Abstract
Astragalus mongholicus (AM) derived from the dry root of Astragalus membranaceus Bge. var. mongolicus (Bge.) Hsiao is a widely used traditional Chinese medicine. The present study investigated the potential role of AM on renal fibrosis on a rat model of unilateral ureteral obstruction (UUO). We divided 48 Sprague-Dawley rats randomly into 4 groups: sham-operated group (Sham), untreated UUO group, AM-treated (10 g/(kg·d)) UUO group, and losartan-treated (20 mg/(kg·d)) UUO group as positive control. Haematoxylin & eosin (HE) and Masson staining were used to study the dynamic histological changes of the kidneys 7 and 14 d after operation. The expressions of fibronectin (FN), type I collagen (colI), hepatocyte growth factor (HGF), transforming growth factor-β1 (TGF-β1), and α-smooth muscle actin (α-SMA) were analyzed by real-time polymerase chain reaction (PCR), immunohistochemistry staining, and Western blot. Results show that, similar to losartan, AM alleviated the renal damage and decreased the deposition of FN and colI from UUO by reducing the expressions of TGF-β1 and α-SMA (P<0.05), whereas HGF increased greatly with AM treatment (P<0.05). Our findings reveal that AM could retard the progression of renal fibrosis. The renoprotective effect of AM might be related to inhibition of myofibroblast activation, inducing of HGF and reducing of TGF-β1 expression.
Keywords: Astragalus mongholicus (AM), Myofibroblast, Transforming growth factor-β1 (TGF-β1), Renal interstitial fibrosis, Hepatocyte growth factor (HGF)
INTRODUCTION
Renal interstitial fibrosis (RIF) is a common histopathological change and the major determinant in the progression of chronic kidney diseases that lead to end-stage renal failure. It is characterized by the accumulation of extracellular matrix (ECM) proteins in the renal tubulointerstitium (Eddy, 1996; Remuzzi and Bertani, 1998). The key event in the progression of RIF is the activation of α-smooth muscle actin (α-SMA)-positive myofibroblast cells, which is identified to be the main source of excessive ECM deposition and the best prognostic indicator of renal diseases both in human and experimental animal models (Roberts et al., 1997; Essawy et al., 1997). However, the origin of myofibroblast cells is still undefined. Emerging evidence indicates that a large proportion of interstitial fibroblasts actually originate from tubular epithelial cells via epithelial to myofibroblast transition (EMT) in diseased kidney (Liu, 2004a).
Previous studies from our laboratory showed that transforming growth factor-β1 (TGF-β1), a multifunctional cytokine with profibrogenic properties, was a central mediator that regulated transdifferentiation of tubular epithelial cells into α-SMA-positive myofibroblasts (Fan et al., 1999). Furthermore, we demonstrated that hepatocyte growth factor (HGF) exhibited a remarkable ability to block this phenotypic transition both in vitro and in vivo (Zhong et al., 2005; Xue et al., 2005). These observations suggested that upregulation of endogenous HGF expression and reducing of TGF-β1 expression could be essential for maintaining the tubular epithelial cell phenotype by blocking EMT. This could provide a potential strategy for the treatment of renal fibrosis.
During the recent decades, Chinese medicinal herbs and their extracts have drawn great attention to the prevention of renal fibrosis. Astragalus mongholicus (AM) derived from the dry root of Astragalus membranaceus Bge. var. mongolicus (Bge.) Hsiao, which belongs to leguminous plant of the Astragalus family, is one of the most popular traditional Chinese medicines. AM is composed of glycoside, astragalus polysaccharides, multi-amino acids, astragalus total saponin, caritinoid, astragalus total flavonoids, and microelement. It has been widely used in China and East Asia area for many years to treat myocardial ischemia, liver fibrosis, chronic nephritis, diabetes, etc. (Han et al., 2000). Although the herb is commonly used in treating various diseases in Chinese herbal formula, scientific reports on its alleviation of tubulointerstitial fibrosis are limited. The mechanism of its renoprotection is lacking. In recent reports, AM has been shown to possess renoprotective activity by attenuating glomerular sclerotic injury in experimental diabetic nephropathy (Yang and Zhu, 2005; Mou et al., 2002). In the present study, we sought to investigate whether the anti-fibrotic action of AM goes beyond the glomeruli by using a unilateral ureteral obstruction (UUO) animal model. We provide evidence to support that AM may attenuate renal fibrosis likely by derivation of HGF and suppression of TGF-β1.
MATERIALS AND METHODS
Drugs
Astragalus mongholicus injections, which were made of the extract of Astragalus mongholicus, were purchased from Chiatai Qingchunbao Pharmaceutical Co., Ltd. (Hangzhou, China) with batch No. 0508081 containing crude drug 2 g/ml.
Animals and experimental design
A total of 48 male Sprague-Dawley (SD) rats (6 weeks old, weighing 180~200 g) were obtained from the Laboratory Animal Center of Sichuan University (Chengdu, China), and were treated strictly according to the Health Guide for the Care and Use of Laboratory Animals of the National Institutes.
UUO was induced by left ureteral ligation with an established procedure as previously described (Chevalier et al., 2000). Sham-operated rats had their ureters manipulated but not ligated. The animals that underwent surgery were randomly divided into four groups: UUO, Sham, AM, and losartan (an angiotensin type-1 receptor blocker, ARB) groups, with 12 rats each group. Treatment started within 1 h after the surgical procedure. Rats in the AM group were intraperitoneally injected with AM at 10 g/(kg·d) (this dose of AM has been shown to be effective in attenuating renal fibrosis without obviously adverse effect in UUO model in the pilot study from our laboratory), while rats in the Sham, UUO, and ARB groups were intraperitoneally injected with identical voluminal normal saline. Rats in the ARB group were intragastricly administrated with losartan (Merck Sharp & Dohme, USA) 20 mg/(kg·d), while those of the Sham, UUO, and AM groups were intragastricly administrated with identical volume of normal saline. Days 7 and 14 after surgery, 6 rats selected randomly from each group were sacrificed, and the left kidneys were harvested and kept at −80 °C. Some kidney tissue samples were immersed with 10% (w/v) neutral-buffered formalin for histopathological and immunohistochemical evaluations.
Histopathological examination
Fixed tissues were dehydrated in graded alcohol and embedded in paraffin. Sections were stained with hematoxylin & eosin (HE) for general histology. Injury score was evaluated with a scale of 0~4 as previously reported (Taal et al., 2000).
Masson-Trichrome staining was performed in accordance with the standard protocol using a reagent kit (Sigma, USA). Ten separate fields of each section were scanned at 400× magnification and put into digital form using imaging software (Photoshop, Adobe, San Jose, California). The number of points covering the blue collagen staining was counted and the extent of collagen deposition was evaluated (Mizuguchi et al., 2004; Xie et al., 2008).
Immunohistochemical staining analysis
Paraffin-embedded sections (5 μm) were deparaffinized, washed with phosphate-buffered saline (PBS), treated with 3% (v/v) H2O2 for 5 min, blocked with 10% (w/v) normal goat serum for 1 h, and then incubated with primary antibodies, including rabbit antibodies to α-SMA (ABCAM, UK), rabbit antibodies to HGF (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit antibodies to TGF-β1 (Santa Cruz Biotechnology), rabbit antibodies to collagen I (colI), and rabbit antibodies to fibronectin (FN) (Boshide Biological Technology Co., Ltd., Wuhan, China), at 4 °C overnight as described previously (Xie et al., 2008). Slides then were incubated with appropriate biotin-conjugated secondary immunoglobulin G, and treated with reagents from a Vecta-Elite streptavidin-peroxidase kit (Vector Laboratories, Burlingame, CA, USA). The scores were determined as previously reported (Fu et al., 2006). Briefly, the percentage of positive stained area in the examined tubulointerstitium was measured and scored in a blind manner on slides.
