Abstract
Objective: Large segmental bone defect repair remains a clinical and scientific challenge with increasing interest focusing on combining gene transfection with tissue engineering techniques. The aim of this study is to investigate the effect of connective tissue growth factor (CTGF) on the proliferation and osteogenic differentiation of the bone marrow mesenchymal stem cells (MSCs). Methods: A CTGF-expressing plasmid (pCTGF) was constructed and transfected into MSCs. Then expressions of bone morphogenesis-related genes, proliferation rate, alkaline phosphatase activity, and mineralization were examined to evaluate the osteogenic potential of the CTGF gene-modified MSCs. Results: Overexpression of CTGF was confirmed in pCTGF-MSCs. pCTGF transfection significantly enhanced the proliferation rates of pCTGF-MSCs (P<0.05). CTGF induced a 7.5-fold increase in cell migration over control (P<0.05). pCTGF transfection enhanced the expression of bone matrix proteins, such as bone sialoprotein, osteocalcin, and collagen type I in MSCs. The levels of alkaline phosphatase (ALP) activities of pCTGF-MSCs at the 1st and 2nd weeks were 4.0- and 3.0-fold higher than those of MSCs cultured in OS-medium, significantly higher than those of mock-MSCs and normal control MSCs (P<0.05). Overexpression of CTGF in MSCs enhanced the capability to form mineralized nodules. Conclusion: Overexpression of CTGF could improve the osteogenic differentiation ability of MSCs, and the CTGF gene-modified MSCs are potential as novel cell resources of bone tissue engineering.
Keywords: Mesenchymal stem cells (MSCs), Connective tissue growth factor (CTGF), Osteogenic differentiation, Osteoblasts, Overexpression, Gene modification
INTRODUCTION
Reconstruction of large skeletal defects is still a challenge for orthopaedic, oral and maxillofacial surgeons. Regeneration with either autograft or allograft provides excellent results, but it is limited by donor-site morbidity and supply. Bone tissue engineering might be a novel way to solve these problems (Rose and Oreffo, 2002; Kofron and Laurencin, 2006; Kanczler and Oreffo, 2008). Based on the principle of bone tissue engineering, seeding cells, scaffolds, and signaling molecules are administered, individually or in combination, to regenerate new bone. Mesenchymal stem cells (MSCs), for both self renewal and being multipotent, have been regarded as the most hopeful cell sources for bone tissue engineering and regenerative medicine (Abreu et al., 2002; Howard et al., 2008; Slater et al., 2008; Xiang et al., 2007). To enhance the proliferation and orientational differentiation ability of stem cells by gene modification is hot in bone tissue engineering (Mauney et al., 2005; Goessler et al., 2006; Meijer et al., 2007).
Bone marrow MSCs are attractive in bone tissue engineering, for it can be easily isolated and further modified by gene modification (Pelled et al., 2002; Pountos et al., 2007; Slater et al., 2008). Many genes had been regulated or overexpressed in MSCs to improve their ability of differentiation into osteoblastic cells. These genes include Runx2, bone morphogenetic protein, fibroblastic growth factor-2 (FGF-2), and so on. Connective tissue growth factor (CTGF) is a secreted, extracellular matrix-associated signaling molecule that belongs to the CCN (CTGF, Cyr61/Cef10, and Nov) family (Nishida et al., 2007; Luft, 2008; Yamanaka et al., 2008). The CCN family of proteins is composed of several cysteine rich proteins with diverse functions including regulation of angiogenesis, cell adhesion, proliferation, skeletal development, tooth development, and apoptosis (Brigstock, 2003; Perbal et al., 2003; Rachfal and Brigstock, 2005). CTGF has mitogenic and chemotactic activities, and also stimulates synthesis of extracellular matrix components. CTGF is capable of promoting both the growth and the differentiation of most mesenchymal cells involved in endochondral ossification. This indicates that CTGF acts as a central driver in cartilage/bone growth and regeneration (Heng et al., 2006; Kanaan et al., 2006; Kubota and Takigawa, 2007; Ono et al., 2007). The importance of CTGF in skeletal development is demonstrated with the generation of CTGF knockout mice (Yamaai et al., 2005). CTGF ablation results in mice with misshappened skeleton, craniofacial abnormalities, and defects associated with endochondral ossification, and attributes to defects in cell proliferation, matrix formation, and remodeling during endochondral ossification. Growing evidence indicates that CTGF plays an essential role in the differentiation of osteoblasts and bone formation. However, little attention has been paid to the effect of CTGF on the proliferation and differentiation of MSCs. In this paper, we modified MSCs by transfection of a plasmid containing the full length of human CTGF cDNA, and further investigated the proliferation and differentiation of CTGF gene-modified MSCs.
MATERIALS AND METHODS
Isolation and culture of MSCs
The human MSCs were isolated and cultured as previously described (Lee et al., 2003; Duan et al., 2008; Jiang et al., 2008). Briefly, bone marrow was harvested from proximal femur samples removed during hip replacement surgery with the consent of patients and the approval by local Ethical Committee. Bone mononuclear cells (MNCs) were isolated from bone marrow aspirates by Percoll-gradient centrifugation and plated at 2×105 cells/cm2 in T-75 culture flasks. The non-adhered cells were removed after 24~48 h incubated at 37 °C in 5% CO2 atmosphere and adherent cells were further cultured. Upon 80% confluence, adherent cells were detached by 0.2% (w/v) trypsin-0.02% (v/v) ethylenediamine tetraacetic acid (EDTA, Sigma, USA), and then seeded at 8×103 cells/cm2 into T-75 flask. Cells used in subsequent experiments were between the 3rd and 7th passages.
MSCs were confirmed by adipogenic, chondrogenic, and osteogenic differentiation assays (Caterson et al., 2002; Beyer Nardi and da Silva Meirelles, 2006). Briefly, osteogenic differentiation was induced by culturing MSCs monolayer in osteogenic (OS) medium containing 10 mmol/L β-glycerol phosphate, 10−8 mol/L dexamethasone, and 50 mg/L ascorbic acid 2-phosphate. Mineralization was evaluated by alkaline phosphatase (ALP) staining and von Kossa staining. For chondrogenic differentiation, MSCs were centrifuged at 800×g for 5 min and cultured in the chondrogenic inductive medium (high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM), 1% (v/v) ITS+ (insulin, transferrin, selenious acid, plus linoleic acid and bovine serum albumin), 10−6 mol/L dexamethasone, 35 μg/ml praline, 0.17 mmol/L ascorbic acid 2-phosphate, and 5 ng/ml transforming growth factor-β1 (TGF-β1) for 21 d. Then frozen sections were obtained and immunostained for type II collagen. Adipogenic differentiation was induced by culturing MSCs monolayer in the adipogenic inductive medium (high-glucose DMEM, 0.5 mmol/L 3-isobutyl-1-methylxanthine, 10−6 mol/L dexamethasone, 100 μg/ml indomethacin, and 10 μg/ml insulin) for 21 d. Adipogenic induction was confirmed by oil red O staining.
Construction of CTGF-expressing vector
Plasmids containing full-length human CTGF cDNA were kindly provided by Prof. Tong-chuan He, Molecular Oncology Laboratory, University of Chicago Medical Center, USA. The full-length CTGF cDNA fragment was excised from the plasmid by digesting with restriction endonucleases, and then subcloned into HindIII/XhoI sites of pcDNA3.1(+) vector (Invitrogen, USA). The plasmid was confirmed by polymerase chain reaction (PCR), restriction endonucleases digestion, and DNA sequence. The resulting plasmid was named after pcDNA3.1-CTGF (pCTGF) containing the full-length of human CTGF cDNA fragment under the control of the cytomegalovirus (CMV) promoter.
Plasmid transfection
For plasmid transfection, MSCs were plated in six-well plates at a density of 2.5×105 cells/well. Transfection of MSCs was performed using Lipofectamine-2000 (Invitrogen, USA) according to the protocol provided by the manufacturer. Briefly, 2 μg purified pCTGF diluted in 100 μl of serum-free medium was mixed with 4 μg Lipofectamine-2000 diluted in 100 μl of serum-free medium. The DNA-Lipofectamine-2000 mixture was incubated for 30 min at room temperature. Then 800 μl of cell growth medium without fetal calf serum (FCS) was added into the mixed solution. When the cells reached 70% confluence, they were washed with serum-free growth medium, and the transfection solution was added. After incubated at 37 °C for 5 h, the transfection solution was replaced with normal cell growth medium. After 24 h, the cells were passaged and subjected to G418 (500 μg/ml) selection for 2 weeks (pCTGF-MSCs). Control cells were mock transfected with “empty” vector (mock-MSCs). The expression of CTGF was detected by Western blotting and real time PCR.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting
Culture supernatant was collected and precipitated with 10% (v/v) trichloroacetic acid (TCA). The proteins in the supernatant were then electrophoresed on 12% (v/v) SDS-PAGE. The separated proteins were transferred electrophoretically onto a polyvinylidene fluoride (PVDF) membrane using a semidry blotting apparatus (Multiphor II, Pharmacia Biotech, USA). Then the membrane was blocked with a Tris-buffered saline (TBS) solution containing 2% (w/v) skim milk at 37 °C for 5 h. Then the membrane was incubated with mouse anti-human CTGF monoclonal antibody (R&D, USA) diluted (1:500, v/v) in TBS-Tween 20 (TBST) solution containing 2% (w/v) skim milk at room temperature for 2 h. The membrane was washed 3 times with TBST solution and then incubated with horseradish peroxidase (HRP)-conjugated rabbit anti-human IgG diluted (1:2000, v/v) into TBST solution containing 2% (w/v) skim milk at 37 °C for 90 min. After washed by TBST solution, protein bands were visualized by the electrochemiluminescence (ECL) kit (Amersham Pharmacia Biotech, UK).
