Abstract
Intense stress and fear have long been known to give rise to a suppression of pain termed “stress-induced analgesia,” mediated by brainstem pain-modulating circuitry, including pain-inhibiting neurons of the rostral ventromedial medulla. However, stress does not invariably suppress pain, and indeed, may exacerbate it. Although there is a growing support for the idea of “stress-induced hyperalgesia,” the neurobiological basis for this effect remains almost entirely unknown. Using simultaneous single-cell recording and functional analysis, we show here that stimulation of the dorsomedial nucleus of the hypothalamus, known to be a critical component of central mechanisms mediating neuroendocrine, cardiovascular and thermogenic responses to mild or “emotional” stressors such as air puff, also triggers thermal hyperalgesia by recruiting pain-facilitating neurons, “ON-cells”, in the rostral ventromedial medulla.
Activity of identified RVM ON-cells, OFF-cells and NEUTRAL cells, nociceptive withdrawal thresholds, rectal temperature, and heart rate were recorded in lightly anesthetized rats. In addition to expected increases in body temperature and heart rate, disinhibition of the DMH induced a robust activation of ON-cells, suppression of OFF-cell firing and behavioral hyperalgesia. Blocking ON-cell activation prevented hyperalgesia, but did not interfere with DMH-induced thermogenesis or tachycardia, pointing to differentiation of neural substrates for autonomic and nociceptive modulation within the RVM.
These data demonstrate top-down activation of brainstem pain-facilitating neurons, and suggest a possible neural circuit for stress-induced hyperalgesia.
Keywords: hyperalgesia, pain-modulation, rostral ventromedial medulla, brainstem, ON cells, OFF cells
Introduction
Intense stress and fear are known to suppress pain, a phenomenon termed “stress-induced analgesia” [41, 72]. However, stress can also give rise to an increase in pain sensitivity, referred to as “stress-induced hyperalgesia.” Thus, repeated swim stress, acute and chronic restraint, novelty, horizontal rotation, and social defeat have all been shown to induce hyperalgesia in animals [2, 10, 17, 19, 33, 39, 42, 51, 54, 55, 64, 70]. This phenomenon is not simple hyper-reflexia, since anxiety or anticipation of pain can be shown to enhance pain sensitivity in humans [1, 4, 52, 53, 67], and since stress is often asserted to exacerbate chronic clinical pain (see [9, 40, 71] for examples). The parameters that differentiate stress-induced analgesia from hyperalgesia are as yet unknown, but intensity or aversiveness of the stressor may interact with arousal level in determining whether the net effect is to enhance or suppress pain [18, 36, 46, 73].
Stress-induced analgesia is mediated by brainstem pain-modulating systems [5, 72], whereas the neural circuitry responsible for stress-induced hyperalgesia remains almost unknown. The dorsomedial nucleus of the hypothalamus (DMH) is a critical component of the central mechanisms mediating neuroendocrine, cardiovascular and thermogenic responses to various stressors [11, 12]. This raises the possibility that the DMH also contributes to stress-induced hyperalgesia through direct and indirect connections with the rostral ventromedial medulla (RVM), a region long implicated in descending control of nociception [13, 20, 57, 59].
The present experiments were designed to determine whether activation of the DMH would recruit pain-modulating circuitry of the RVM to facilitate nociception. Because of the functional overlap of autonomic control and pain-modulation in the RVM region, we used electrophysiological methods to record responses of physiologically characterized RVM neurons to stimulation of the DMH, and pharmacological manipulation to test their roles in behavioral hyperalgesia. RVM neurons can be classified as NEUTRAL cells, ON-cells, or OFF-cells. The physiological function of NEUTRAL cells remains unknown, but OFF-cells suppress, and ON-cells facilitate, nociception [20]. OFF-cells have been implicated in stress-induced analgesia related to conditioned fear [45]. By contrast, ON-cells are activated by the anxiogenic peptide cholecystokinin, which has been implicated in stress-induced hyperalgesia [24, 42]. These later findings point to a possible role for ON-cells in stress-induced hyperalgesia. Any or all of these three cell classes could also in theory contribute to the autonomic sequelae of DMH stimulation.