Quantitative real-time polymerase chain reaction (PCR) analysis
Total RNA was extracted by RNeasy Mini RNA extracting kit (QIAGEN, USA) following the manufacturer’s protocol. The cDNA was synthesized and real-time PCR was run as described previously (Xie et al., 2008). Primers and probes of α-SMA, TGF-β1, HGF, fibronectin, collagen I, and the internal calibrator glyceraldehyde-3-phosphate dehydrogenase (GAPDH, synthesized by Sangon, Shanghai, China) were listed in Table 1.
Table 1.
Primers and probes of fibronectin (FN), collagen I (colI), α-SMA, HGF, TGF-β1 and the internal calibrator GAPDH
| Primers | Probes | |
| FN | Forward: 5′-CCATTGCAAATCGCTGCCAT-3′ | TaqMan: 5′-CTCATGTGGCCTCCTCCAC-3′ |
| Reverse: 5′-AACATTTCTCAGCTATTGGCTT-3′ | ||
| ColI | Forward: 5′-TGGCGCTTCAGGTCCAAT-3′ | TaqMan: 5′-CCAGCTTCCCCATCATCTCC-3′ |
| Reverse: 5′-TGTTCCAGGCAATCCACGAG-3′ | ||
| α-SMA | Forward: 5′-GTTCGAAACCTTCAATGTTCCT-3′ | TaqMan: 5′-ATCTACGAGGGCTATGCACTG-3′ |
| Reverse: 5′-CCATTCAAGCTGTGCTCTCGCT-3′ | ||
| HGF | Forward: 5′-GACCTTGTGAGGGAGATTAT-3′ | TaqMan: 5′-CTTGGTGTCATTGTTCCTGGTC-3′ |
| Reverse: 5′-ATGTGCCATCCCAAATCGTCC-3′ | ||
| TGF-β1 | Forward: 5′-CCAACTATTGCTTCAGCTCCA-3′ | TaqMan: 5′-CCAAGGGCTACCATGCCAAC-3′ |
| Reverse: 5′-GTGTCCAGGCTCCAAATGT-3′ | ||
| GAPDH | Forward: 5′-TGGGTGTGAACCAGGAGAA-3′ | TaqMan: 5′-ACTGCACCACCAACTGCTTAGC-3′ |
| Reverse: 5′-GGCATGGACTGTGGTCATGA-3′ |
A 30 μl of PCR reaction solution was amplified in Roche Lightcycler (Roche Diagnostics, USA). The thermocycles of PCR reaction were performed as reported previously (Xie et al., 2008). PCR products then went through agarose-gel electrophoretic analysis, and fluorescence signal were collected to calculate Ct (cycles of threshold). Serial dilutions of sample RNA (500 ng), including α-SMA, TGF-β1, HGF, fibronectin, collagen I (10× dilution), GAPDH (10× dilution), were performed to establish the standard curves for determining the efficiency of real-time PCR. The method of Pfaffl et al.(2002) was used to analyze the real-time PCR results.
Western blot analysis
Protein from kidney tissues was extracted and electrophoresed on SDS-polyacrylamide gel for Western blot analysis as previously reported (Fu et al., 2006). In brief, samples were heated at 99 °C for 5 min and then transferred to a polyvinylidene difluoride membrane. Nonspecific binding to the membrane was blocked for 1 h at room temperature with 5% (w/v) bovine serum albumin (BSA) in Tris-buffered saline buffer (20 mmol/L Tris-HCl, 150 mmol/L NaCl, and 0.1% (v/v) Tween 20). The membranes were then incubated overnight at 4 °C with primary antibodies, including rabbit antibodies to α-SMA, rabbit antibodies to HGF, rabbit antibodies to TGF-β1, and GAPDH (Chemicon, Temecula, CA). After washing, the membranes were incubated with appropriate secondary immunoglobulin G-peroxidase conjugates (1:10 000 dilution; Kirkegaard Perry Laboratories, Gaithersburg, MD, USA), and developed using the enhanced chemiluminescence method. Band intensities were analyzed using a software named Quantity one. For quantification, the densities of the signals were normalized to that for GAPDH.
Statistical analyses
All data were expressed as mean±SD. One-way analysis of variance (ANOVA) was used to perform comparisons between the different groups, and a P value <0.05 was considered as statistically significant. All analyses were performed with the SPSS statistical software package (SPSS, Chicago, IL, USA).
RESULTS
Effect of AM on the histological changes
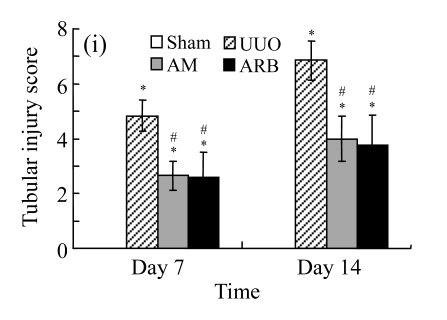
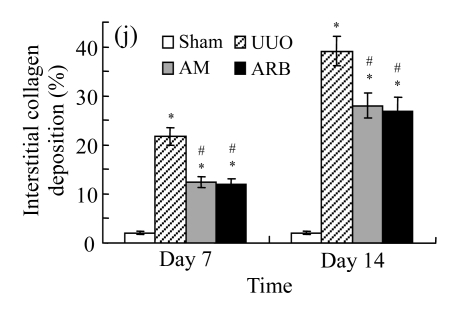
On Day 7, the kidneys of rats subjected to ureteral obstruction developed a conspicuous tubulointerstitial injury characterized by tubular dilatation and atrophy, interstitial inflammation, and a marked interstitial fibrosis. The damage was more prominent on Day 14. Renal injury was ameliorated after treatments with AM and losartan as indicated by the lower tubulointerstitial injury scores and interstitial fibrosis expansion of these groups, with P<0.05 for all comparisons (Table 2 and Fig.1 in page 385). There was no significant difference between AM and ARB groups (P>0.05).
Table 2.
