google Scholar]
- 25.Swisher SG, Roth JA. Clinical update of Ad-p53 gene therapy for lung cancer. Surg Oncol Clin N Am. 2002;11(3):521–535. doi: 10.1016/S1055-3207(02)00028-5. [DOI] [PubMed] [Google Scholar]
- 26.Swisher SG, Roth JA, Nemunaitis J, Lawrence DD, Kemp BL, Carrasco CH, Connors DG, El-naggar AK, Fossella F, Glisson BS, et al. Adenovirus-mediated p53 gene transfer in advanced nonsmall-cell lung cancer. J Natl Cancer Inst. 1999;91(9):763–771. doi: 10.1093/jnci/91.9.763. [DOI] [PubMed] [Google Scholar]
- 27.Swisher SG, Roth JA, Komaki R, Gu J, Lee JJ, Hicks M, Ro JY, Hong WK, Merritt JA, Ahrar K, et al. Induction of p53-regulated genes and tumor regression in ling cancer patients after intratumoral delivery of adenoviral p53 (INGN 201) and radiation therapy. Clin Cancer Res. 2003;9(1):93–101. [PubMed] [Google Scholar]
- 28.Takahashi T, Nau MM, Chiba I, Birrer MJ, Rosenberg RK, Vinocour M, Levitt M, Pass H, Gazadr AF, Minna JD. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989;246(4929):491–499. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- 29.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, van Glabbeke M, van Oostrom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumor. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 30.Vogelstein B, Kinzler KW. Through the glass lightly. Science. 1995;267(5204):1613. doi: 10.1126/science.267.5204.1613-b. [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Song M, Xu H, Fang Y. Prospective trial of combined hyperfractionated radiotherapy and bronchial arterial infusion of chemotherapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1996;34(2):309–313. doi: 10.1016/0360-3016(95)02111-6. [DOI] [PubMed] [Google Scholar]