The present experiments show that activation of the DMH recruits pain-facilitating ON-cells in the RVM to produce behavioral hyperalgesia. In addition, the pronociceptive effects of DMH activation are mediated by a medullary substrate that is at least in part distinct from that responsible for the accompanying thermogenesis and tachycardia. Activation of pain-facilitating neurons in the RVM may therefore play an important role in stress-induced hyperalgesia.
Methods
Animals and surgical preparation
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University and followed the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. Male Sprague-Dawley rats (Taconic, 250–300g) were anesthetized with pentobarbital (60 mg/kg, i.p.), and a catheter inserted into an external jugular vein for administration of anesthetic. The rat was placed in a stereotaxic apparatus, a hole drilled in the skull over the cerebellum, and the dura removed to allow placement of an electrode in the RVM (using surface landmarks for guidance). A second small craniotomy was made to allow placement of a microinjection pipette into the DMH (stereotaxic coordinates: 5.8 anterior to interaural line, 0.3 mm lateral to midline at an angle of 4°, 8.4 mm ventral to brain surface). Body temperature was supported by placing the animals on a circulating water pad at 35 °C.
Following surgery, the anesthetic level was allowed to lighten until a paw withdrawal reflex could be elicited by application of noxious heat using a feedback-controlled projector lamp focused on the blackened plantar surface of the left hindpaw. The animals were subsequently maintained in a lightly anesthetized state using a continuous infusion of methohexital at a rate (15–30 mg/kg per h, i.v.) that allowed a stable paw withdrawal latency and that prevented any signs of discomfort. The animals did not move spontaneously, nor did they vocalize or produce vigorous or prolonged withdrawal reflexes following noxious pinch. The rate was adjusted for each animal to allow a baseline paw withdrawal latency of approximately 4.5 s. The protocol was begun after a stabilization period of at least 30 min, and infusion rate was not altered during the protocol.
Nociceptive responsiveness, rectal temperature, and heart rate
Paw withdrawal latency to heat was used as the primary measure of nociceptive responsiveness. Each trial consisted of a linear increase in temperature at approximately 1.2 °C/s from a holding temperature of 35 °C until the paw withdrawal occurred or to a maximum of 48 °C at approximately 10.6 s. Trials were carried out at 5 min intervals throughout the experiment. The holding temperature obviates any concern that apparent effects on paw withdrawal latency were due to changes in plantar skin temperature potentially produced by stimulation of the DMH. Mechanical nociceptive threshold was determined using von Frey filaments (1.0 g up to 100 g) applied to the lateral plantar surface of the hindpaw until the paw was withdrawn. The withdrawal threshold was defined as the minimum force eliciting a withdrawal on two consecutive trials. Heart rate was derived from EKG. Rectal temperature was measured using the Physitemp Thermalert TH-5 thermometer and the rat rectal probe inserted 5–6 cm.
Recording and drug administration
A gold- and platinum-plated stainless steel recording microelectrode (Frederick Haer Co., Brunswick, ME) was lowered into the RVM for extracellular single unit recording. A fresh glass infusion micropipette (75–100 μm, OD) was attached to a 1 μl Hamilton syringe with a length of PE-50 tubing and lowered into the DMH for bicuculline infusion or into the RVM for infusions of kynurenate or muscimol. In some cases, the RVM infusion pipette was attached to a recording electrode to monitor the effect of the drug on cell activity (see Heinricher et al. [23] and Xu et al. [74] for detailed description of chemical stimulation/recording methods).
RVM neurons were classified as previously described [14]. Spike waveforms were monitored and stored for off-line analysis (Spike2, CED, Cambridge, UK) to ensure that the unit under study was unambiguously discriminated throughout the experiment, and cell data are included only in cases where we successfully maintained isolation for the entire protocol. OFF-cells were characterized by an abrupt pause in ongoing activity beginning just prior to the occurrence of the paw withdrawal. ON-cells were identified by a sudden burst of activity beginning just prior to the occurrence of the paw withdrawal. Cells of a third class, “NEUTRAL cells,” were identified by no change in activity associated with paw withdrawal, and they did not respond to noxious or innocuous cutaneous stimulation.