Comparison of tubulointerstitial injury score (TIS) and interstitial collagen deposition (ICD) in renal tissues of rats in different groups
| Group |
TIS |
ICD (%) |
||
| Day 7 | Day 14 | Day 7 | Day 14 | |
| Sham | 0.01±0.02 | 0.01±0.01 | 2.09±0.20 | 2.09±0.25 |
| UUO | 4.83±0.72* | 6.85±1.12* | 21.78±2.95* | 39.11±3.87* |
| AM | 2.65±1.11*# | 4.00±0.65*# | 12.39±2.50*# | 28.00±3.20*# |
| ARB | 2.61±0.82*# | 3.76±0.45*# | 11.99±3.05*# | 26.74±3.19*# |
Compared with the Sham group, P<0.05;
Compared with the UUO group, P<0.05
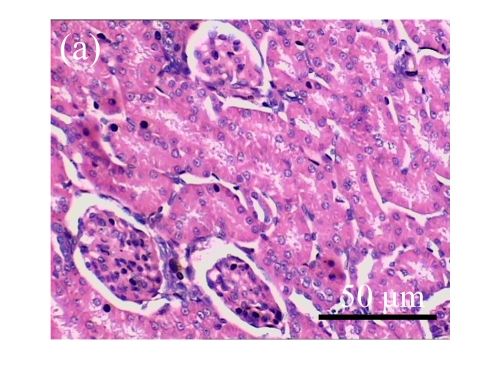
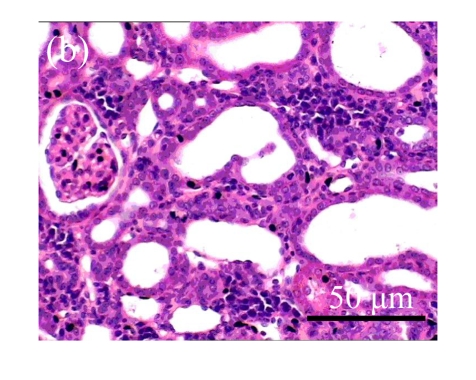
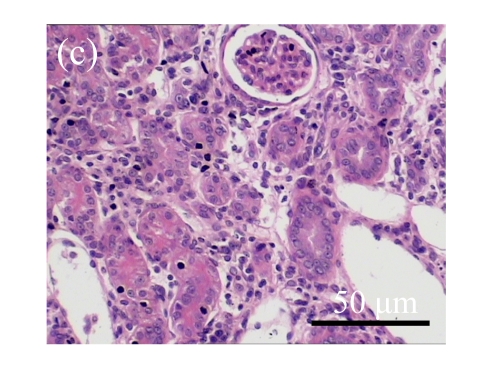
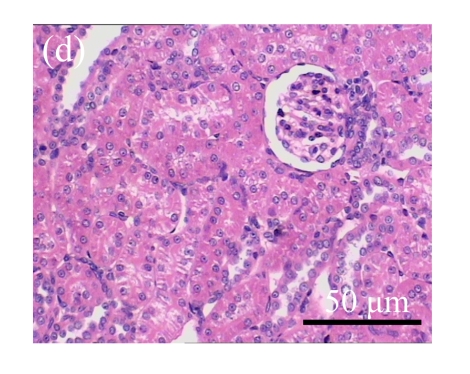
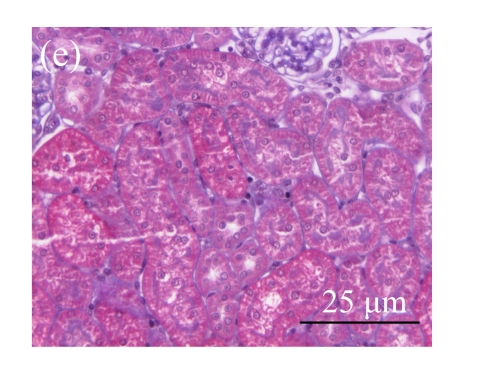
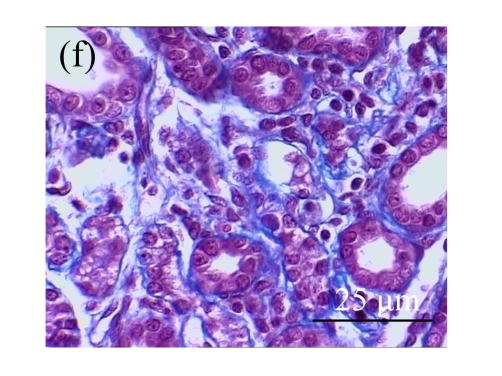
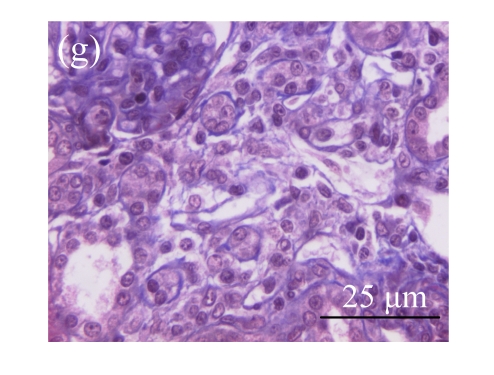
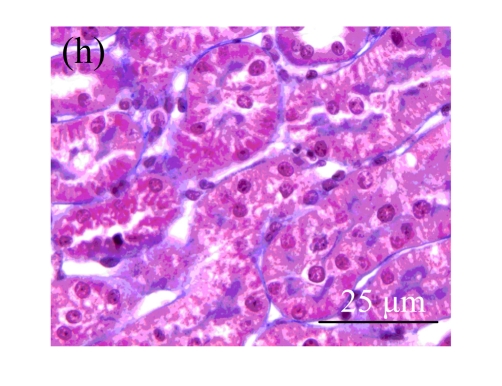
Fig. 1.
Sections of the rat kidneys stained with hematoxylin & eosin and Masson of different groups on Day 14 after operation. (a~d) Representative photographs of histologic changes visualized by hematoxylin & eosin staining; (e~h) Representative photographs of histologic changes visualized by Masson staining. (a) & (e) Sham-operated control rats; (b) & (f) Untreated UUO control rats; (c) & (g) AM-treated rats; (d) & (h) Losartan-treated rats. Changes in tubular injury scores (i) and interstitial collagen deposition (j) in renal tissues of rats in different groups were shown in histograms. Histologic damage including tubular injury scores and interstitial collagen deposition was exacerbated in the obstructed kidneys, and was greatly attenuated by the intervention of AM and losartan. Data are expressed as mean±SD (n=6); * P<0.05 vs the Sham group, # P<0.05 vs the UUO group
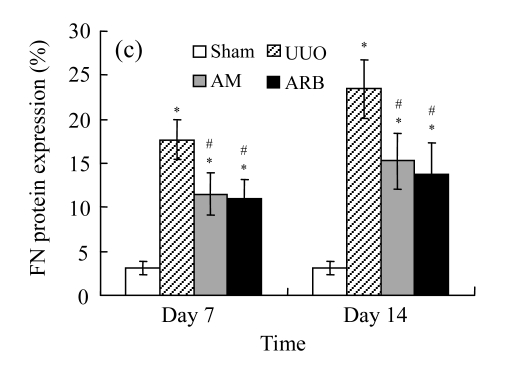
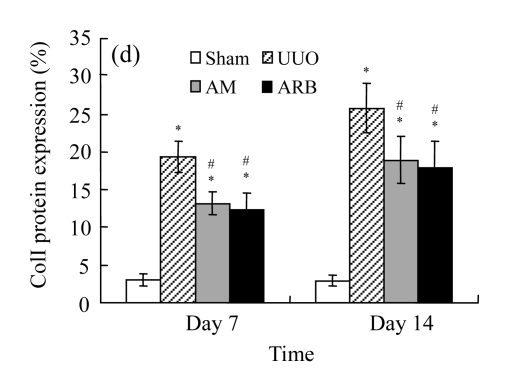
Effect of AM on the expressions of fibronectin and collagen I
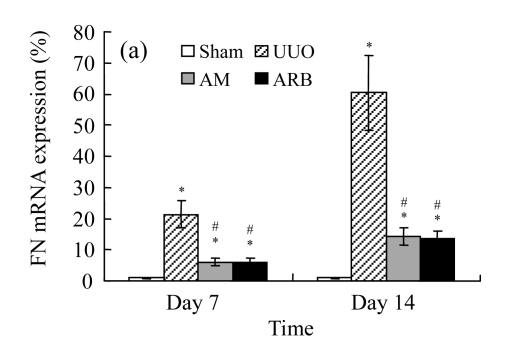
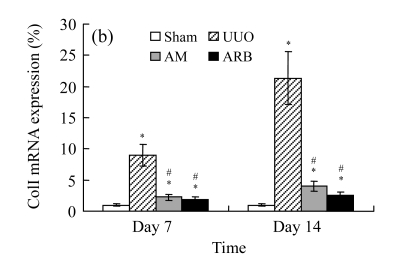
A marked increase in tubulointerstitial fibronectin and collagen I accumulation was observed on Days 7 and 14 after UUO compared with the sham-operated animals. On Days 7 and 14, the mRNA and protein levels of collagen I and fibronectin reduced greatly in the AM and ARB groups as compared with the UUO group (Table 3 and Fig.2), with P<0.05 for all comparisons. Again, there was no significant difference between AM and ARB groups (P>0.05).
