Abstract
Objective: To study the relationship between plasma adiponectin concentration and the functional activities of circulating endothelial progenitor cells (EPCs) in patients with coronary artery disease (CAD). Methods: Circulating EPCs were enumerated as AC133+/KDR+ cells via flow cytometry and identified by co-staining with DiI-acLDL and fluorescein isothiocyanate (FITC)-conjugated lectin under a fluorescent microscope. The migratory capacity of EPCs was measured by modified Boyden chamber assay. Adhesion capacity was performed to count adherent cells after replating EPCs on six-well culture dishes coated with fibronectin. Results: The number of circulating EPCs (AC133+/KDR+ cells) decreased significantly in CAD patients, compared with control subjects [(74.2±12.3) vs (83.5±12.9) cells/ml blood, P<0.01]. In addition, the number of EPCs also decreased in CAD patients after ex vivo cultivation [(54.4±8.6) vs (71.9±11.6) EPCs/field, P<0.01]. Both circulating EPCs and differentiated EPCs were positively correlated with plasma adiponectin concentration. The functional activities of EPCs from CAD patients, such as migratory and adherent capacities, were also impaired, compared with control subjects, and positively correlated with plasma adiponectin concentration. Conclusion: The study demonstrates that the impairment of the number and functional activities of EPCs in CAD patients is correlated with their lower plasma adiponectin concentrations.
Keywords: Adiponectin, Adipokines, Endothelial progenitor cells (EPCs), Coronary artery disease (CAD)
INTRODUCTION
Adiponectin is a novel collagen-like plasma protein, which is specifically and highly expressed in human adipose tissue (Arita et al., 1999). It is present abundantly in circulating blood. Adiponectin exhibits the beneficial role in regulating insulin action. Several studies showed a negative relation between plasma adiponectin level and the degree of insulin resistance (Higashiura et al., 2004; Tirziu and Simons, 2005). Plasma concentration of adiponectin is reduced in association with obesity-linked disease (Ouchi et al., 1999; Higashiura et al., 2004), including type 2 diabetes mellitus (Hotta et al., 2000; Higashiura et al., 2004) and coronary artery disease (CAD) (Otake et al., 2008). Several clinical and epidemiological studies have indicated that hypoadiponectinemia is a common and independent risk factor for cardiovascular disorders (Iwashima et al., 2006a; Otake et al., 2008). The mechanism underlying the physiology of adiponectin appears to modulate endothelial function. For example, adiponectin stimulates the production of nitric oxide and suppresses the adhesion molecule expression in vascular endothelial cells (Chen et al., 2003; Motoshima et al., 2004). Decreasing adiponectin levels contribute to the endothelial dysfunction (Ouchi et al., 2003a). Several clinical studies have also demonstrated that hypoadiponectinemia correlated with endothelial dysfunction in peripheral arteries and CAD (Iwashima et al., 2006a; 2006b; Otake et al., 2008). Endothelial dysfunction is an early pivotal event in the development, progression, and manifestation of atherosclerosis and ultimately represents an imbalance between the magnitude of injury process and repair capacity (Zhu et al., 2008). Previous studies suggested that circulating endothelial progenitor cells (EPCs) participate in this endothelial repair process (Hill et al., 2003; Zhu et al., 2008).
EPCs have the capacity of homing from bone marrow, and circulate, proliferate, and differentiate into mature endothelial cells, but they have neither acquired characteristics of mature endothelial markers, nor formed a lumen (Asahara et al., 1997; Zhu et al., 2008). EPCs coexpress hematopoietic stem or progenitor cell markers (CD34 or AC133) and endothelial surface markers (VE-Cadherin or VEGFR-2) (Peichev et al., 2000; Gill et al., 2001; Zhu et al., 2008). Laboratory evidence suggests that these precursor cells participate in the processes of postnatal neovascularization and re-endothelialization (Asahara et al., 1999; Hill et al., 2003). Recently, studies have also shown that risk factors for CAD correlate with reduced number and functional activity of circulating EPCs (Vasa et al., 2001). However, the relationship between adiponection and circulating EPCs remains unclear. If a correlation between plasma adiponectin levels and the number and activity of circulating EPCs was confirmed, drug and/or non-drug treatments that aim at increasing circulating adiponectin levels could be useful for treating CAD.