Cell proliferation assay
Cells were seeded into 96-well microplates at a density of 1×103 cells/well. During the continuous 7 d, cell proliferation was measured by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay every day. Briefly, 20 μl MTT (5 mg/ml) was added to each well and the microplate was further incubated at 37 °C for 4 h. After the supernatant was removed, 200 μl dimethyl sulfoxide (DMSO) was added into each well and the absorbance was examined by using an HTS 7000 Plus Bio Assay Reader (PerkinElmer Life Sciences, USA) at the wavelength of 570 nm.
Migration assay
The effect of CTGF on cell migration was measured by using transwell chamber described previously (Karp et al., 2005). In brief, identical numbers of untransfected MSCs, mock-MSCs or pCTGF-MSCs were plated into the bottom chamber of 12-transwell culture plates (Corning Costar, Cambridge, USA) individually. And about 2×104 cells of the untransfected MSCs were seeded into the upper chamber of transwell, which consists of collagencoated microporous membrane (8.0 μm). Then the plate containing transwells was placed back to the incubator. After incubated for 4 h, the upper chamber of the transwell was taken out and the unattached cells on the underside of the membrane were wiped by a damp cotton swab. The cells on the underside of the membrane were fixed in 10% (v/v) phosphate-buffered formalin for 10 min and stained with hematoxylin for 30 min. The stained membrane was then mounted onto a glass slide with Permount (Fisher Scientific, Pittsburgh, PA) and dried. The migrating cells were then observed under microscope and the cell number was counted from 10 random fields with 200× magnifications. The transwell assays were performed in duplicate under each experimental condition.
Quantitative real-time PCR analysis for the expressions of bone-related genes
Cells were washed twice in phosphate buffered saline (PBS), and total RNA of normal control MSCs, mock-MSCs, and pCTGF-MSCs was isolated on Days 3 and 14 after transfection by using TRIZOL Reagent (Invitrogen Life Technologies, NY, USA). Total RNA of MSCs induced by OS-medium was also extracted on Days 3 and 14 after induction. The concentration of extracted RNA was quantified by spectrophotometer (GeneQuant Pro; Amersham Biosciences, USA) at wavelengths of 260 and 280 nm. The quality of RNA was determined by agarose gel electrophoresis. Total RNA was reversely transcribed into first-strand cDNA by using the TaKaRa reverse transcriptase (RT)-PCR Kit (TaKaRa Bio. Inc., Shiga, Japan).
The first-strand cDNA products were used as templates. The expressions of bone-related genes were determined by SYBR green-based real-time PCR analysis using TaKaRa PrimeScript™ RT Regent Kit (TaKaRa Bio. Inc.). Each reaction contained 12.5 μl 2× SYBR Premix Ex Taq™, 1 μl forward primer, 1 μl reverse primer, 2 μl cDNA template, and ddH2O to a final volume of 25 μl per reaction. The sample was analyzed on the Bio-Rad iCycler iQ™ Multicolor Real-Time Detection System (Bio-Rad Laboratories Inc., Richmond, California, USA), while the RT-PCR was performed and fluorescence signal was detected simultaneously. The specific primers were displayed in Table 1. The PCR was performed as followings: denatured at 95 °C for 2 min at the beginning, then denatured at 95 °C for 5 s, annealing at 55~57 °C for 30 s, and extending at 72 °C for 20 s, for 30 cycles. The specificity of real-time PCR was verified by melting curve analysis (55~95 °C, with 0.5 °C increment each cycle), and further confirmed by resolving the PCR products on 1.5% (w/v) agarose gels. The baseline cycles and the threshold were automatically calculated by Bio-Rad iCycler software. In order to quantify the expression of each gene, standard curves were constructed using the purified, gradient diluted relevant PCR product. Gene expression was normalized by the expression of house keeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the same sample. The data represented the average fold changes with standard deviation. All experiments were carried out in triplicate.
Table 1.
Specific primers for real-time PCR with optimal annealing temperature
| Gene | Primer | Annealing temperature (°C) | GenBank No. |
| GAPDH | Forward: 5′-TGTGTCCGTCGTGGATCTGA-3′ | 57.4 | NM_001001303 |
| Reverse: 5′-TTGCTGTTGAAGTCGCAGGAG-3′ | 57.8 | ||
| AKP2 | Forward: 5′-TGCCTACTTGTGTGGCGTGAA-3′ | 57.8 | NM_007432 |
| Reverse: 5′-TCACCCGAGTGGTAGTCACAATG-3′ | 60.2 | ||
| ColIα1 | Forward: 5′-ATGCCGCGACCTCAAGATG-3′ | 57.1 | NM_007742 |
| Reverse: 5′-TGAGGCACAGACGGCTGAGTA-3′ | 59.8 | ||
| IBSP | Forward: 5′-TGGAGACTGCGATAGTTCCGAAG-3′ | 60.2 | NM_008318 |
| Reverse: 5′-CGTAGCTAGCTGTTACACCCGAGAG-3′ | 63.9 | ||
| Runx2 | Forward: 5′-CACTGGCGGTGCAACAAGA-3′ | 57.1 | NM_009821 |
| Reverse: 5′-TTTCATAACAGCGGAGGCATTTC-3′ | 56.6 | ||
| OCN | Forward: 5′-CCCTCTCTCTGCTCACTCTGCT-3′ | 61.9 | L24430 |
| Reverse: 5′-TGTGTTGTCCCTTCCCCTCT-3′ | 57.4 |
GAPDH: glyceraldehyde-3-phosphate dehydrogenase; ColIα1: collagen type I α1; AKP2: alkaline phosphatase 2; IBSP: integrin-binding sialoprotein; Runx2: Runt-related transcription factor 2; OCN: osteocalcin
Immunohistochemistry
Immunohistochemical analysis was carried out as described previously (Lin et al., 2005). In brief, MSCs on the slides were fixed with 4% (v/v) buffered paraformaldehyde. After blocking of endogenous peroxidase activity with 3% (v/v) hydrogen peroxide in methanol for 30 min, the slides were washed with PBS, and non-specific binding was blocked with 1% (w/v) bovine serum albumin and 1.5% (w/v) normal goat serum at room temperature for 30 min. Slides were subsequently incubated with goat polyclonal antibody against osteocalcin (OCN) (Santa Cruz, USA) at 4 °C overnight. After washed with PBS, slides were incubated with the secondary biotinylated antibodies and HRP-conjugated streptavidin to detect the primary antibody. Staining was done with 3,3′-diaminobenzidine tetrahydrochloride (DAB) as chromogens and counter staining was performed in Mayer’s hematoxylin. After rinsed with distilled water, slides were dehydrated through an ethanol series, cleared in xylene, and covered with slips for microscopy.
ALP activity assay
The cells of identical numbers were seeded. Cells cultured on the substrates were washed twice with PBS and fixed in 4% (v/v) paraformaldehyde for 5 min at room temperature, then incubated for 10 min with the staining solution including 0.1% (w/v) naphthol AS_MX phosphate, 56 mmol/L 2-amino-2-methyl-1-3-propanediol, and 0.1% (w/v) fast red violet LB salt, finally washed with distilled water and kept in water for 20 min. Staining solutions were freshly prepared before use. Quantitative analysis of ALP activity was determined by colorimetric assay of enzyme activity using the ALP kit (BioAssay System, USA), following the manufacturer’s instructions. Before cell dissolving, cell layers were washed three times with PBS, and then total proteins were extracted by Protein Extract Reagents (Pierce, USA), followed by centrifugation to remove cellular debris. 100 μl of lysate was then mixed with 100 μl of the freshly prepared colorimetric substrate para-nitrophenyl phosphate, and incubated at 37 °C for 30 min. The enzymatic reaction was stopped by adding 100 μl of 0.2 mol/L NaOH. The optical density of the yellow product para-nitrophenol was determined by a HTS 7000 Plus Bio Assay reader (Perkin Elmer Life Sciences, USA) at a wavelength of 405 nm. The protein concentration of the cell lysate was measured with a bicinchoninic acid (BCA) Protein Assay Kit (Pierce, USA) and the ALP activity was then expressed as OD 405 per mg protein.
Mineralization nodule staining
The mineralization nodules formed after osteogenic induction or plasmid transfection were stained with alizarin red S (AR-S). The cells on slides were rinsed in PBS and incubated with 40 mmol/L AR-S (pH 4.2) with rotation for 30 min. Then they were rinsed 5 times with water followed by a 15 min washing with PBS by rotation to reduce nonspecific AR-S staining. The stained nodules were observed through a microscope.
Statistical analysis
Results were expressed as mean±SD and analyzed by a paired analysis of variance. P values were described in figures and P<0.05 was considered as statistically significant.