Table 3.
Comparisons of protein expressions of α-SMA, fibronectin (FN), and collagen I (colI) in renal tissues of different groups by immunohistochemical analysis
| Group | Protein expression (%) |
|||||
| α-SMA |
FN |
ColI |
||||
| Day 7 | Day 14 | Day 7 | Day 14 | Day 7 | Day 14 | |
| Sham | 0.37±0.12 | 0.43±0.12 | 3.05±0.73 | 3.06±0.73 | 3.04±0.71 | 2.93±0.50 |
| UUO | 8.69±2.21* | 18.80±3.31* | 17.65±3.29* | 23.43±4.69* | 19.31±3.25* | 25.77±3.77* |
| AM | 4.67±1.46*# | 13.45±2.13*# | 11.50±3.14*# | 15.27±4.01*# | 13.12±3.12*# | 18.91±3.37*# |
| ARB | 4.20±1.37*# | 11.12±3.12*# | 10.94±3.49*# | 13.84±4.77*# | 12.36±3.54*# | 17.95±3.19*# |
Compared with the Sham group, P<0.05;
Compared with the UUO group, P<0.05
Fig. 2.
The effect of AM on fibronectin (FN) and collagen I (colI) expressions by immunohistochemical staining and RT-PCR detection: FN mRNA (a) and colI mRNA (b) expressions in renal tissues of different groups by semi-quantitative RT-PCR analysis; change in FN (c) and colI (d) protein expressions in renal tissues of different groups by immunohistochemical analysis
Compared with the UUO group, AM and ARB groups had a greatly reduced deposition of FN and colI; Data are expressed as mean±SD (n=6); * P<0.05 vs the Sham group, # P<0.05 vs the UUO group
Effect of AM on α-SMA expression
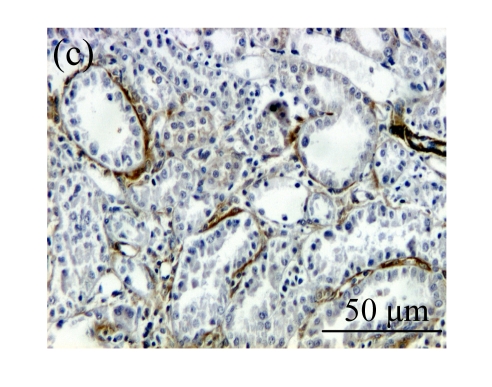
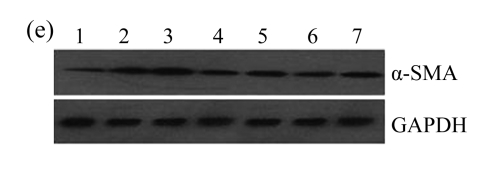
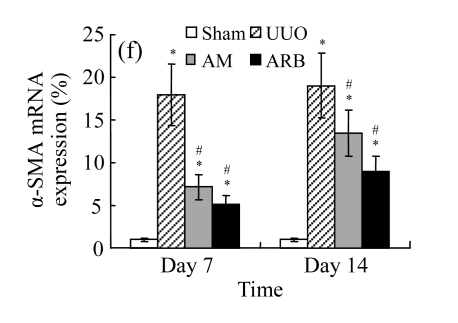
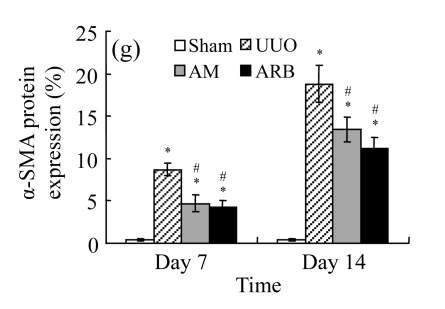
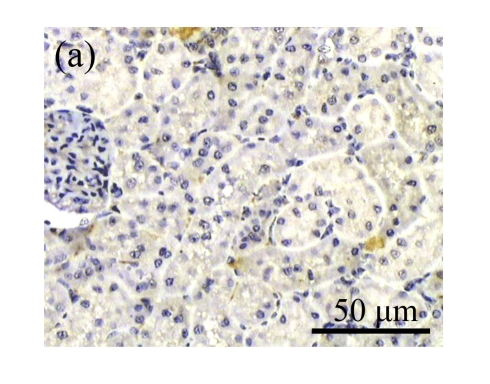
The obstructed kidneys displayed a marked activation of myofibroblasts on Day 7 after surgery, characterized by a dramatic increase of α-SMA. This change was more prominent on Day 14. AM and losartan largely reduced renal α-SMA mRNA expression in the kidneys of UUO rats. Furthermore, Western blot and immunohistochemical findings proved that the protein level of α-SMA decreased in the UUO kidneys treated with AM or losartan compared with UUO rats without treatment (Table 3 and Fig.3) (P<0.05 for all comparisons). Again, there was no significant difference between AM and ARB groups (P>0.05). Our results demonstrate that AM retarded renal myofibroblast activation in vivo.
Fig. 3.
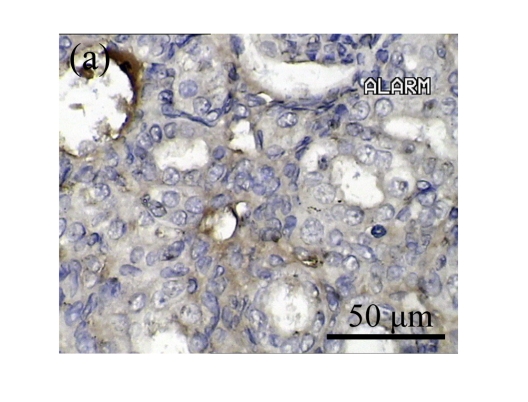
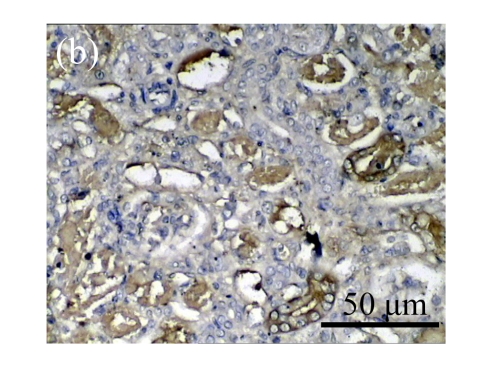
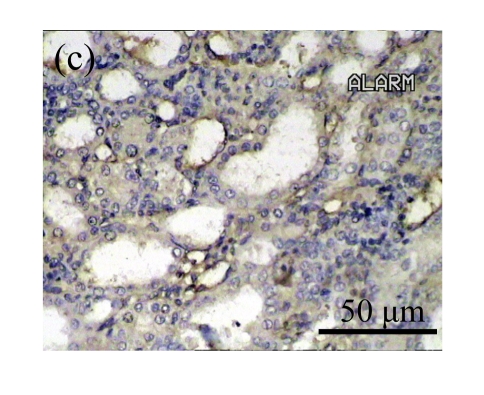