Based on these considerations, we hypothesize that EPCs of patients with hypoadiponectinemia are impaired, and these damaged EPCs may lead to endothelial dysfunction and promote the progression of the disease. Therefore, we investigated the relationships among plasma adiponectin concentrations and the number and activity of circulating EPCs from CAD patients and control subjects.
MATERIALS AND METHODS
Patients
Fifty-eight CAD patients and 50 control subjects were prospectively studied. CAD was defined angiographically (>50% diameter reduction). Plasma adiponectin concentration was determined by enzyme-linked immunosorbent assay (ELISA) (Linco Research Inc., Missouri, USA). Other basal characteristics such as age, sex, blood pressure, body mass index (BMI), and lipid profiles were also included in clinical data. Patients with acute inflammation, wounds, retinopathy, recent surgery, ulcers, malignant disease, and unstable angina or myocardial infraction within 3 months, which may influence EPC kinetics, were excluded. All patients and control subjects were not treated with vitamins or folate. All participants provided informed consent and the study protocol was approved by the local research ethics committee.
Isolation, cultivation, and characterization of circulating EPCs
EPCs were isolated, cultured, and characterized according to previously described techniques (Hill et al., 2003; Zhu et al., 2006; 2008). Briefly, mononuclear cells were isolated by density gradient centrifugation and seeded on six-well culture dishes coated with fibronectin (Chemicon, Temecula, CA, USA) in endothelial basal medium (EBM)-2 (Clonetics, Walkersville, MD, USA) supplemented with endothelial basal medium (EBM)-2 with single aliquot of EGM-2MV, which contains 5% fetal bovine serum (FBS), vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), epidermal growth factor (EGF), insulin-like growth factor (IGF), and ascorbic acid. After 7 d in culture, adherent cells were incubated with DiI-acLDL (Molecular Probes, Eugene, OR, USA) and stained with fluorescein isothiocyanate (FITC)-labelled ulex europaeus agglutinin (UEA-1) (Sigma Chemical Co., St. Louis, MO, USA). Cells co-stained with DiI-acLDL and FITC-conjugated lectin were judged as EPCs. Under fluorescent microscope, the number of EPCs per well was evaluated by three independent investigators by counting five randomly selected high-power fields (×200) (Chen et al., 2006; Zhu et al., 2006; 2008).
Flow cytometry analysis
Cell surface marker proteins of circulating EPCs (AC133+/KDR+) were measured by flow cytometry (Chen et al., 2006; Zhu et al., 2006; 2008). A volume of 100 μl of peripheral blood was obtained and incubated for 30 min at 4 °C with antihuman-KDR-PE (R&D Systems, Minneapolis, USA) and antihuman-AC133-APC (eBioscence, San Diego, USA). The suspension was collected and incubated with fluorescence-activated cell sorter (FACS) lysing solution for 10~15 min (Chen et al., 2006; Zhu et al., 2008). FACS-Calibur instrument (Becton-Dickson, New Jersey, USA) was used to analyze cell surface marker proteins. Surface markers of EPCs, AC133, and KDR were detected on cells in the lymphocyte gate (Chen et al., 2006; Zhu et al., 2006; 2008). Isotype-matched antibodies served as controls. The percentages of AC133+/KDR+ positive cells were converted to cell per ml peripheral blood using the total blood count (Vasa et al., 2001; Chen et al., 2006; Zhu et al., 2006; 2008).
Migration assay
A modified Boyden chamber assay (Jiangsu Qilin Medical Equipment, China) was used to evaluate EPC migration (Chen et al., 2006; Zhu et al., 2006; 2008). Isolated EPCs were detached and resuspended in 500 μl EBM-2 and counted. A total of 2×104 EPCs were placed in the upper chamber, and the lower compartment was filled with medium containing EBM-2 and VEGF (50 ng/ml). After 24 h incubation, the lower side of the filter was washed with phosphate buffered saline (PBS) and fixed with 2% paraformaldehyde (Chen et al., 2006; Zhu et al., 2006; 2008). Cells migrating into the lower chamber were counted manually in three random microscopic fields (×200) (Vasa et al., 2001; Chen et al., 2006; Zhu et al., 2006; 2008).