Protocol and data analysis
We determined the effects of microinjection of bicuculline methiodide (BIC) into the DMH on paw withdrawal latency or von Frey threshold, rectal temperature, heart rate and the firing of RVM neurons. Rectal temperature was noted just prior to each paw withdrawal trial. Following three baseline nociceptive trials, BIC (Tocris Bioscience, St. Louis, MO, 10 pmol in 100 nl saline vehicle) was infused into the DMH over a period of approximately 2 min. Control injections were made in surrounding areas. All parameters were then monitored for a period of 55 min. Only one protocol was performed in each animal. On nine occasions, more than one neuron was isolated with the electrode. Because OFF-cells and ON-cells often show irregular alternations between periods of silence and activity, cell activity integrated over the 30 s prior to each paw withdrawal trial was used as an overall index of ongoing firing.
To test the role of ON-cells in hyperalgesia, and in increased body temperature and heart rate induced by application of BIC in the DMH, a microinjection pipette was glued adjacent to the recording electrode in the RVM such that the tips were separated by 100–300 μm. The broad-spectrum excitatory amino acid antagonist kynurenate (1 nmol/200 nl) or the GABAA receptor agonist muscimol (8.8 pmol/200 nl), both from Tocris Bioscience (St. Louis, MO) was infused into the RVM over a period of approximately 4 min following three baseline trials. The dose of kynurenate was chosen as sufficient to suppress ON-cell activity selectively, with no affect on OFF-cell or NEUTRAL cell discharge in normal animals [22, 23]. Higher doses/concentrations evoke second-order or non-specific effects in the RVM (Heinricher and McGaraughty, unpublished observations). Muscimol was employed as a positive control, with the dose chosen as sufficient to inhibit all three RVM cell classes uniformly. Null control (vehicle) was not employed, as our intention was not to study excitatory amino acid transmission per se, and because we have repeatedly shown that saline injections in the RVM do not alter paw withdrawal latency or the activity of ON-cells, OFF-cells or NEUTRAL cells (e.g., Xu et al. [74]). Paw withdrawal latency, heart rate, and rectal temperature were recorded again 1 min later, and BIC was then infused into the DMH. All parameters were then monitored as above for an additional 50 min.
Three cell parameters were analyzed. 1) Overall ongoing activity. Because OFF-cells and ON-cells often show irregular alternations between periods of silence and activity, total cell count integrated over the 30 s prior to each withdrawal trial was used as an overall index of ongoing firing. 2) ON-cell reflex—related firing rate. Average firing rate in the 3 s period beginning 0.5 s before the paw withdrawal was recorded for all trials. This approach, rather than counting the number of spikes or duration of the reflex-related burst, was necessary because a burst can only be identified in cases in which the ON-cell is inactive at the time of heat onset. Because OFF-cell firing was decreased or suppressed for prolonged periods after DMH stimulation, a reflex-related pause in firing could not be identified. Reflex-related inhibition of OFF-cells was therefore not studied. 3) Cycling. The proportion of time that a given cell was considered to be in an “active” or “silent” period was defined as described previously [3]. Briefly, an active period was defined as any epoch lasting at least 2 s with a minimum of 1 spike/s, and a silent period as any epoch of at least 2 s without any cell activity. The proportion of time in which each cell was “active” or “silent” was then calculated for the baseline and post-treatment periods.
Data are presented as mean ±SEM, and in some cases converted to change from baseline for illustration purposes. Because RVM cell discharge rates are not normally distributed, Friedman’s analysis of variance by ranks with comparison of post-treatment time points to baseline was used for statistical analysis of neuronal firing rates [61]; and t-test for correlated means for comparing pre- and post-DMH stimulation parameters related to firing pattern. Repeated-measures ANOVA followed by Dunnett’s test or Tukey’s tests (Graphpad Prism, 5.0) were used for comparing the mean of the baseline with post-injection cell cycling parameters, heart rate, rectal temperature, paw withdrawal latencies, and mechanical thresholds. P < 0.05 was considered significant.