RESULTS
Culture and identification of MSCs
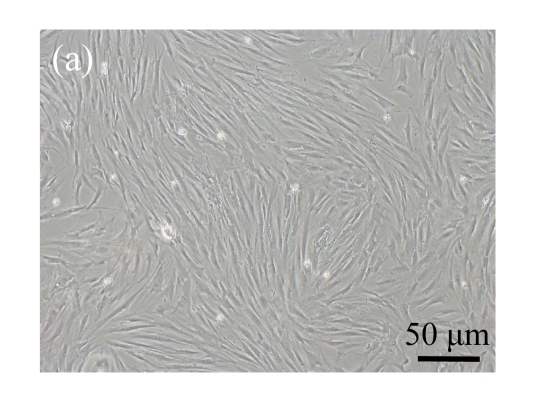
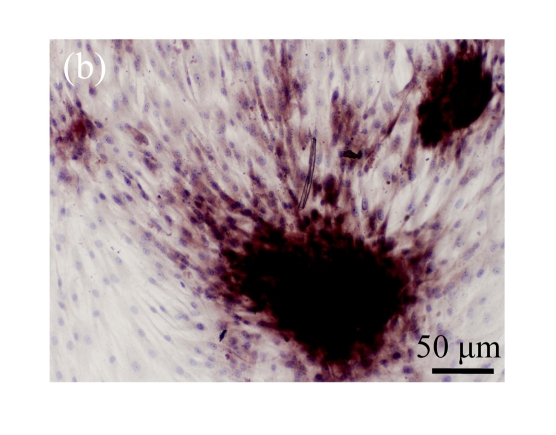
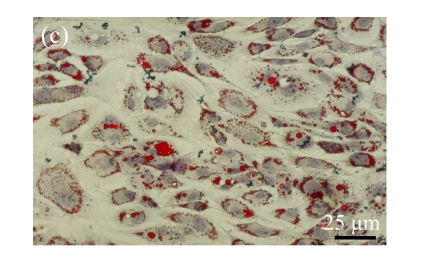
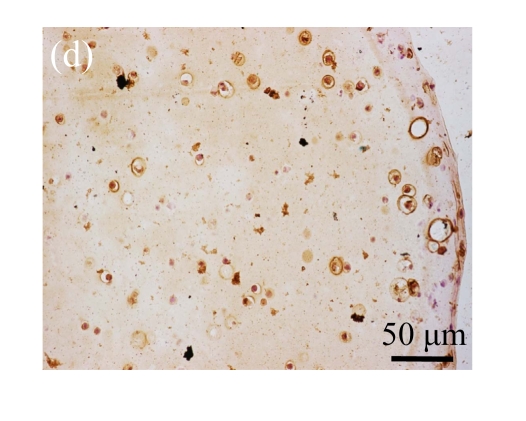
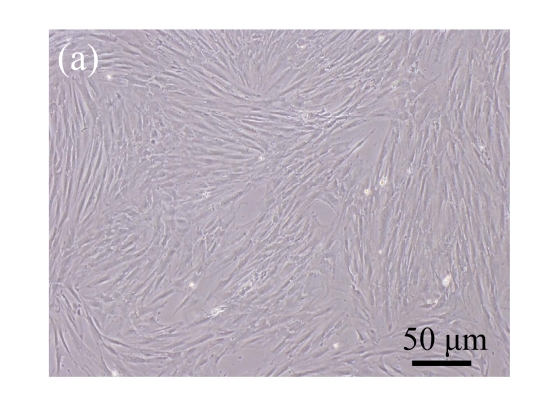
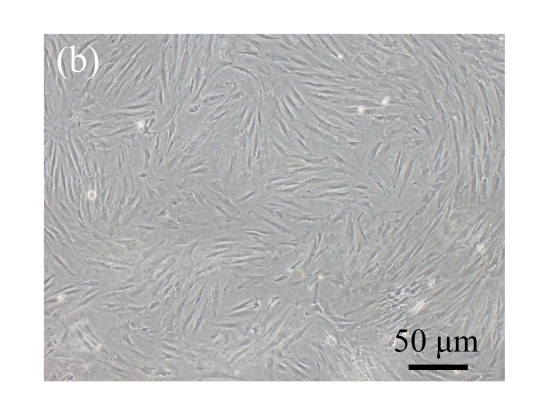
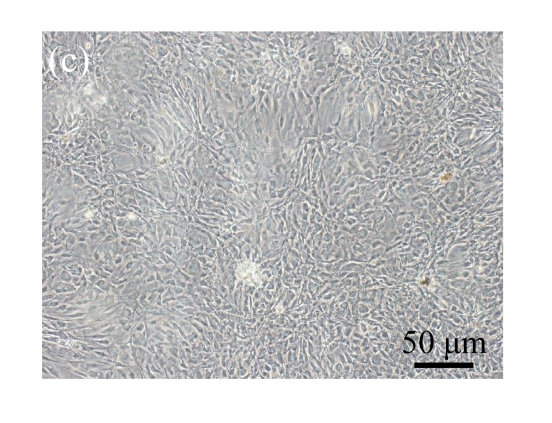
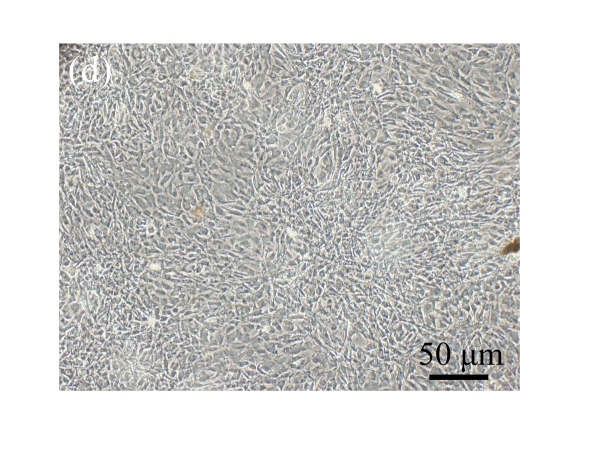
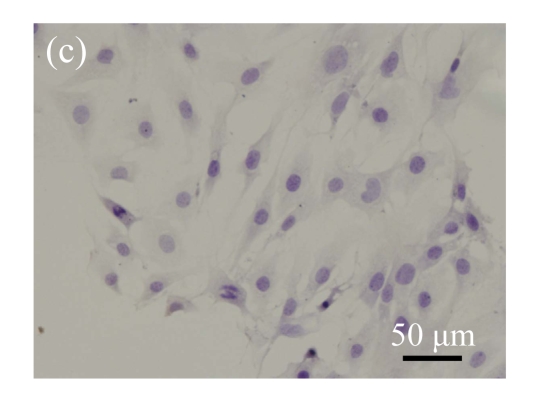
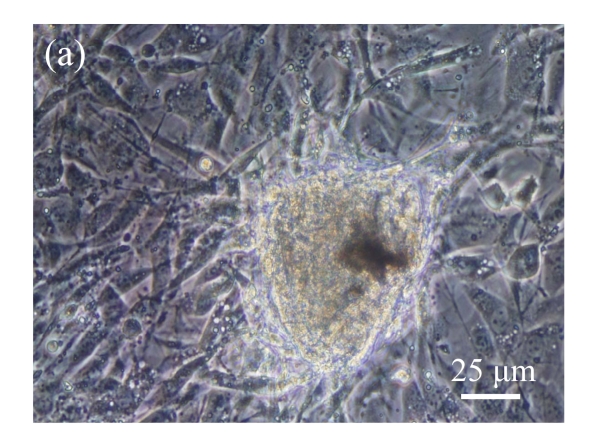
Human bone marrow MSCs were isolated from bone marrow tissue and cultured at 37 °C in humidified atmosphere with 5% CO2. After primarily cultured for 5 d, individual colonies formed. Twelve days later, 80% confluence of cells was achieved, and then the cells were trypsinized and passaged approximately every 5~7 d for 12 passages. During the culture period, primary or passaged MSCs displayed fibroblast-like morphological features, without visible morphologic alteration. The multi-linage differentiation of cultured MSCs was confirmed when these cells have the ability of osteogenic, chondrogenic, and adipogenic potential (Fig.1 in page 362).