Cell adhesion assay
EPC adhesion was measured by replacing into fibronectin-coated culture dishes as described previously (Chen et al., 2006; Zhu et al., 2006; 2008). Isolated EPCs were resuspended and counted. After the identical number of cells were reseeded into culture dishes coated with fibronectin for incubation for 30 min at 37 °C in 5% CO2, the adherent cells averagely were counted by three independent investigators (Vasa et al., 2001; Chen et al., 2006; Zhu et al., 2006; 2008).
Statistical analysis
Date are presented as percentages or mean±SD. Student’s t-test was used to assess differences between two groups, χ 2 text to compare the categorical variables of CAD patients and control subjects, and Pearson’s correlation analysis to analyze the correlations between adiponectin levels and the number and activities of circulating EPCs. All statistical analyses were performed with SPSS 11.5, and values of P<0.05 were considered significant.
RESULTS
Plasma adiponectin levels and patient characteristics
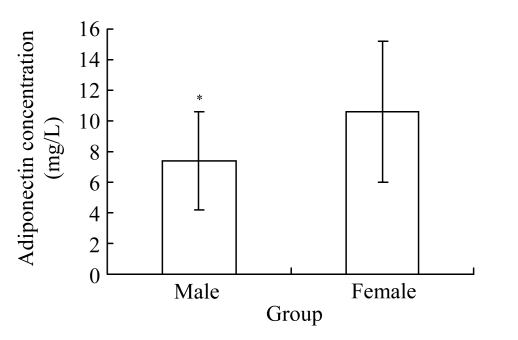
A total of 108 participants (58 CAD patients and 50 control subjects) were enrolled in the present study. Patient characteristics and biochemical measurement data are shown in Table 1. A significant decrease was observed in adiponectin in CAD patients compared with matched control subjects [(8.3±3.9) vs (11.5±4.3) mg/L, P<0.01]. The concentration of plasma adiponectin was significantly lower in males compared with that in females in both CAD and control groups [(7.3±3.2) vs (10.6±4.6) mg/L, P<0.01; Fig.1].
Table 1.
Clinical data for patients with CAD and control subjects
| Characteristics | CAD (n=58) | Control (n=50) |
| Age (year) | 65.8±8.2 | 64.8±6.6 |
| Patient number | ||
| Female | 17 (29.3%) | 16 (32%) |
| Current smoking | 25 (43.1%) | 20 (40%) |
| Hypertension | 34 (58.6%) | 26 (52%) |
| Diabetes mellitus | 13 (22.4%) | 9 (18%) |
| BMI (kg/m2) | 24.2±1.70 | 23.6±2.10 |
| Triglyceride (mmol/L) | 1.73±0.29 | 1.65±0.31 |
| Uric acid (μmol/L) | 377.6±52.1 | 373.3±73.4 |
| hs-CRP (mg/L) | 3.21±1.94* | 2.19±0.99 |
| Adiponectin (mg/L) | 8.3±3.9* | 11.5±4.30 |
Values are percentages or mean±SD;
P<0.01 vs control subjects
Fig. 1.
Plasma adiponectin concentration was significantly lower in males compared with that in females (* P<0.01) in CAD patients
Association of adiponectin with EPC levels
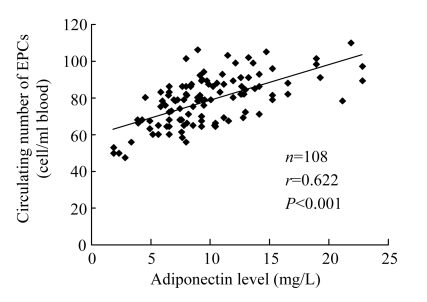
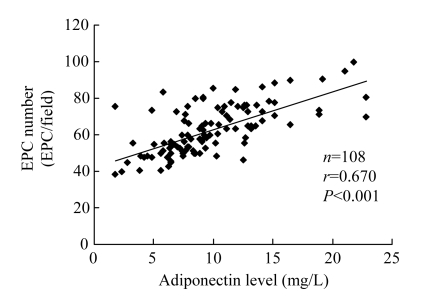
Fluorescence-activated cell sorter analysis was used to assay the number of EPCs in the peripheral blood. Circulating EPCs were enumerated as AC133+/KDR+ cells (Vasa et al., 2001; Chen et al., 2006; Zhu et al., 2008). A significant decrease was observed in circulating EPCs in CAD patients compared with matched control subjects [(74.2±12.3) vs (83.5±12.9) cells/ml blood, P<0.01], and a positive correlation was shown between the number of EPCs with plasma adiponectin concentration (r=0.622, P<0.001; Fig.2). In addition, the number of EPCs decreased in CAD patients compared with matched control subjects after ex vivo cultivation [(54.4±8.6) vs (71.9±11.6) EPCs/field, P<0.01], and a positive correlation between plasma adiponectin concentration and the number of EPCs was also demonstrated (r=0.670, P<0.001; Fig.3).