Histology
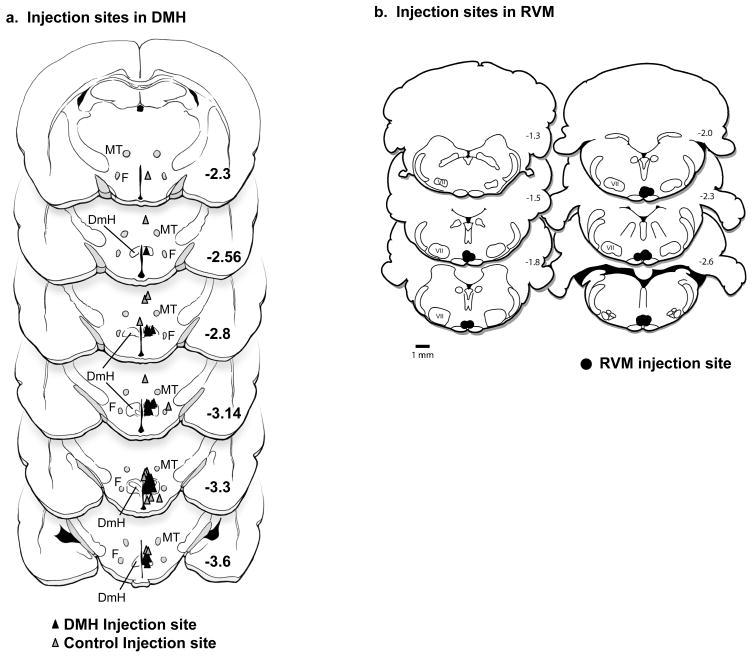
At the conclusion of the experiments, recording sites were marked with an electrolytic lesion, and infusion sites by injection of pontamine sky blue dye. Animals were euthanized with an overdose of methohexital, and perfused intracardially with physiological saline followed by 10% formalin. Recording and infusion sites were histologically verified and plotted on standardized sections adapted from Paxinos and Watson [49]. Injection sites in the DMH and control placements in surrounding regions are shown in Figure 1a. The RVM was defined as the nucleus raphe magnus and adjacent reticular formation at the level of the facial nucleus. Recording sites were distributed in this region as in previous publications from this laboratory [25, 26]. Kynurenate infusion sites are shown in Figure 1b.
Figure 1.
Histologically verified locations of infusion sites in the DMH and RVM.
a. Histologically verified locations of infusion sites in the DMH (n = 40, black filled triangles) and surrounding area (n = 17, gray triangles). DmH: dorsomedial hypothalamus, F: fornix, MT: mammilothalamic tract. Distance from bregma indicated on each section, as adapted from the atlas of Paxinos and Watson [49].
b. Histologically verified locations of kynurenate infusion sites in the RVM. Distance from bregma indicated on each section, as adapted from the atlas of Paxinos and Watson [49].
Results
Stimulation of the DMH gives rise to a significant thermal and mechanical hyperalgesia
We used a lightly anesthetized rat preparation in which we could record activity of physiologically identified pain-modulating neurons in the RVM while monitoring nociceptive threshold, rectal temperature, and heart rate. Focal microinjection of the GABAA receptor antagonist bicuculline was used to activate the DMH. This approach has been adopted by other investigators as a model for acute or “emotional” stress in awake behaving and anesthetized animals [11]. We first determined whether stimulation of the DMH that mimicked stress in evoking increased heart rate and hyperthermia also gave rise to behavioral hyperalgesia. As expected, activation of the DMH resulted in an increase in both body temperature and heart rate (Figures. 2, 3a). The new finding in the present experiments was that these animals also showed robust thermal and mechanical hyperalgesia (Figure 3b). Withdrawal latency to heat was shortened by an average of 1.3 ±0.3 s, which represents a decrease in threshold of approximately 1.6 °C. Mechanical withdrawal threshold was also substantially reduced following activation of DMH, so that animals responded to a von Frey filament intensity normally considered innocuous. The effect of bicuculline was localized to the DMH, since injection at control sites surrounding the DMH did not result in a significant change in paw withdrawal latency or increase in body temperature (Fig 3a, b, open symbols).