The effect of AM on α-SMA protein and mRNA expressions by immunohistochemical staining, Western blot, and RT-PCR detection. (a~d) Representative photographs of α-SMA visualized by immunohistochemical staining in different groups on Day 14 after operation: (a) sham-operated control rats, (b) untreated UUO control rats, (c) AM-treated rats, and (d) losartan-treated rats; (e) Comparison of α-SMA protein expression in renal tissues of different groups by Western blot analysis (Lane 1: Sham group; Lane 2: UUO group on Day 7; Lane 3: UUO group on Day 14; Lane 4: AM group on Day 7; Lane 5: AM group on Day 14; Lane 6: ABR group on Day 7; Lane 7: ABR group on Day 14); (f) α-SMA mRNA expression in renal tissues of different groups by semi-quantitative RT-PCR analysis; (g) Change in α-SMA protein expression in renal tissues of different groups by immunohistochemical analysis. The overexpressions of α-SMA mRNA and protein in the obstructed kidneys were significantly decreased in the AM and ARB groups. Data are expressed as mean±SD (n=6); * P<0.05 vs the Sham group, # P<0.05 vs the UUO group
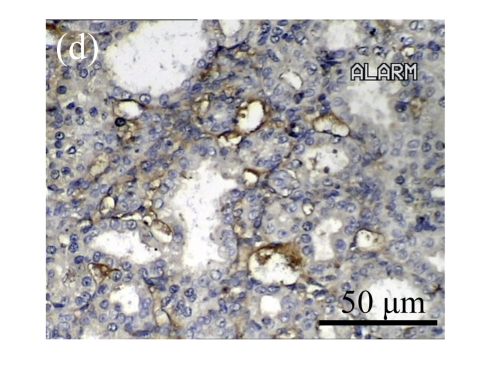
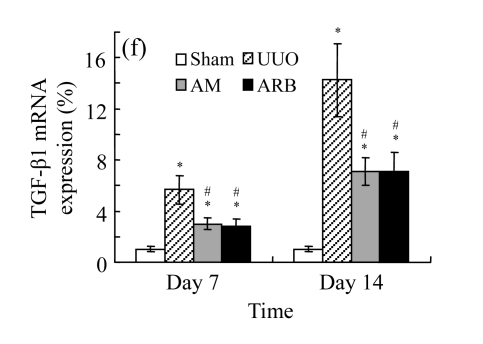
Effect of AM on TGF-β1 expression
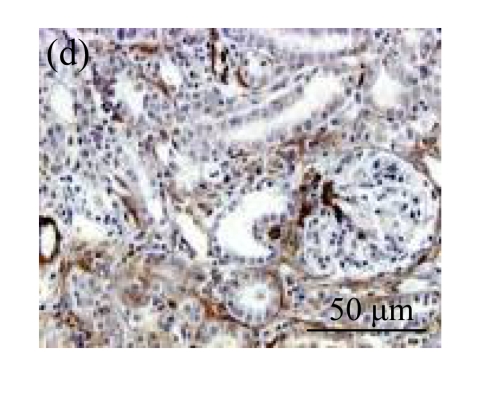
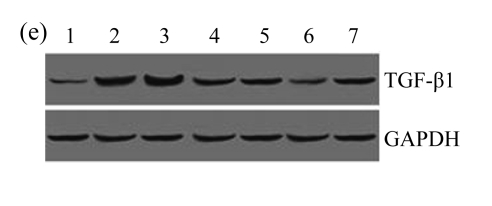
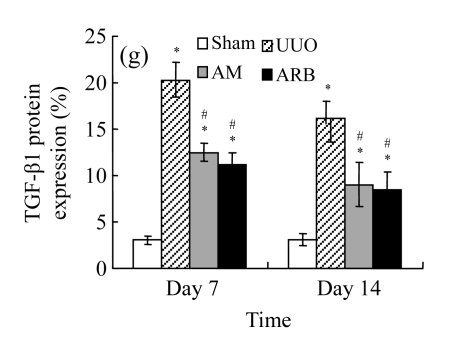
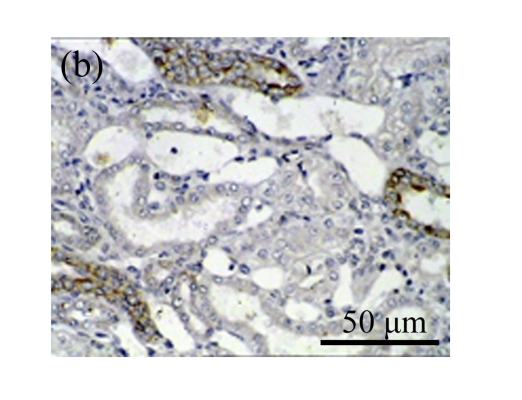
A drastic increase of TGF-β1 is a key feature of the UUO kidney. We found that the levels of TGF-β1 mRNA in obstructed kidneys were increased by 5.657 and 14.254 times than those in the Sham group on Days 7 and 14, respectively, after ureteral ligation. Treatments with AM and losartan significantly decreased the expression of TGF-β1 mRNA, compared with the UUO control kidneys on Days 7 and 14. Immunohistochemical staining revealed that the expressions of TGF-β1 in tubular and interstitial cells were increased in the UUO control kidneys on Days 7 and 14 as compared with the sham-operated kidneys, which showed only the background with antibody against TGF-β1. AM and losartan treatments reduced the expression of TGF-β1 in tubulointerstitium on Days 7 and 14, in together with the reduced tubulointerstitial fibrosis. These results were consistent with the findings of Western blot analysis of TGF-β1 (Table 4 and Fig.4) (P<0.05 for all comparisons). There was no significant difference between the AM and ARB groups (P>0.05).
Table 4.
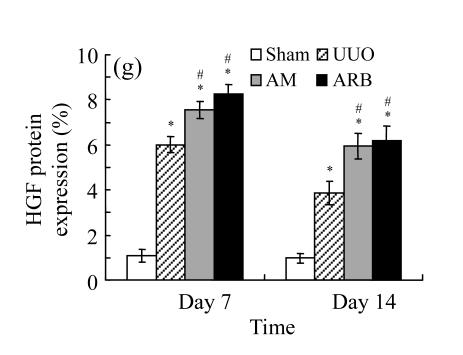
Comparisons of protein expressions of HGF and TGF-β1 in renal tissues of different groups by immunohistochemical analysis
| Group | Protein expression (%) |
|||
| HGF |
TGF-β1 |
|||
| Day 7 | Day 14 | Day 7 | Day 14 | |
| Sham | 1.08±0.21 | 1.09±0.15 | 3.18±0.65 | 3.07±0.78 |
| UUO | 6.01±0.54* | 2.41±0.49* | 12.90±2.51* | 20.30±2.55* |
| AM | 7.54±0.57*# | 3.94±0.55*# | 7.73±2.39*# | 12.45±3.80*# |
| ARB | 8.26±0.64*# | 4.33±0.37*# | 6.96±1.90*# | 11.11±3.85*# |
Compared with the Sham group, P<0.05;
Compared with the UUO group, P<0.05
Fig. 4.