Fig. 2.
Positive relation between plasma adiponectin concentration and circulating EPC numbers
Fig. 3.
Positive relation between plasma adiponectin concentration and EPC numbers after ex vivo cultivation
Association of adiponectin with migratory capacity of EPCs
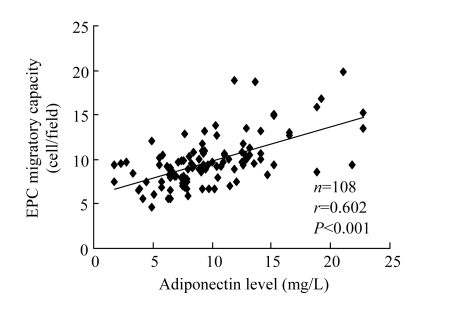
The migratory capacity of EPCs isolated from CAD patients was significantly damaged compared with matched control subjects [(9.1±2.3) vs (10.5±3.2) cells/fields, P<0.001]. A significant positive correlation between adiponectin concentration and EPC migratory activity was detectable (r=0.602, P<0.001; Fig.4).
Fig. 4.
Positive relation between plasma adiponectin concentration and EPC migratory activity after ex vivo cultivation after ex vivo cultivation
Association of adiponectin with adhesion capacity of EPCs
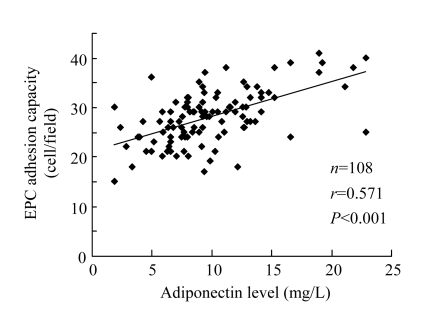
EPCs from CAD patients were significantly impaired in their ability to adhere to fibronectin [(26.7±5.3) vs (29.6±5.0) cells/fields, P<0.001]. Plasma adiponectin concentration showed a significantly positive correlation with the ability of EPCs to adhere to fibronectin (r=0.571, P<0.001; Fig.5).
Fig. 5.
Positive relation between plasma adiponectin concentration and EPC adhesion activity after ex vivo cultivation
DISCUSSION
The present study demonstrates the relationships of plasma adiponectin concentration with number and functional activity of EPCs from peripheral blood of CAD patients. The results indicate that plasma adiponectin concentration significantly decreased in CAD patients compared with matched control subjects, and positively correlated with the number of circulating ECPs. The plasma adiponectin concentration was significantly lower in males, compared with females, in both CAD and control groups. In addition, the present study also shows that adiponectin levels had significantly positive correlations with the functional activities of EPCs such as adhesive and migratory capacities. These findings indicate that lower circulating adiponectin level reflected the decreased number and impaired functional activities of EPCs in CAD patients. Therefore, therapeutic approaches, drug and/or non-drug, aiming at increasing circulating adiponectin levels could be use to treat CAD.
Adipose tissue is not only a simple energy storage compartment, but also an important secretary organ for bioactive molecules which contribute to the pathophysiology of cardiovascular diseases (Funahashi et al., 1999). Adiponectin is one of adipokines and is synthesized and secreted exclusively by the adipose tissue. Plasma adiponectin level was decreased in CAD patients (Ouchi et al., 2001). Adiponectin has anti-atherogenic property, and hypoadiponectinemia is associated with the development of cardiovascular diseases. Okamoto et al.(2002) reported that adiponectin overexpression decreased atherosclerosis by attenuating the endothelial inflammatory response and macrophage-to-foam cell transformation in vivo. Further, the clinical study showed that adiponectin concentration rapidly declined following acute myocardial infarction (Kojima et al., 2003). In our study, the number and activity of circulating EPCs were significantly reduced and impaired in the CAD patients with lower plasma adiponectin. This suggests that adiponectin has beneficial effects on vascular function and plays a protective role against atherosclerotic vascular change, and low plasma adiponectin, a well-known independent risk factor for CAD, may enhance endothelial dysfunction.