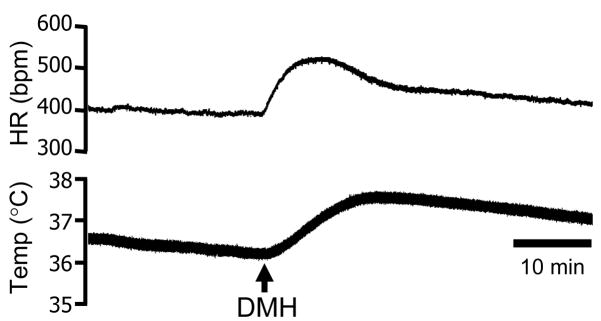
Figure 2.
Raw traces document changes in heart rate (HR) and rectal temperature (Temp) triggered by microinjection of bicuculline in the DMH (arrow).
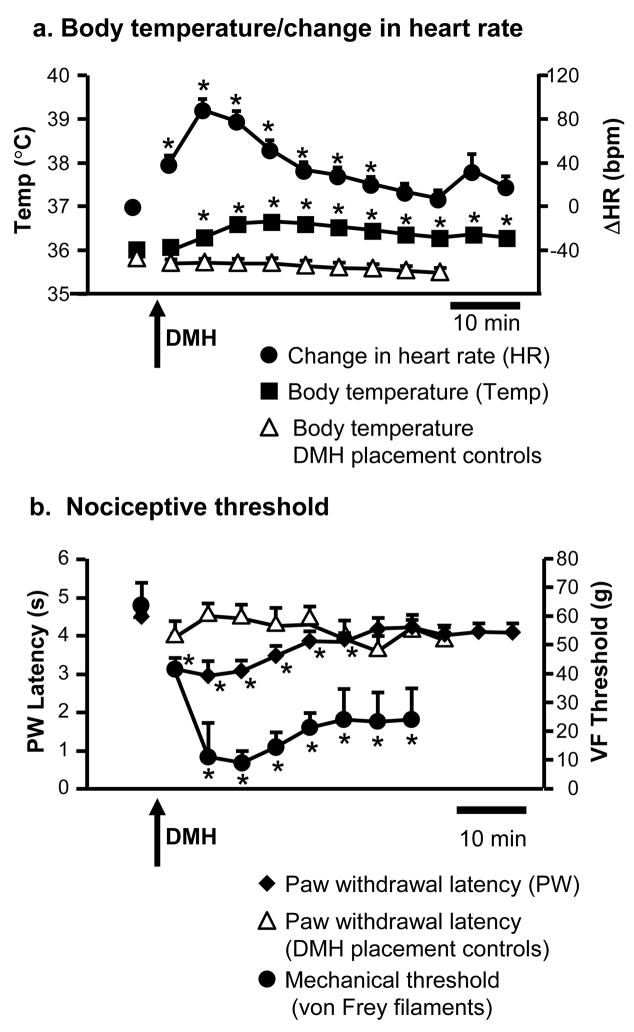
Figure 3.
Thermogenesis and tachycardia (a) evoked by activation of the dorsomedial hypothalamus are accompanied by thermal and mechanical hyperalgesia (b).
a. Rectal temperature (Temp, left axis) and change in heart rate (ΔHR, right axis, baseline 399.8 ± 6.7 bpm) as a function of time following microinjection of bicuculline (10 pmol in 100 nl) into the DMH (arrow). Bicuculline injected into areas surrounding the DMH (placement controls, open triangles) did not result in increased body temperature. This placement control also supports the stability of this parameter over the course of the experiment.
b. Paw withdrawal latency to heat (left axis) and threshold for withdrawal to probing with von Frey filaments (VF threshold, right axis) as a function of time following DMH disinhibition. Bicuculline injected into areas surrounding the DMH (placement controls, open triangles) did not result in thermal hyperalgesia. This placement control also supports the stability of paw withdrawal latency over the course of the experiment.