The effect of AM on TGF-β1 protein and mRNA expressions by immunohistochemical staining, Western blot, and RT-PCR detection. (a~d) Representative photographs of TGF-β1 visualized by immunohistochemical staining in different groups on Day 14 after operation: (a) sham-operated control rats, (b) untreated UUO control rats, (c) AM-treated rats, and (d) losartan-treated rats; (e) Comparison of TGF-β1 protein expression in renal tissues of different groups by Western blot analysis (Lane 1: Sham group; Lane 2: UUO group on Day 7; Lane 3: UUO group on Day 14; Lane 4: AM group on Day 7; Lane 5: AM group on Day 14; Lane 6: ABR group on Day 7; Lane 7: ABR group on Day 14); (f) TGF-β1 mRNA expression in renal tissues of different groups by semi-quantitative RT-PCR analysis; (g) Change in TGF-β1 protein expression in renal tissues of different groups by immunohistochemical analysis. The overexpressions of TGF-β1 mRNA and protein in the obstructed kidneys were significantly decreased in the AM and ARB groups. Data are expressed as mean±SD (n=6); * P<0.05 vs the Sham group, # P<0.05 vs the UUO group
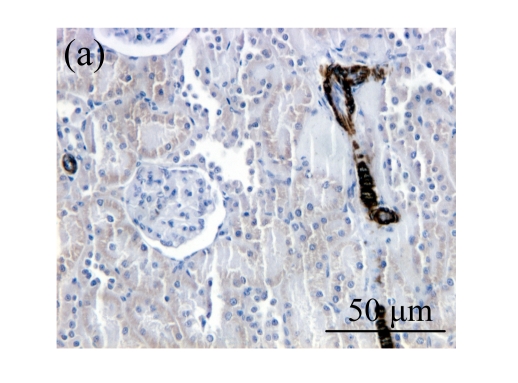
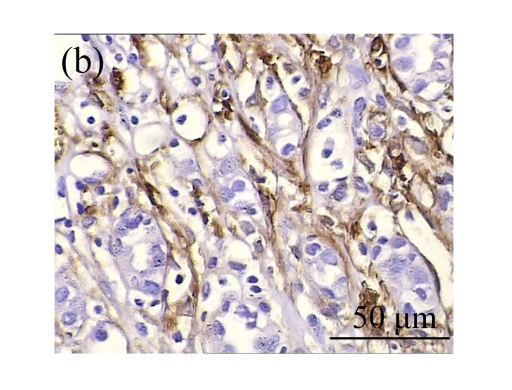
Effect of AM on HGF expression
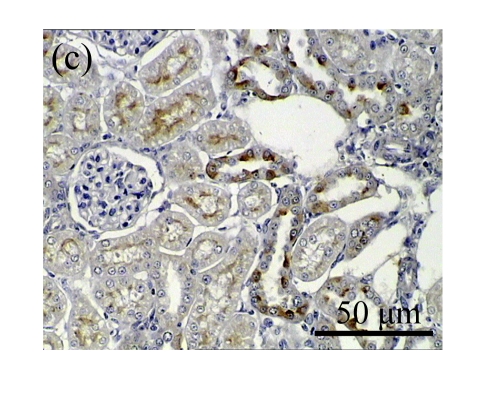
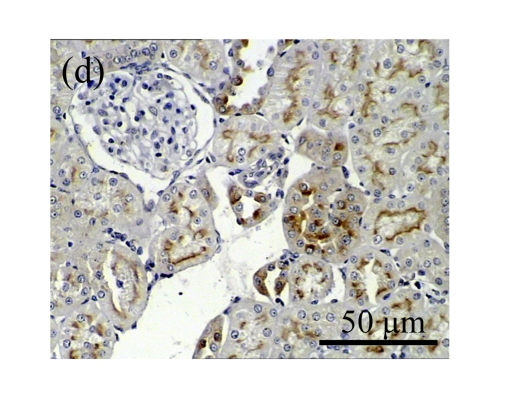
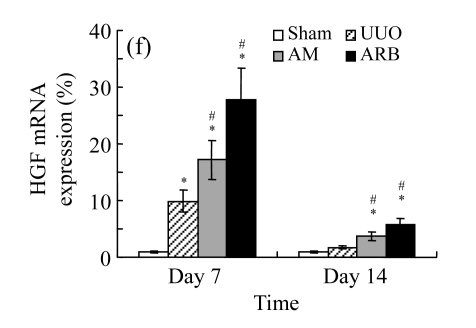
We next investigated the effect of AM on the expression of HGF, which has been identified as an antifibrotic molecular and an antagonist of TGF-β1. We found that the HGF mRNA and protein levels in UUO animals increased to peak on Day 7 after operation and decreased dramatically thereafter. HGF mRNA in the obstructed kidneys increased by 9.849 and 1.741 times than that in the Sham group on Days 7 and 14, respectively. But there was no significant difference between the UUO and Sham groups on Day 14. Both AM and losartan treatments evidently increased HGF mRNA levels to 1.741, 2.144 and 2.828, 3.249 times over those of the UUO control kidneys on Days 7 and 14, respectively. Furthermore, Western blot analysis of HGF in the UUO rats was also significantly induced after the treatments with AM and losartan, and this was in agreement with immunohistochemical evidence (Table 4 and Fig.5). There was no significant difference between the AM and ARB groups (P>0.05).
Fig. 5.
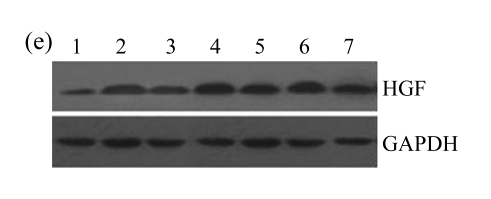
The effect of AM on HGF protein and mRNA expressions by immunohistochemical staining, Western blot, and RT-PCR detection. (a~d) Representative photographs of HGF visualized by immunohistochemical staining in different groups on Day 14 after operation: (a) sham-operated control rats, (b) untreated UUO control rats, (c) AM-treated rats, and (d) losartan-treated rats; (e) Comparison of HGF protein expression in renal tissues of different groups by Western blot analysis (Lane 1: Sham group; Lane 2: UUO group on Day 7; Lane 3: UUO group on Day 14; Lane 4: AM group on Day 7; Lane 5: AM group on Day 14; Lane 6: ABR group on Day 7; Lane 7: ABR group on Day 14); (f) HGF mRNA expression in renal tissues of different groups by semi-quantitative RT-PCR analysis; (g) Change in HGF protein expression in renal tissues of different groups by immunohistochemical analysis. The deficient expressions of HGF mRNA and protein in the obstructed kidneys were significantly increased in the AM and ARB groups. Data are expressed as mean±SD (n=6); * P<0.05 vs the Sham group, # P<0.05 vs the UUO group
DISCUSSION
The progression of chronic kidney diseases is considered irreversible and eventually leads to end-stage renal failure. Renal interstitial fibrosis is the common final outcome and the remarkably histologic hallmark of chronic renal disease irrespective of diverse etiology. It is the result of destruction in architecture of the kidney and an imbalance of fibrogenesis and antifibrogenesis (Iwano and Neilson, 2004).
Angiotensin II (Ang II) was identified to be essential in the pathogenesis of renal fibrosis (Taal and Brenner, 2000). Ang II inhibitors may suppress the expression of TGF-β1, block activation of myofibroblast cells via EMT, and ameliorate ECM accumulation (Nakao et al., 2003; Gross et al., 2004). Blocking Ang II has become a standard therapy in treating renal fibrosis. Nevertheless, it does not appear that they will entirely halt fibrosis (Peters et al., 1998). It is thus worthwhile to seek other anti-fibrotic therapies, including herbal medicine. We report here that AM exhibited antifibrotic effect on UUO rats. This effect is comparable with losartan, an angiotensin type-1 receptor blocker (ARB) agent.
UUO is an experimental model widely used to study the pathogenesis of tubulointerstitial fibrosis, because it is highly reproducible and induces pathogenetic events similar to those observed in human renal fibrosis (Chevalier, 1999; Moon et al., 2006; Wen et al., 1999). In agreement with previous studies (Chevalier, 1999; Klahr and Morrissey, 2002; Moon et al., 2006), the obstructed kidneys on Days 7 and 14 after UUO in the present study showed typical features of obstructive nephropathy including the presence of myofibroblasts in the interstitium, infiltration of inflammatory cells, tubular atrophy, and interstitial extracellular matrix deposition. Our study shows that both AM and losartan evidently suppressed the expressions of collagen and fibronectin, the specific biochemical markers for fibrosis, and attenuated the morphological changes of tubulointerstitial fibrosis in the UUO kidneys, although neither of them bestowed a complete protection from renal fibrosis in the UUO kidneys.