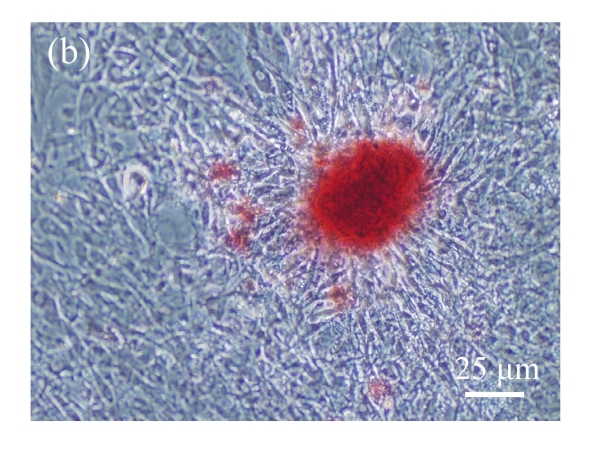
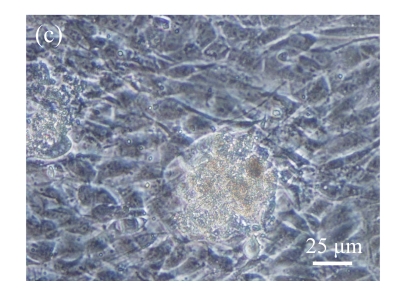
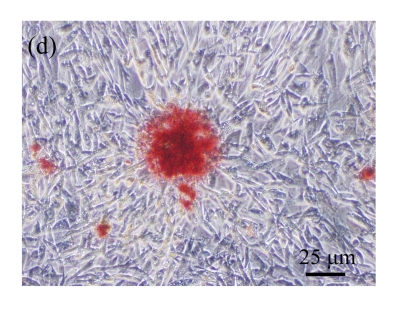
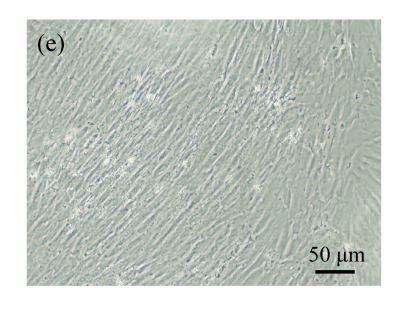
Fig. 1.
Identification of MSCs. (a) The undifferentiated MSCs cultured in monolayer culture; (b) The osteogenic differentiation of MSCs was confirmed by staining with alkaline phosphatase histochemistry; (c) The adipogenic differentiation of MSCs was confirmed by staining with oil red O histochemistry; (d) The chondrogenic differentiation of MSCs was confirmed by immunostaining with antibody specifically against type II collagen
Identification of pcDNA3.1(+)/CTGF
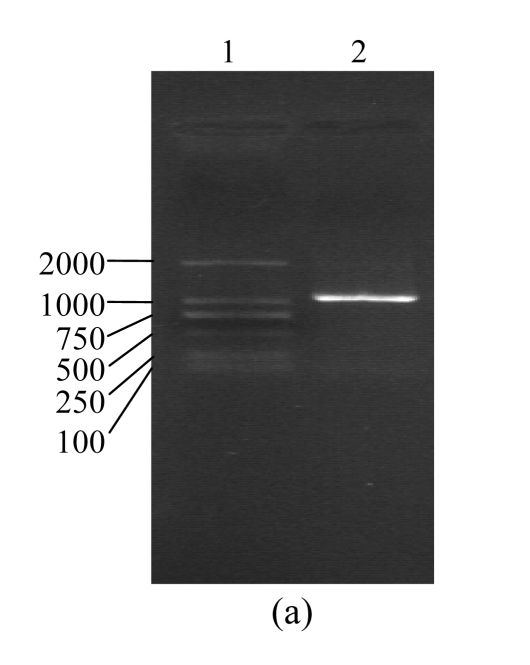
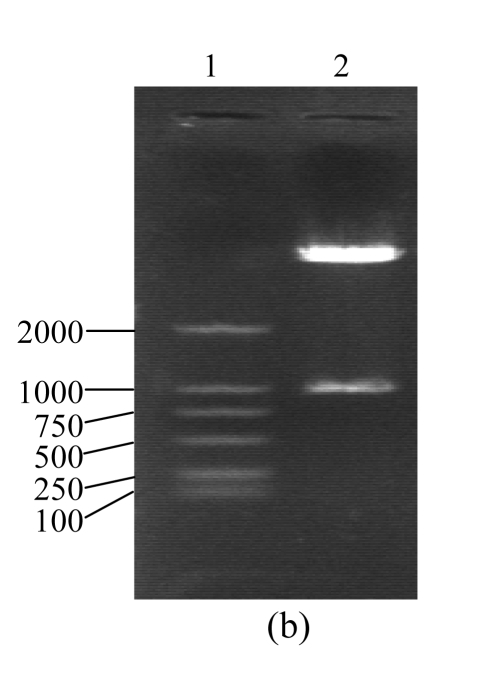
According to the background reference of human CTGF cDNA record by GenBank and the trait of the designed specific primers, the whole length of PCR product fragments identified by agarose gel electrophoresis was consisted with the analysis of background reference (Fig.2a). There were two fragments of 5.4 and 1.05 kb when pcDNA3.1(+)/CTGF was digested by HindIII and XhoI and electrophoresed on agarose gel. The fragment of 5.4 kb was accorded with pcDNA3.1(+), another fragment of 1.05 kb was accorded with human CTGF cDNA fragment produced by RT-PCR. These results demonstrate that CTGF cDNA has been inserted into pcDNA3.1(+)/CTGF (Fig.2b). The DNA sequence analysis confirmed that positively reformed plasmid contains the full length of human CTGF cDNA, which is the same as the one described in the GenBank with accession No. NM_001901. The CTGF cDNA fragment containing entire coding region (corresponding to the 1.05 kb covering from the AUG start codon to the UGA stop codon) was placed downstream of the CMV promoter element. The eukaryotic expression vector pCTGF has been constructed successfully.
Fig. 2.
Identification of pCTGF
(a) Agarose gel electrophoresis assay of CTGF PCR products. Lane 1: DNA marker, DL2000; Lane 2: CTGF (1.05 kb); (b) Digestion assay of pCTGF with XbaI and HindIII. Lane 1: DNA marker, DL2000; Lane 2: pCTGF
Expression of CTGF in MSCs by DNA transfection
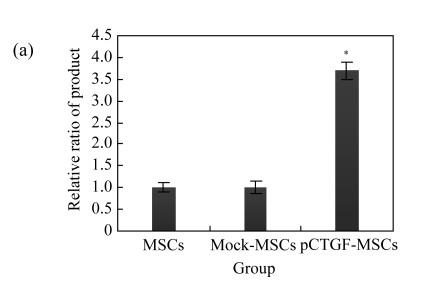
Real-time PCR and Western blotting analysis confirmed that pCTGF-MSCs overexpressed CTGF when compared with mock-MSCs and normal control cells (Fig.3a). The expression of CTGF mRNA was 3.7-fold higher than those of mock-MSCs and normal control. The cell culture supernatant after 48 h post-transfection was used for Western blotting. CTGF peptide was detectable in the cell culture supernatant of pCTGF-MSCs. However, the expression of CTGF peptide was not detectable in vector control and normal control (Fig.3b). It was suggested that CTGF was expressed effectively in the cells transfected with pCTGF.
Fig. 3.
Expression of CTGF in MSCs detected by real-time PCR and Western blotting
(a) The expression of CTGF mRNA of pCTGF-MSCs, mock-MSCs, and normal control cells. The amount of real-time PCR product in mock-MSCs was taken as 1.0, and the relative ratio of the products is indicated in the ordinate. The value was corrected using the expression of GAPDH as internal control. * P<0.05, significantly different from mock-MSCs and normal cells; (b) The expression of CTGF peptide of pCTGF-MSCs, mock-MSCs, and normal control cells. In the cell supernatant from pCTGF-MSCs, a large amount of CTGF protein with an apparent molecular mass of 38 kDa was illustrated. However, the expression of CTGF peptide was not detectable in vector control and normal control
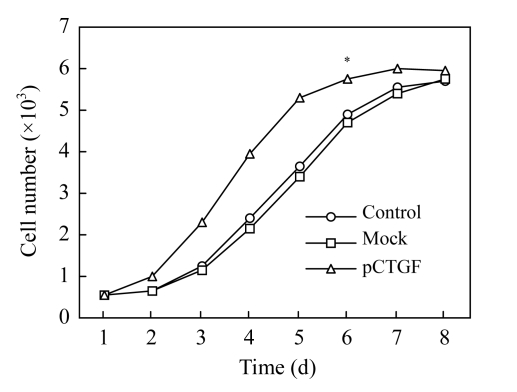
Proliferation of MSCs promoted by pCTGF
MTT assays were performed to evaluate the proliferative abilities of pCTGF-MSCs, mock-MSCs, and normal control MSCs. As shown in Fig.4, proliferation rate of pCTGF-MSCs was significantly higher than those of mock-transfected cells and normal control cells (P<0.05).
Fig. 4.
Proliferation rates of normal control MSCs, mock-MSCs, and pCTGF-MSCs
* P<0.05, compared with mock-transfected cells and normal control cells
Migration of MSCs promoted by pCTGF transfection
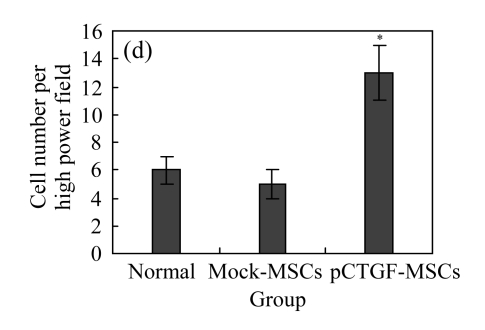
The transwell chamber assay is one of the frequently used methods to evaluate cell adhesion and migration in vitro. Here we conducted transwell chamber experiment to investigate whether pCTGF transfection exerted any effect on the migration of MSCs. As shown in Fig.5c (in page 362), cells that were cocultured with CTGF-expressing MSCs migrated to the underside of the membrane of the upper chamber were more than those cocultured with control cells. This indicates that CTGF secreted from pCTGF-MSCs showed a strong ability to induce the migration of the MSCs in the upper chamber as compared with normal controls. CTGF induced a 7.5-fold increase in cell migration over the empty vector-transfected controls (Fig.5d), which was significantly higher than those cocultured with vector control and normal control (P<0.05). These findings suggest that pCTGF transfection could indeed promote the migration of MSCs, a potentially important feature of CTGF functions in osteoblast differentiation.