The integrity and functional activity of endothelial monolayer play a critical role in atherosclerosis. Risk factors in CAD patients may induce endothelial injury and impair endothelial function, which also predicts subsequent cardiovascular events (Davignon and Ganz, 2004). Endothelium is a barrier between blood and vessel. The extent of endothelial injury may represent a balance between the magnitude of injury and the capacity for repair (Hill et al., 2003). Recent studies suggest that the injured endothelial monolayer is regenerated by circulating bone marrow-derived EPCs (Hill et al., 2003; Walter et al., 2002). These reports have suggested that levels of circulating EPCs are associated with endothelial function and contribute to atherosclerotic disease progression. In the present study, adiponectin levels of CAD patients had significantly positive correlations with the number and functional activities of circulating EPCs. In concordance with our findings, hypoadiponectinemia patients with CAD have a decreased number of circulating EPCs with impaired functional activities (Satoh et al., 2008). Therefore, lower adiponectin may decrease the number and impair the activity of circulating EPCs, reducing the capacity for endothelial repair, ultimately contributing to the progression of atherosclerosis, and leading to CAD.
Hypoadiponectinemia was often observed in patients with obesity-linked disease (Arita et al., 1999). Okui et al.(2008) reported that obesity-linked disease was associated with increased risk of CAD, and low adiponectin levels were considered to be an independent risk factor for CAD and a better predictor of coronary endothelial function than other factors such as HOMA-R, body mass index, immunoreactive insulin, and triglycerides. Although the exact mechanisms by which the number and functional activity of EPCs were damaged in CAD patients with low adiponectin levels remain unclear up to now, several possible reasons could account for the impaired EPCs. Firstly, it is suggested in in vitro experiments that C reactive protein (CRP) attenuates the survival of EPCs, and it has been recently demonstrated that CRP has the potential to decrease the angiogenic function of EPCs (Fujii et al., 2006; Wang et al., 2007; Zhu et al., 2008). Ouchi et al.(2003b) reported that human adipose tissue expressed CRP, and that there was an inversed relation between CRP and adiponectin in both plasma and adipose tissue. Therefore, increasing of CRP and decreasing of adiponectin in plasma might contribute to the damage of EPCs. The second explanation is that the decreased number and functional activity of EPCs in CAD patients with low adiponectin levels might have relation with adipovascular axis, which, through genomic and environmental influences, affects the supply of EPCs.
There are a few limitations in our study. First, patients in this study were recruited from ones scheduled for elective coronary angiography; therefore, they might not be representative of the population at large. However, none of the patients had myocardial infarction and acute coronary syndrome. Future studies on larger scales will be needed. Second, the present study was limited to the measurement of plasma adiponectin concentration, and it did not assess whether high molecular forms of this protein would be associated with the number and activity of circulating EPCs. Third, the exact mechanisms, especially the molecular pathway(s), responsible for decreasing the number and impairing the functional activity of EPCs in CAD patients with hypoadiponectinemia have not been determined, to which further studies are needed to address.
In conclusion, correlations between adiponectin and the number and activity of circulating EPCs were significantly positive in CAD patients. Thus, therapeutic approaches, drug and/or non-drug treatments, aiming at increasing circulating adiponectin levels could be useful for treating CAD and other diseases associated with vascular insufficiency.