Activation of α-SMA positive myofibroblasts plays a key role in the progression of chronic renal fibrosis. Therefore, the inhibition of α-SMA positive myofibroblasts becomes a potential antifibrotic strategy. On account of their absence in the renal interstitium, the exact origins and activation process of interstitial myofibroblasts under pathologic conditions remain largely unclear. Possible sources of renal myofibroblasts include interstitial fibroblasts, transdifferentiated tubular epithelial cells, and circulating mesenchymal precursor cells (Iwano et al., 2002; Yang and Liu, 2002; Yang et al., 2002). Whether only one or all of these potential sources contribute to the myofibroblast pool in a given disease condition remains undefined. Yang et al.(2002; 2005)’s study proposed that myofibroblast activation in the obstructive kidney is a dynamic process, in which myofibroblasts are derived from local activation of residential fibroblasts at the earlier stage of obstructive nephropathy (3 d after obstruction) and are mainly derived from tubular epithelial cells via EMT at the later stage (7 d). Previous evidence indicated that more than 30% interstitial fibroblasts were actually from tubular epithelial cells via EMT in diseased kidney (Strutz et al., 2002). In the process of EMT, tubular epithelial cells acquired mesenchymal features such as α-SMA expression and produced interstitial matrix components such as fibronectin and collagen I (Liu, 2004a). In the present study, the obstructed kidneys displayed a marked activation of myofibroblasts on Day 7 after ureteral ligation, characterized by a dramatic increase in α-SMA abundance (Yang and Liu, 2002). This change was more prominent on Day 14. AM largely reduced both renal α-SMA mRNA and protein. Given the data and previous reports, the results from our study demonstrate that AM prevented renal myofibroblast activation at least in part through the prevention of EMT in obstructive nephropathy, as did losartan.
Our previous report showed that TGF-β1 was a key mediator in regulating transdifferentiation of tubular epithelial cells into α-SMA positive myofibroblasts (Fan et al., 1999). A drastic increase of TGF-β1 was observed in obstructed kidneys in the present study. Administration of AM considerably inhibited the expression of TGF-β1 in the level of mRNA as well as protein, indicating that AM decreased the level of TGF-β1 in vivo.
HGF is a pleiotropic factor that plays an imperative role in tubular repair and regeneration after acute renal injury (Yang and Liu, 2002). Growing evidence indicates that HGF is a regenerative, cytoprotective molecule as well as a endogenous antifibrotic factor that shows an impressive efficacy in ameliorating tissue fibrosis in a wide variety of animal models, regulating motility, mitogenesis, and morphogenesis in a cell type-dependent fashion (Liu, 2004b; Liu and Yang, 2006). HGF and TGF-β1 regulate diversified cellular functions and often act antagonistically against each other. It has been reported that the reciprocal imbalance between TGF-β1 and HGF is closely involved in the progression of tissue fibrosis (Mizuno et al., 2000). Previous studies indicated that TGF-β1 and HGF are counteracting in their biological activities (Mizuno et al., 2001). TGF-β1 strongly stimulates the synthesis of ECM proteins and inhibits the degradation of ECM (Esposito et al., 2005; Taipale et al., 1996). In contrast, HGF accelerates the degradation of ECM by modulating proteinases involved in the breakdown of ECM proteins. These proteinases include matrix metalloproteinases/tissue inhibitors of metalloproteinases, and plasminogen activators/plasminogen activator inhibitors (Gong et al., 2003; Mizuno et al., 2000). TGF-β1 induces growth arrest and apoptosis in renal tubular cells and endothelial cells (Nowak and Schnellmann, 1996; Taipale and Keski-Oja, 1996), while HGF exhibits mitogenic, motogenic, morphogenic, and anti-apoptotic activities in these cell types (Bowes et al., 1999; Dai et al., 2004; Liu, 1999). Furthermore, the epithelial to myofibroblast transition can be induced by TGF-β1 and reversed by HGF (Blobe et al., 2000; Fan et al., 1999; Yang and Liu, 2002). Yang et al.(2005) reported a novel mode of interaction between the signals activated by HGF receptor tyrosine kinase and TGF-β1 receptor serine/threonine kinases. They found that HGF blocks EMT by antagonizing TGF-β1’s action via upregulating Smad transcriptional corepressor SnoN expression.
In view of the fact that HGF expression increased at early stage and then decreased greatly later in a manner reciprocal to the increase in expression of TGF-β1 in obstructed kidney, our results likely provide novel insights into the mechanisms of impairment in the balance of profibrogenic and antifibrogenic cytokines in promoting renal fibrosis. In the AM-treated group, the enhancement of HGF expression and the reduction of TGF-β1 in kidney cells of rats might have contributed to the reduced fibrosis in the kidneys of these rats. However, the definitive mechanisms how AM suppresses TGF-β1 expression and augments HGF expression remain obscure. Future study is needed to provide more complete answers.
CONCLUSION
Our results suggest that AM can evidently inhibit renal interstitial fibrosis in vivo, which might be related with inhibition of myofibroblast activation, inducing HGF expression and suppressing TGF-β1 expression. AM could be a rational strategy to combat renal fibrosis.
Acknowledgments
The authors are grateful to Prof. Qing-jie Xia (Laboratory of Molecular Genetics, West China Hospital of Sichuan University, China) for technical advice.
Footnotes
Project (No. 30170437) supported by the National Natural Science Foundation of China
References
- 1.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 2.Bowes RC3rd, Lightfoot RT, van de Water B, Stevens JL. Hepatocyte growth factor induces tubulogenesis of primary renal proximal tubular epithelial cells. J Cell Physiol. 1999;180(1):81–90. doi: 10.1002/(SICI)1097-4652(199907)180:1<81::AID-JCP9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Chevalier RL. Molecular and cellular pathophysiology of obstructive nephropathy. Pediatr Nephrol. 1999;13(7):612–619. doi: 10.1007/s004670050756. [DOI] [PubMed] [Google Scholar]
- 4.Chevalier RL, Goyal S, Kim A, Chang AY, Landau D, LeRoith D. Renal tubulointerstitial injury from ureteral obstruction in the neonatal rat is attenuated by IGF-1. Kidney Int. 2000;57(3):882–890. doi: 10.1046/j.1523-1755.2000.057003882.x. [DOI] [PubMed] [Google Scholar]
- 5.Dai C, Yang J, Bastacky S, Xia J, Li Y, Liu Y. Intravenous administration of hepatocyte growth factor gene ameliorates diabetic nephropathy in mice. J Am Soc Nephrol. 2004;15(10):2637–2647. doi: 10.1097/01.ASN.0000139479.09658.EE. [DOI] [PubMed] [Google Scholar]
- 6.Eddy AA. Molecular insithts into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7(12):2495–2508. doi: 10.1681/ASN.V7122495. [DOI] [PubMed] [Google Scholar]
- 7.Esposito C, Parrilla B, de Mauri A, Cornacchia F, Fasoli G, Foschi A, Mazzullo T, Plati A, Scudellaro R, Dal Canton A. Hepatocyte growth factor (HGF) modulates matrix turnover in human glomeruli. Kidney Int. 2005;67(6):2143–2150. doi: 10.1111/j.1523-1755.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 8.Essawy M, Soylemezoglu O, Muchaneta-Kubara EC, Shortland J, Brown CB, el Nahas AM. Myofibroblasts and the progression of diabetic nephropathy. Nephrol Dial Transplant. 1997;12(1):43–50. doi: 10.1093/ndt/12.1.43. [DOI] [PubMed] [Google Scholar]