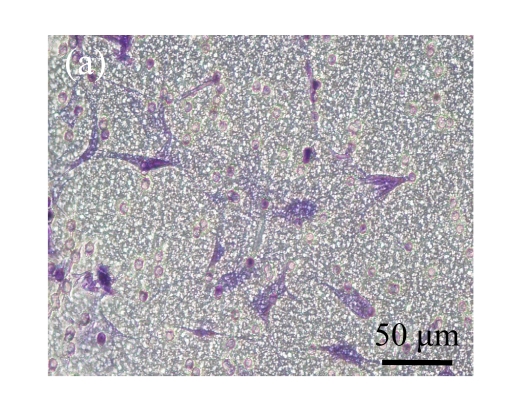
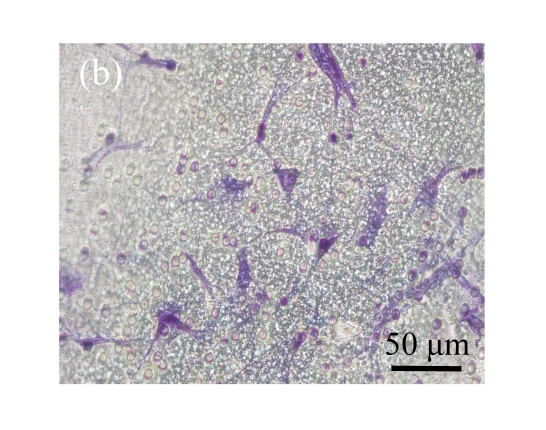
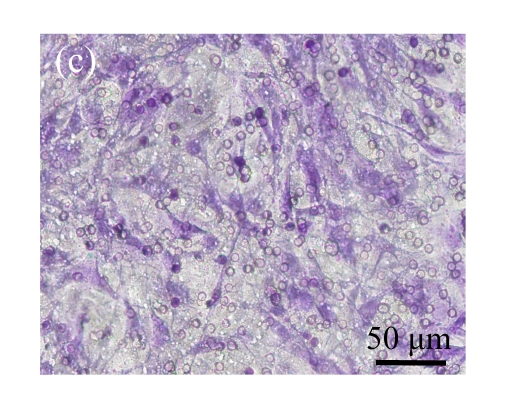
Fig. 5.
Effect of CTGF on migration of MSCs. (a) MSCs cocultured with normal control MSCs; (b) MSCs cocultured with mock-MSCs; (c) MSCs cocultured with pCTGF-MSCs; (d) Quantitative analysis of the effect of CTGF on migration of MSCs. The migration rate of MSCs cocultured with pCTGF-MSCs was significantly higher than those of MSCs cocultured with vector control and normal control. * P<0.05, compared with vector control and normal control
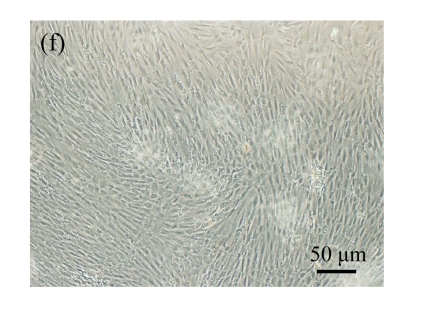
Morphologic changes of MSCs caused by pCTGF transfection
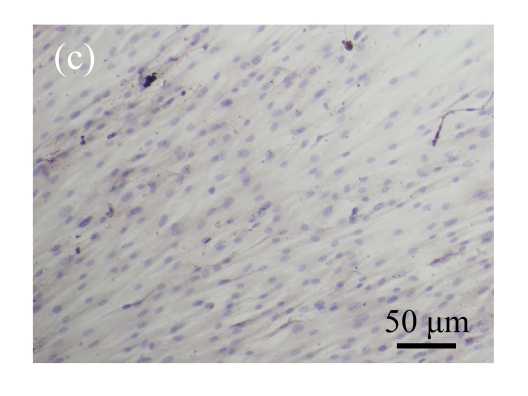
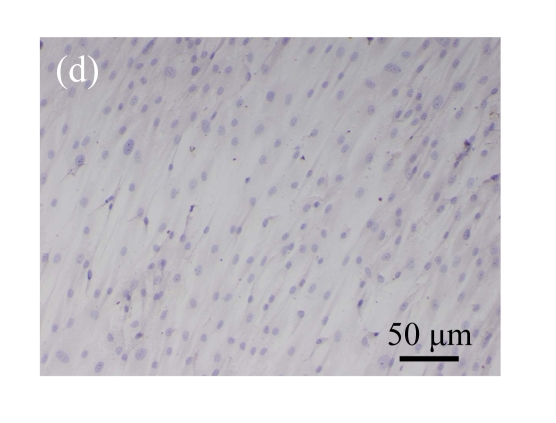
Normal MSCs and mock-MSCs maintained a fibroblasts-like appearance with a spindle shape at confluent (Fig.6 in page 362). At the same time, pCTGF-MSCs exhibited obvious morphological alteration after 2 weeks, becoming columnar and polarized, tending to align in straight parallel lines, all of which were typical morphologic features of osteoblast-like cells (Fig.6c). These cells were very much in appearance like MSCs cultured in OS-medium (Fig.6d).
Fig. 6.
Morphology of MSCs with plasmid transfection. (a) Normal MSCs maintained fibroblasts-like appearance cultured in growth medium; (b) Mock-MSCs maintained fibroblasts-like appearance; (c) pCTGF-MSCs became columnar and polarized, and tended to align themselves in straight parallel lines after two weeks of selection in growth medium containing G418; (d) MSCs became columnar and polarized, and tended to align themselves in straight parallel lines after two weeks in osteoinductive medium
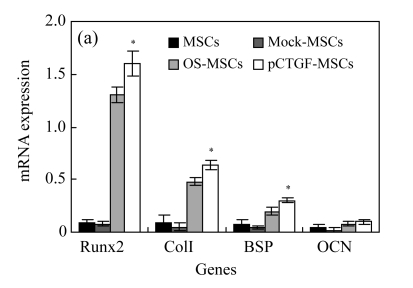
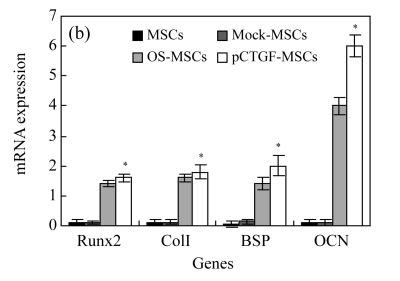
Expression patterns of bone-related genes
We focused on the expression of a set of genes related to bone development and mineralization in both pCTGF-MSCs and MSCs cultured in OS-medium. Expressions of osteoblastic marker genes including transcription factor Runx2, collagen type I (ColI), bone sialoprotein (BSP), and OCN in cells were analysized by real-time PCR on Days 3 and 14 after MSCs transfected with pCTGF or OS-induction. Overexpression of CTGF induced the expressions of Runx2, ColI, BSP, and OCN (Fig.7). Similar to pCTGF-MSCs, bone-related genes were also expressed in OS-medium–induced MSCs. However, mock-MSCs and normal control MSCs only had basal expressions of these osteoblastic phenotype genes. GAPDH was used as internal control.
Fig. 7.
Real-time PCR analyses for mRNA expressions of bone-related genes in different cells after cultured for (a) 3 d and (b) 14 d
For Runx2, collagen I (colI) and bone sialoprotein (BSP) (Days 3 and 14) and osteocalcin (OCN) (Day 14), the increased expression on pCTGF-MSCs or MSCs cultured in OS-medium was observed. However, mock-MSCs and normal control MSCs only have basal expressions of these osteoblastic phenotype genes. GAPDH was used as internal control. * P<0.05, statistically significant difference between control cells and pCTGF-MSCs or MSCs cultured in OS-medium
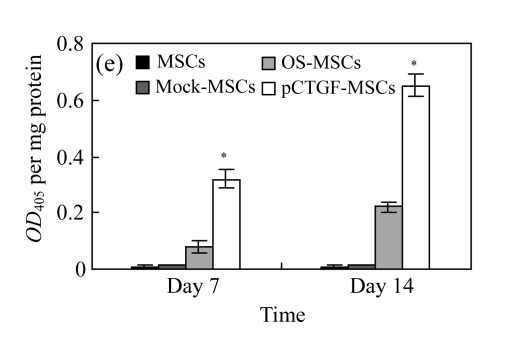
ALP activity in MSCs
We analyzed whether overexpression of CTGF could increase ALP activity in MSCs. Our data indicate that pCTGF-MSCs exhibited a linear increase in ALP activity within the first two weeks in selective medium. The levels of ALP activities of pCTGF-sMSCs at the 1st and 2nd weeks were 4.0- and 3.0-fold higher than those of MSCs cultured in OS-medium, respectively. In contrast, ALP activities of mock-MSCs and normal control MSCs remained at background levels when cultured in control medium for up to two weeks (Fig.8 in page 362).