References
- 1.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278(45):45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 5.Chen TG, Chen JZ, Wang XX. Effects of rapamycin on number activity and eNOS of endothelial progenitor cells from peripheral blood. Cell Prolif. 2006;39(2):117–125. doi: 10.1111/j.1365-2184.2006.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl. 1):III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 7.Fujii H, Li SH, Szmitko PE, Fedak PW, Verma S. C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2006;26(11):2476–2482. doi: 10.1161/01.ATV.0000242794.65541.02. [DOI] [PubMed] [Google Scholar]
- 8.Funahashi T, Nakamura T, Shimomura I, Maeda K, Kuriyama H, Takahashi M, Arita Y, Kihara S, Matsuzawa Y. Role of adipocytokines on the pathogenesis of atherosclerosis in visceral obesity. Intern Med. 1999;38(2):202–206. doi: 10.2169/internalmedicine.38.202. [DOI] [PubMed] [Google Scholar]
- 9.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88(2):167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 10.Higashiura K, Ura N, Ohata J, Togashi N, Takagi S, Saitoh S, Murakami H, Takagawa Y, Shimamoto K. Correlations of adiponectin level with insulin resistance and atherosclerosis in Japanese male populations. Clin Endocrinol (Oxf) 2004;61(6):753–759. doi: 10.1111/j.1365-2265.2004.02165.x. [DOI] [PubMed] [Google Scholar]
- 11.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 12.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20(6):1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 13.Iwashima Y, Horio T, Kumada M, Suzuki Y, Kihara S, Rakugi H, Kawano Y, Funahashi T, Ogihara T. Adiponectin and renal function, and implication as a risk of cardiovascular disease. Am J Cardiol. 2006;98(12):1603–1608. doi: 10.1016/j.amjcard.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 14.Iwashima Y, Horio T, Suzuki Y, Kihara S, Rakugi H, Kangawa K, Funahashi T, Ogihara T, Kawano Y. Adiponectin and inflammatory markers in peripheral arterial occlusive disease. Atherosclerosis. 2006;188(2):384–390. doi: 10.1016/j.atherosclerosis.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 15.Kojima S, Funahashi T, Sakamoto T, Miyamoto S, Soejima H, Hokamaki J, Kajiwara I, Sugiyama S, Yoshimura M, Fujimoto K, et al. The variation of plasma concentrations of a novel, adipocyte derived protein, adiponectin, in patients with acute myocardial infarction. Heart. 2003;89(6):667. doi: 10.1136/heart.89.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motoshima H, Wu X, Mahadev K, Goldstein BJ. Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun. 2004;315(2):264–271. doi: 10.1016/j.bbrc.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106(22):2767–2770. doi: 10.1161/01.CIR.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 18.Okui H, Hamasaki S, Ishida S, Kataoka T, Orihara K, Fukudome T, Ogawa M, Oketani N, Saihara K, Shinsato T, et al. Adiponectin is a better predictor of endothelial function of the coronary artery than HOMA-R, body mass index, immunoreactive insulin, or triglycerides. Int J Cardiol. 2008;126(1):53–61. doi: 10.1016/j.ijcard.2007.03.116. [DOI] [PubMed] [Google Scholar]
- 19.Otake H, Shite J, Shinke T, Watanabe S, Tanino Y, Ogasawara D, Sawada T, Hirata K, Yokoyama M. Relation between plasma adiponectin, high-sensitivity C-reactive protein, and coronary plaque components in patients with acute coronary syndrome. Am J Cardiol. 2008;101(1):1–7. doi: 10.1016/j.amjcard.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 20.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100(25):2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 21.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103(8):1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 22.Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42(3):231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- 23.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107(5):671–674. doi: 10.1161/01.CIR.0000055188.83694.B3. [DOI] [PubMed] [Google Scholar]
- 24.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–958. [PubMed] [Google Scholar]
- 25.Satoh M, Ishikawa Y, Takahashi Y, Itoh T, Minami Y, Nakamura M. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 2008;198(2):347–353. doi: 10.1016/j.atherosclerosis.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 26.Tirziu D, Simons M. Angiogenesis in the human heart: gene and cell therapy. Angiogenesis. 2005;8(3):241–251. doi: 10.1007/s10456-005-9011-z. [DOI] [PubMed] [Google Scholar]
- 27.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 28.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105(25):3017–3024. doi: 10.1161/01.CIR.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 29.Wang HY, Gao PJ, Ji KD, Shen WF, Fan CL, Lu L, Zhu DL. Circulating endothelial progenitor cells, C-reactive protein and severity of coronary stenosis in Chinese patients with coronary artery disease. Hypertens Res. 2007;30(2):133–141. doi: 10.1291/hypres.30.133. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Wang X, Chen J, Sun J, Zhang F. Reduced number and activity of circulating endothelial progenitor cells from patients with hyperhomocysteinemia. Arch Med Res. 2006;37(4):484–489. doi: 10.1016/j.arcmed.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Zhu JH, Wang XX, Fu GS, Shang YP, Zhang FR, Chen JZ. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med. 2008;102(7):1073–1079. doi: 10.1016/j.rmed.2007.12.030. [DOI] [PubMed] [Google Scholar]