- 9.Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56(4):1455–1467. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- 10.Fu P, Liu F, Su S, Wang W, Huang XR, Entman ML, Schwartz RJ, Wei L, Lan HY. Signaling mechanism of renal fibrosis in unilateral ureteral obstructive kidney disease in ROCK1 knockout mice. J Am Soc Nephrol. 2006;17(11):3105–3114. doi: 10.1681/ASN.2005121366. [DOI] [PubMed] [Google Scholar]
- 11.Gong R, Rifai A, Tolbert EM, Centracchio JN, Dworkin LD. Hepatocyte growth factor modulates matrix metalloproteinases and plasminogen activator/plasmin proteolytic pathways in progressive renal interstitial fibrosis. J Am Soc Nephrol. 2003;14(12):3047–3060. doi: 10.1097/01.ASN.0000098686.72971.DB. [DOI] [PubMed] [Google Scholar]
- 12.Gross O, Schulze-Lohoff E, Koepke ML, Beirowski B, Addicks K, Bloch W, Smyth N, Weber M. Antifibrotic, nephroprotective potential of ACE inhibitor vs AT1 antagonist in a murine model of renal fibrosis. Nephrol Dial Transplant. 2004;19(7):1716–1723. doi: 10.1093/ndt/gfh219. [DOI] [PubMed] [Google Scholar]
- 13.Han X, Wang JJ, Fan SK. Study on pharmacology of Astragalus injection. Beijing Zhong Yi. 2000;19(1):44–45. (in Chinese) [Google Scholar]
- 14.Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens. 2004;13(3):279–284. doi: 10.1097/00041552-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110(3):341–350. doi: 10.1172/JCI200215518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 2002;283(5):F861–F875. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y. Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am J Physiol. 1999;277(4 Pt 2):F624–F633. doi: 10.1152/ajprenal.1999.277.4.F624. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15(1):1–12. doi: 10.1097/01.ASN.0000106015.29070.E7. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol. 2004;287(1):F7–F16. doi: 10.1152/ajprenal.00451.2003. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Yang J. Hepatocyte growth factor: new arsenal in the fights against renal fibrosis? Kidney Int. 2006;70(2):238–240. doi: 10.1038/sj.ki.5001661. [DOI] [PubMed] [Google Scholar]
- 21.Mizuguchi Y, Miyajima A, Kosaka T, Asano T, Asano T, Hayakawa M. Atorvastatin ameliorates renal tissue damage in unilateral ureteral obstruction. J Urol. 2004;172(6):2456–2459. doi: 10.1097/01.ju.0000138473.38447.f0. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno S, Matsumoto K, Kurosawa T, Mizuno-Horikawa Y, Nakamura T. Reciprocal balance of hepatocyte growth factor and transforming growth factor-beta 1 in renal fibrosis in mice. Kidney Int. 2000;57(3):937–948. doi: 10.1046/j.1523-1755.2000.00910.x. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno S, Matsumoto K, Nakamura T. Hepatocyte growth factor suppresses interstitial fibrosis in a mouse model of obstructive nephropathy. Kidney Int. 2001;59(4):1304–1314. doi: 10.1046/j.1523-1755.2001.0590041304.x. [DOI] [PubMed] [Google Scholar]
- 24.Moon JA, Kim HT, Cho IS, Sheen YY, Kim DK. IN-1130, a novel transforming growth factor-β type I receptor kinase (ALK5) inhibitor, suppresses renal fibrosis in obstructive nephropathy. Kidney Int. 2006;70(7):1234–1243. doi: 10.1038/sj.ki.5001775. [DOI] [PubMed] [Google Scholar]
- 25.Mou S, Zhang QY, Ni ZH, Tong JF. Effect of Astragalus membranaceus on HGF by renal interstitial fibroblasts in the high glucose. Zhong Guo Zhong Xi Yi Jie He Shen Bing Za Zhi. 2002;3(1):7–9. (in Chinese) [Google Scholar]
- 26.Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003;361(9352):117–124. doi: 10.1016/S0140-6736(03)12229-5. [DOI] [PubMed] [Google Scholar]
- 27.Nowak G, Schnellmann RG. Autocrine production and TGF-beta 1-mediated effects on metabolism and viability in renal cells. Am J Physiol. 1996;271(3 Pt 2):F689–F697. doi: 10.1152/ajprenal.1996.271.3.F689. [DOI] [PubMed] [Google Scholar]
- 28.Peters H, Border WA, Noble NA. Targeting TGF-beta overexpression in renal disease: maximizing the antifibrotic action of angiotensin II blockade. Kidney Int. 1998;54(5):1570–1580. doi: 10.1046/j.1523-1755.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339(20):1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 31.Roberts IS, Burrows C, Shanks JH, Venning M, McWilliam LJ. Interstitial myofibroblasts: predictors of progression in membranous nephropathy. J Clin Pathol. 1997;50(2):123–127. doi: 10.1136/jcp.50.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Müller GA, Neilson EG. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61(5):1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 33.Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int. 2000;57(5):1803–1817. doi: 10.1046/j.1523-1755.2000.00031.x. [DOI] [PubMed] [Google Scholar]
- 34.Taal MW, Zandi-Nejad K, Weening B, Shahsafaei A, Kato S, Lee KW, Ziai F, Jiang T, Brenner BM, MacKenzie HS. Proinflammatory gene expression and macrophage recruitment in the rat remnant kidney. Kidney Int. 2000;58(4):1664–1676. doi: 10.1111/j.1523-1755.2000.00327.x. [DOI] [PubMed] [Google Scholar]
- 35.Taipale J, Keski-Oja J. Hepatocyte growth factor releases epithelial and endothelial cells from growth arrest induced by transforming growth factor-β1. J Biol Chem. 1996;271(8):4342–4348. doi: 10.1074/jbc.271.8.4342. [DOI] [PubMed] [Google Scholar]
- 36.Taipale J, Saharinen J, Hedman K, Keski-Oja J. Latent transforming growth factor-beta 1 and its binding protein are components of extracellular matrix microfibrils. J Histochem Cytochem. 1996;44(8):875–889. doi: 10.1177/44.8.8756760. [DOI] [PubMed] [Google Scholar]
- 37.Wen JG, Frøkiaer J, Jørgensen TM, Djurhuus JC. Obstructive nephropathy: an update of the experimental research. Urol Res. 1999;27(1):29–39. doi: 10.1007/s002400050086. [DOI] [PubMed] [Google Scholar]
- 38.Xie X, Yang M, Liu H, Zuo C, Li Z, Deng Y, Fan J. Influence of ginsenoside Rg1, a panaxatriol saponin from Panax notoginseng, on renal fibrosis in rats with unilateral ureteral obstruction. J Zhejiang Univ Sci B. 2008;9(11):885–894. doi: 10.1631/jzus.B0820024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue H, Fan JM, Chen L, Li Z, Hu ZX, Liu XR. Identification and characterization of hepatocyte growth factor in counteracting tubular epithelial-myofibroblast transdifferentiation in renal interstitial fibrosis. Zhong Hua Shen Zang Bing Za Zhi. 2005;21(8):458–463. (in Chinese) [Google Scholar]
- 40.Yang HX, Zhu MY. Renoprotective effect of Astragalus membranaceus on rats with diabetic nephropathy. Shi Yong Yi Xue Za Zhi. 2005;21(17):1964–1965. (in Chinese) [Google Scholar]
- 41.Yang J, Liu Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol. 2002;13(1):96–107. doi: 10.1681/ASN.V13196. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Shultz RW, Mars WM, Rodney EW, Li Y, Dai C, Kari N, Liu Y. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest. 2002;110(10):1525–1538. doi: 10.1172/JCI16219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J, Dai C, Liu Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol. 2005;16(1):68–78. doi: 10.1681/ASN.2003090795. [DOI] [PubMed] [Google Scholar]
- 44.Zhong H, Fan JM, Li Z, Liu F, Yang LC, Ji L, Sha ZH, Ma XY. Effects of hepatocyte growth factor on TGF-beta1 triggered tubular epithelial-myofibroblast transdifferentiation by CTGF in vitro. Sichuan Da Xue Xue Bao Yi Xue Ban. 2005;36(5):653–656. 653. (in Chinese) [PubMed] [Google Scholar]