Fig. 8.
Comparison of the expressions of ALP in (a) pCTGF-MSCs, (b) OS-induced MSCs, (c) mock-MSCs, and (d) normal control MSCs after 14-d culture. Note that there was no visible ALP activity in mock-MSCs and normal control MSCs. (e) Quantitative analyses of ALP activities in MSCs after 7- and 14-d culture. ALP activity was only found in pCTGF-MSCs and OS-induced MSCs. Data were displayed as mean±SD. * P<0.05, statistically significant difference compared with mock-MSCs and normal control MSCs
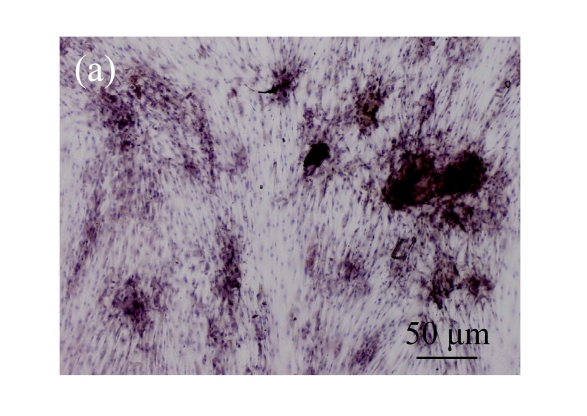
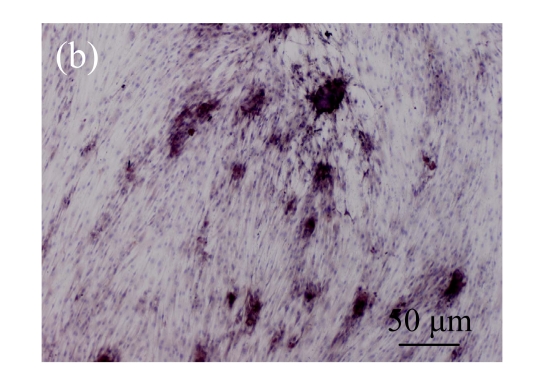
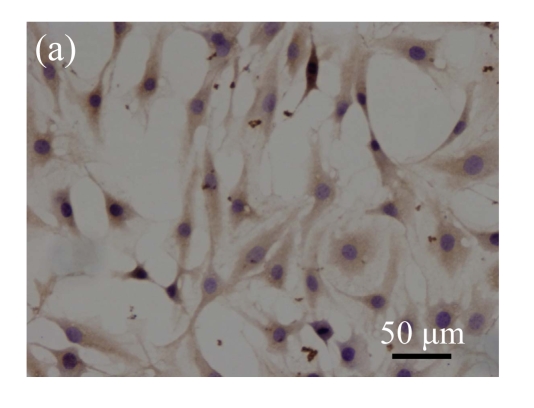
Immunohistochemical analysis of the expression of OCN
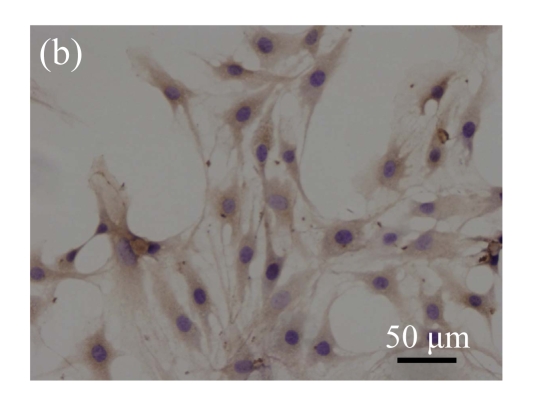
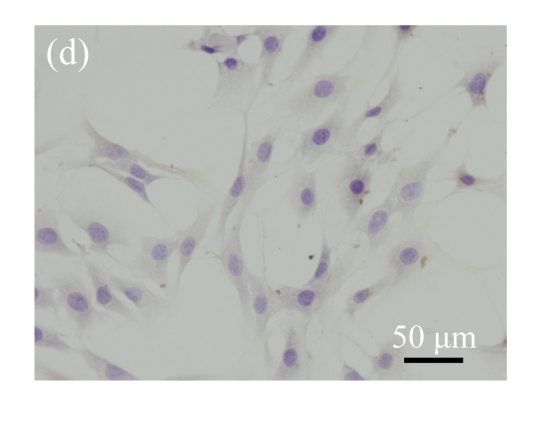
Immunohistochemical staining was performed to detect the expression of OCN in pCTGF-MSCs, OS-induced MSCs, mock-MSCs, and normal control MSCs. As shown in Fig.9 (in page 363), almost all of pCTGF-MSCs and OS-induced MSCs were positive with immunocytochemical staining. As negative control, normal control cells and mock-MSCs showed no apparent expression of OCN, when cultured under the same conditions as pCTGF-MSCs.
Fig. 9.
Immunohistochemical detection of OCN. (a) Nearly all pCTGF-MSCs were positive with the immunohistochemistry of OCN after two-week culture; (b) Most of the OS-medium−induced cells were positive with the immunohistochemistry of OCN after two weeks; No apparent expression of OCN was detected in (c) mock-MSCs and (d) normal control MSCs
Mineralized nodule formation
Alizarin red staining was performed to investigate whether the expression of bone-related genes resulted in a mineralized nodule formation. For pCTGF-MSCs, the mineralized nodules formed on Day 14 after confluence demonstrated positive AR-S staining (Figs.10a~10b in page 363). For MSCs cultured in OS-medium, mineralized nodules appeared two weeks later after confluence (Fig.10c), and these nodules were also positive with alizarin red staining (Fig.10d). On the other hand, mock-MSCs and normal control MSCs could not form any mineralized nodules in the control medium even three weeks later after confluence (Figs.10e~10f).
Fig. 10.
Mineralized nodule formation. (a) Mineralized nodules formed in pCTGF-MSCs 14 d after confluence; (b) Mineralized nodules formed in pCTGF-MSCs 14 d after confluence were positive with alizarin red staining; (c) Mineralized nodules formed in OS-medium–cultured MSCs 14 d after confluence; (d) Mineralized nodules formed in OS-medium–cultured MSCs were positive with alizarin red staining; (e) Normal control MSCs cultured in growth medium have no any mineralized nodule formed; (f) Mock-MSCs cultured in growth medium have no any mineralized nodule formed
DISCUSSION
MSCs are considered as a readily accepted source of stem cells in bone tissue regeneration because they are easy to be isolated and expanded in vitro. It is still a challenge to enhance the proliferation and differentiation of seed cells in the application of bone tissue engineering, and this problem can be solved by gene therapy approaches (van Damme et al., 2002; Gamradt and Lieberman, 2004; Chan et al., 2005; Betz et al., 2008). A number of signaling molecules have been shown to participate in MSCs osteogenesis. These signaling molecules include bone morphogenetic proteins (BMPs), WNT, and FGF families, transcription factors of Runx2 and sox9 (Oakes and Lieberman, 2000; Alden et al., 2002; Gersbach et al., 2006). Former studies have shown that cells of the osteoblastic lineage express transcripts for CTGF and other members of the CCN family (Arnott et al., 2007; Edgar et al., 2007). However, the function of CTGF in skeletal cells has been controversial, and both stimulatory and inhibitory effects on osteoblastogenesis have been reported (Abreu et al., 2002; Luo et al., 2004). In present study, we demonstrate that the constitutive overexpression of CTGF enhanced the osteoblastic lineage differentiation of MSCs. CTGF overexpression induced the formation of mineralized nodules, ALP, and osteocalcin expression in MSCs. On the crucial function of CTGF, we reported the osteoblastic differentiation of MSCs initiated by CTGF overexpression.
We found that transfection of cDNA encoding the human CTGF in MSCs induced these cells to differentiate into functional osteoblasts with definite morphological alterations, which were also observed in MSCs cultured in OS-medium. Our data also indicate that these osteoblasts-like cells derived from pCTGF-MSCs showed high levels of ALP activity, and well-defined mineralized nodules formed just like MSCs cultured in OS-medium. Expression of several bone-related genes confirmed pCTGF-MSCs differentiated toward osteoblasts. pCTGF-transfected MSCs had the ability to express BSP, OCN, osteopontin (OPN), and colI, which are responsible for the deposition and maturation of bone extracellular matrix. Runx2, the earliest transcription factor proven essential for commitment to osteoblastogenesis, which is essential for the expression of a number of bone-specific genes, is primarily considered a master regulator of bone development. BSP is a member of the SIBLING gene family of glycoproteins involved in the formation of mineral crystal in calcified dentin and bone matrix. Expression of BSP is restricted to bone and other mineralized tissues. On the other hand, OCN is produced by mature osteoblasts during mineralization and is the one of the most abundant noncollagenous proteins both in mineralized dentin and bone extracellular matrix. ALP is the most often used early marker of osteoblast differentiation. As demonstrated in this study, ALP activity was up-regulated in CTGF-transfected MSCs. The coexpression of these genes, together with the characteristic morphological and polarization changes, suggests the differentiation of the CTGF-transfected MSCs into osteoblasts.
Similarly to our results, CTGF had been proved to enhance the differentiation and function of osteoblast-like Saos-2 cells, MC3T3-E1 cells, and primary osteoblast cultures (Nishida et al., 2000; Abreu et al., 2002; Ivkovic et al., 2003; Safadi et al., 2003; Kawaki et al., 2008; Ono et al., 2008). In contrast, CTGF overexpression did not promote osteoblastic differentiation or mineralization in fibroblasts NIH3T3 cell line. Transduction of C3H10T1/2 mesenchymal cells with adenoviral vectors for the constitutive expression of CTGF or addition of CTGF to these cells results in impaired osteoblastogenesis, which suggests that the promotion of osteogenic differentiation was specific in multipotent stem cells or cells of osteoblastic lineage (Luo et al., 2004; Liu et al., 2007). The different effects of CTGF on cells of the osteoblastic lineage may reflect the fact that different mechanisms may be operational in the various cells. It has been reported that overexpression of BMP-2 inhibited the growth of a cell line derived from rat osteosarcoma, though it promoted their osteogenic differentiation (Huang et al., 2002). Other reports also indicated that proliferative abilities of osteoblasts and MSCs are influenced by osteogenic induction (Lee et al., 2004; Igarashi et al., 2004; Cho et al., 2005). It is generally believed that osteogenic induction on pluripotent cells with gene therapy approaches or chemical inducement decreases their proliferation rate. However, our results in this study show that CTGF overexpression obviously increased the proliferation rate of MSCs, as significant difference between CTGF-transfected MSCs and mock-MSCs was found. Overexpression of CTGF increases the migration of MSCs. These findings are profound in the possible application of hard tissue engineering with genetically modified MSCs. Although it has been also reported that overexpression of CTGF induces proliferation of BMSCs (bone marrow stromal cells) and NIH3T3 (Liu et al., 2007; Ono et al., 2008), genetic diversities among different cell sources maybe exist.
Although the exact mechanism by which CTGF is involved in bone development and mineralization is yet not well understood, growing evidence shows the CCN family plays an important regulatory role during proliferation and differentiation in musculoskeletal cells. CTGF is a member of the CCN family of growth factors, which is related to selected BMP antagonists, such as Tsg and chordin (Brigstock, 2002; Brigstock et al., 2003). Previous studies have shown a direct protein-protein interaction between CTGF and BMP-2, and the similar interaction has been reported for Nov (Abreu et al., 2002; Mercurio et al., 2004; Rydziel et al., 2007). However, Nov inhibits osteoblastogenesis by suppressing BMP-2 and WNT signaling and activities, whereas CTGF overexpression in vitro favors osteoblastogenesis despite a modest decrease in BMP/Smad and WNT/β-catenin signaling. Taken together, these results demonstrate that the effect of CTGF on osteoblastogenesis is independent of BMP-2 and WNT 3 signaling. In fact, previous studies have documented direct interactions between CTGF and BMP-2, and as a consequence, an inhibitory effect on osteoblastogenesis (Abreu et al., 2002; Mercurio et al., 2004). Although BMPs can induce the differentiation of mesenchymal cells toward the osteoblastic lineage, they have the ability to enhance the function of the osteoblasts. So the mechanism of CTGF overexpression promoting both proliferation and differentiation of MSCs may be explained by BMP and WNT independent signaling mechanism.
In summary, we constructed pcDNA3.1(+)/CTGF eukaryotic expression vector successfully by molecular clone technology and demonstrated that endogenous CTGF promoted the migration, proliferation, and osteogenic differentiation of MSCs by CTGF overexpression. Transfection of CTGF-expressing plasmid induced MSCs to differentiate into functional osteoblast. Further investigation may be needed to investigate the promotion mechanism of both promoting proliferation and differentiation of MSCs. This observation concerning CTGF overexpression and function has led to the intriguing notion that CTGF may be a novel growth factor for bone tissue engineering.
Acknowledgments
We are grateful to Prof. Tong-chuan He (Molecular Oncology Laboratory, University of Chicago Medical Center, USA) for kindly providing the plasmid containing full-length CTGF cDNA fragment. We are also grateful for the contributions of many people within the Key Laboratory of Transplant Engineering and Immunology, Ministry of Health, West China Hospital, Sichuan University, China. Special thanks must also go to Prof. Xiao-feng Lu (Key Laboratory of Transplant Engineering and Immunology, Ministry of Health, West China Hospital, Sichuan University, China) and Dr. Yan-bao Li (College of Materials Science and Engineering, Nanjing University of Technology, China) for helpful discussion.
Footnotes
Project supported by the National Basic Research Program (973) of China (No. 2005CB623900)
References
- 1.Abreu JG, Ketpura NI, Reversade B, de Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4(8):599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alden TD, Varady P, Kallmes DF, Jane JAJr, Helm GA. Bone morphogenetic protein gene therapy. Spine. 2002;27(16S):S87–S93. doi: 10.1097/00007632-200208151-00016. [DOI] [PubMed] [Google Scholar]
- 3.Arnott JA, Nuglozeh E, Rico MC, Arango HI, Odgren PR, Safadi FF, Popoff SN. Connective tissue growth factor (CTGF/CCN2) is a downstream mediator for TGF-beta1-induced extracellular matrix production in osteoblasts. J Cell Physiol. 2007;210(3):843–852. doi: 10.1002/jcp.20917. [DOI] [PubMed] [Google Scholar]
- 4.Betz VM, Betz OB, Harris MB, Vrahas MS, Evans CH. Bone tissue engineering and repair by gene therapy. Front Biosci. 2008;13(13):833–841. doi: 10.2741/2724. [DOI] [PubMed] [Google Scholar]
- 5.Beyer Nardi N, da Silva Meirelles L. Stem Cells. Handbook of Experimental Pharmacology. Vol. 174. Springer Berlin Heidelberg; 2006. Mesenchymal Stem Cells: Isolation, in Vitro Expansion and Characterization; pp. 249–282. [DOI] [PubMed] [Google Scholar]
- 6.Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61) Angiogenesis. 2002;5(3):153–165. doi: 10.1023/A:1023823803510. [DOI] [PubMed] [Google Scholar]
- 7.Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178(2):169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- 8.Brigstock DR, Goldschmeding R, Katsube KI, Lam SC, Lau LF, Lyons K, Naus C, Perbal B, Riser B, Takigawa M, et al. Proposal for a unified CCN nomenclature. Mol Pathol. 2003;56(2):127–128. doi: 10.1136/mp.56.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caterson E, Nesti L, Danielson K, Tuan R. Human marrow-derived mesenchymal progenitor cells. Mol Biotechnol. 2002;20(3):245–256. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- 10.Chan J, O′Donoghue K, de la Fuente J, Roberts IA, Kumar S, Morgan JE, Fisk NM. Human fetal mesenchymal stem cells as vehicles for gene delivery. Stem Cells. 2005;23(1):93–102. doi: 10.1634/stemcells.2004-0138. [DOI] [PubMed] [Google Scholar]
- 11.Cho HH, Park HT, Kim YJ, Bae YC, Suh KT, Jung JS. Induction of osteogenic differentiation of human mesenchymal stem cells by histone deacetylase inhibitors. J Cell Biochem. 2005;96(3):533–542. doi: 10.1002/jcb.20544. [DOI] [PubMed] [Google Scholar]
- 12.Duan X, Yang L, Dong S, Xin R, Chen G, Guo L. Characterization of EGFP-labeled mesenchymal stem cells and redistribution of allogeneic cells after subcutaneous implantation. Archives of Orthopaedic and Trauma Surgery. 2008;128(7):751–759. doi: 10.1007/s00402-008-0585-y. [DOI] [PubMed] [Google Scholar]
- 13.Edgar CM, Chakravarthy V, Barnes G, Kakar S, Gerstenfeld LC, Einhorn TA. Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone. 2007;40(5):1389–1398. doi: 10.1016/j.bone.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamradt SC, Lieberman JR. Genetic modification of stem cells to enhance bone repair. Ann Biomed Eng. 2004;32(1):136–147. doi: 10.1023/B:ABME.0000007798.78548.b8. [DOI] [PubMed] [Google Scholar]
- 15.Gersbach CA, Le Doux JM, Guldberg RE, Garcia AJ. Inducible regulation of Runx2-stimulated osteogenesis. Gene Ther. 2006;13(11):873–882. doi: 10.1038/sj.gt.3302725. [DOI] [PubMed] [Google Scholar]
- 16.Goessler UR, Riedel K, Hormann K, Riedel F. Perspectives of gene therapy in stem cell tissue engineering. Cells Tissues Organs. 2006;183(4):169–179. doi: 10.1159/000096508. [DOI] [PubMed] [Google Scholar]
- 17.Heng EC, Huang Y, Black SAJr, Trackman PC. CCN2, connective tissue growth factor, stimulates collagen deposition by gingival fibroblasts via module 3 and alpha6- and beta1 integrins. J Cell Biochem. 2006;98(2):409–420. doi: 10.1002/jcb.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard D, Buttery LD, Shakesheff KM, Roberts SJ. Tissue engineering: strategies, stem cells and scaffolds. J Anat. 2008;213(1):66–72. doi: 10.1111/j.1469-7580.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W, Rudkin GH, Carlsen B, Ishida K, Ghasri P, Anvar B, Yamaguchi DT, Miller TA. Overexpression of BMP-2 modulates morphology, growth, and gene expression in osteoblastic cells. Exp Cell Res. 2002;274(2):226–234. doi: 10.1006/excr.2002.5483. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi M, Kamiya N, Hasegawa M, Kasuya T, Takahashi T, Takagi M. Inductive effects of dexamethasone on the gene expression of Cbfa1, osterix and bone matrix proteins during differentiation of cultured primary rat osteoblasts. J Mol Histol. 2004;35(1):3–10. doi: 10.1023/B:HIJO.0000020883.33256.fe. [DOI] [PubMed] [Google Scholar]
- 21.Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130(12):2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang CY, Gui C, He AN, Hu XY, Chen J, Jiang Y, Wang JA. Optimal time for mesenchymal stem cell transplantation in rats with myocardial infarction. J Zhejiang Univ Sci B. 2008;9(8):630–637. doi: 10.1631/jzus.B0820004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanaan RA, Aldwaik M, Al-Hanbali OA. The role of connective tissue growth factor in skeletal growth and development. Med Sci Monit. 2006;12(12):RA277–RA281. [PubMed] [Google Scholar]
- 24.Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100–114. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 25.Karp JM, Tanaka TS, Zohar R, Sodek J, Shoichet MS, Davies JE, Stanford WL. Thrombin mediated migration of osteogenic cells. Bone. 2005;37(3):337–348. doi: 10.1016/j.bone.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Kawaki H, Kubota S, Suzuki A, Yamada T, Matsumura T, Mandai T, Yao M, Maeda T, Lyons KM, Takigawa M. Functional requirement of CCN2 for intramembranous bone formation in embryonic mice. Biochem Biophys Res Commun. 2008;366(2):450–456. doi: 10.1016/j.bbrc.2007.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kofron MD, Laurencin CT. Bone tissue engineering by gene delivery. Adv Drug Deliv Rev. 2006;58(4):555–576. doi: 10.1016/j.addr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Kubota S, Takigawa M. Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol. 2007;257:1–41. doi: 10.1016/S0074-7696(07)57001-4. [DOI] [PubMed] [Google Scholar]
- 29.Lee HS, Huang GT, Chiang H, Chiou LL, Chen MH, Hsieh CH, Jiang CC. Multipotential mesenchymal ctem cells from femoral cone carrow cear the cite of osteonecrosis. Stem Cells. 2003;21(2):190–199. doi: 10.1634/stemcells.21-2-190. [DOI] [PubMed] [Google Scholar]
- 30.Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, Bae YC, Jung JS. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14(4-6):311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y, Luo E, Chen X, Liu L, Qiao J, Yan Z, Li Z, Tang W, Zheng X, Tian W. Molecular and cellular characterization during chondrogenic differentiation of adipose tissue-derived stromal cells in vitro and cartilage formation in vivo. J Cell Mol Med. 2005;9(4):929–939. doi: 10.1111/j.1582-4934.2005.tb00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu LD, Shi HJ, Jiang L, Wang LC, Ma SH, Dong CH, Wang JJ, Zhao HL, Liao Y, Li QH. The repairing effect of a recombinant human connective-tissue growth factor in a burn-wounded rhesus-monkey (Macaca mulatta) model. Biotechnol Appl Biochem. 2007;47(2):105–112. doi: 10.1042/BA20060114. [DOI] [PubMed] [Google Scholar]
- 33.Luft FC. CCN2, the connective tissue growth factor. J Mol Med. 2008;86(1):1–3. doi: 10.1007/s00109-007-0287-x. [DOI] [PubMed] [Google Scholar]
- 34.Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, et al. Connective tissue growth factor (CTGF) is regulated by WNT and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279(53):55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 35.Mauney JR, Volloch V, Kaplan DL. Role of adult mesenchymal stem cells in bone tissue engineering applications: current status and future prospects. Tissue Eng. 2005;11(5-6):787–802. doi: 10.1089/ten.2005.11.787. [DOI] [PubMed] [Google Scholar]
- 36.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell-based bone tissue engineering. PLoS Med. 2007;4(2):e9. doi: 10.1371/journal.pmed.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131(9):2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- 38.Nishida T, Nakanishi T, Asano M, Shimo T, Takigawa M. Effects of CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, on the proliferation and differentiation of osteoblastic cells in vitro. J Cell Physiol. 2000;184(2):197–206. doi: 10.1002/1097-4652(200008)184:2<197::AID-JCP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 39.Nishida T, Kawaki H, Baxter RM, Deyoung RA, Takigawa M, Lyons KM. CCN2 (connective cissue crowth cactor) is essential for extracellular matrix production and integrin signaling in chondrocytes. J Cell Commun Signal. 2007;1(1):45–58. doi: 10.1007/s12079-007-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oakes DA, Lieberman JR. Osteoinductive applications of regional gene therapy: ex vivo gene transfer. Clin Orthop Relat Res. 2000;379(Suppl.):S101–S112. doi: 10.1097/00003086-200010001-00014. [DOI] [PubMed] [Google Scholar]
- 41.Ono M, Kubota S, Fujisawa T, Sonoyama W, Kawaki H, Akiyama K, Oshima M, Nishida T, Yoshida Y, Suzuki K, et al. Promotion of attachment of human bone marrow stromal cells by CCN2. Biochem Biophys Res Commun. 2007;357(1):20–25. doi: 10.1016/j.bbrc.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 42.Ono M, Kubota S, Fujisawa T, Sonoyama W, Kawaki H, Akiyama K, Shimono K, Oshima M, Nishida T, Yoshida Y, et al. Promotion of hydroxyapatite-associated, stem cell-based bone regeneration by CCN2. Cell Transplant. 2008;17(1-2):231–240. doi: 10.3727/000000008783907143. [DOI] [PubMed] [Google Scholar]
- 43.Pelled GG, Turgeman G, Aslan H, Gazit Z, Gazit D. Mesenchymal stem cells for bone gene therapy and tissue engineering. Curr Pharm Des. 2002;8(21):1917–1928. doi: 10.2174/1381612023393666. [DOI] [PubMed] [Google Scholar]
- 44.Perbal B, Brigstock DR, Lau LF. Report on the second international workshop on the CCN family of genes. Mol Pathol. 2003;56(2):80–85. doi: 10.1136/mp.56.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pountos I, Corscadden D, Emery P, Giannoudis PV. Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application. Injury. 2007;38(S4):23–33. doi: 10.1016/S0020-1383(08)70006-8. [DOI] [PubMed] [Google Scholar]
- 46.Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- 47.Rose FR, Oreffo RO. Bone tissue engineering: hope vs hype. Biochem Biophys Res Commun. 2002;292(1):1–7. doi: 10.1006/bbrc.2002.6519. [DOI] [PubMed] [Google Scholar]
- 48.Rydziel S, Stadmeyer L, Zanotti S, Durant D, Smerdel-Ramoya A, Canalis E. Nephroblastoma overexpressed (Nov) inhibits osteoblastogenesis and causes osteopenia. J Biol Chem. 2007;282(27):19762–19772. doi: 10.1074/jbc.M700212200. [DOI] [PubMed] [Google Scholar]
- 49.Safadi FF, Xu J, Smock SL, Kanaan RA, Selim AH, Odgren PR, Marks SCJr, Owen TA, Popoff SN. Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol. 2003;196(1):51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- 50.Slater BJ, Kwan MD, Gupta DM, Panetta NJ, Longaker MT. Mesenchymal cells for skeletal tissue engineering. Expert Opin Biol Ther. 2008;8(7):885–893. doi: 10.1517/14712598.8.7.885. [DOI] [PubMed] [Google Scholar]
- 51.van Damme A, van den Driessche T, Collen D, Chuah MK. Bone marrow stromal cells as targets for gene therapy. Curr Gene Ther. 2002;2(2):195–209. doi: 10.2174/1566523024605645. [DOI] [PubMed] [Google Scholar]
- 52.Xiang Y, Zheng Q, Jia BB, Huang GP, Xu YL, Wang JF, Pan ZJ. Ex vivo expansion and pluripotential differentiation of cryopreserved human bone marrow mesenchymal stem cells. J Zhejiang Univ Sci B. 2007;8(2):136–146. doi: 10.1631/jzus.2007.B0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaai T, Nakanishi T, Asano M, Nawachi K, Yoshimichi G, Ohyama K, Komori T, Sugimoto T, Takigawa M. Gene expression of connective tissue growth factor (CTGF/CCN2) in calcifying tissues of normal and cbfa1-null mutant mice in late stage of embryonic development. J Bone Miner Metab. 2005;23(4):280–288. doi: 10.1007/s00774-004-0600-5. [DOI] [PubMed] [Google Scholar]
- 54.Yamanaka O, Saika S, Ikeda K, Miyazaki K, Kitano A, Ohnishi Y. Connective tissue growth factor modulates extracellular matrix production in human subconjunctival fibroblasts and their proliferation and migration in vitro. Jpn J Ophthalmol. 2008;52(1):8–15. doi: 10.1007/s10384-007-0497-3. [DOI] [PubMed] [Google Scholar]