For rectal temperature and paw withdrawal latency, n = 40 in DMH group, 17 in outside DMH group. For mechanical threshold n = 5, for heart rate n = 12. No difference between groups in baseline. DMH injection sites are shown in Figure 1a. *p < 0.05 compared to baseline, repeated-measures ANOVA for mean baseline and 45-min period after DMH activation (10 time points), followed by Dunnett’s test when overall F was significant. Heart rate: F9,99 = 39.0, p < 0.0001; Temperature: F9,351 = 43.5, p < 0.0001; Temperature/DMH placement controls: F9,144 = −7.5, p < 0.0001; Paw withdrawal latency/DMH placement controls: F9,144 = 1.1, p = 0.36; Paw withdrawal latency: F9,351 = 11.4, p < 0.0001; Mechanical threshold (only 9 time points): F8,32 = 5.06, p = 0.004.
The DMH has not classically been associated with modulation of nociceptive processing. However, these data demonstrate that disinhibition of the DMH, a region critical for physiological responses in various stress paradigms [11, 31, 35, 60, 65], evokes significant thermal and mechanical hyperalgesia in parallel with the autonomic components of the stress response.
Hyperalgesia induced by stimulation of the DMH is associated with activation of RVM ON-cells and suppression of RVM OFF-cell firing
In order to determine whether the RVM pain-modulating system, known to mediate stress-induced analgesia, could also contribute to stress-induced hyperalgesia, we recorded the activity of identified ON-cells, OFF-cells, and NEUTRAL cells in the RVM of a subset of animals before and after DMH activation. Because OFF-cells suppress, whereas ON-cells facilitate, nociception [20], we expected that activation of the DMH that evoked hyperalgesia should activate ON-cells and/or depress the firing of OFF-cells in the RVM.
As predicted, ON-cell and OFF-cell classes displayed reciprocal responses to DMH stimulation. The ON-cell shown in Figure 4a illustrates the typical response of this cell class to activation of the DMH, with a prolonged period of continuous firing starting almost immediately after completing the injection in the DMH. The OFF-cell shown in Figure 4b illustrates the characteristic response of cells of this class to DMH stimulation, with an extended interval of inactivity, again beginning almost immediately after the DMH microinjection was complete.
Figure 4.
Typical responses of RVM ON-cells and OFF-cells to disinhibition of the DMH.
a. Potent activation of an RVM ON-cell following stimulation of the DMH. Ratemeter record (firing rate in spikes/s) shows that, in baseline, this neuron was active 26% of the time, with firing rate during active periods of 13.3 sp/s. After the DMH injection was completed, the cell entered a prolonged period of continuous discharge that lasted over 7 minutes (firing rate 13.4 sp/s). The cell subsequently returned to its original firing pattern, with silent periods again predominating (active 42% of the time, firing rate during active periods was 12.9 sp/s). This neuronal response was temporally correlated with thermal hyperalgesia (paw withdrawal latency decreased to 53% of baseline following DMH stimulation, paw heat trials indicated by triangles below the trace). The animal also manifested a substantial increase in body temperature (1.2 °C over baseline).
Individual paw withdrawal trials shown on an expanded time base below each ratemeter record document the reflex-related burst on one trial in baseline; second trial following DMH disinhibition is also shown.
b. Prolonged suppression of an OFF-cell following stimulation of the DMH. Ratemeter record (firing rate in spikes/s) shows that cell activity was substantially depressed for over 10 minutes before resuming at a lower level (14.5 sp/s in baseline, 5.8 sp/s following silent period). The neuronal response was associated with thermal hyperalgesia (paw withdrawal latency decreased to 79% of baseline following DMH activation, paw heat trials indicated by triangles below the trace). The animal also displayed a 1.4 °C increase in body temperature compared to baseline.
Individual paw withdrawal trials shown on an expanded time base below the ratemeter record depict the reflex-related pause on one trial in baseline and the second trial following DMH disinhibition. No pause is evident in the post-DMH trial, as the cell had no spontaneous activity at that point.
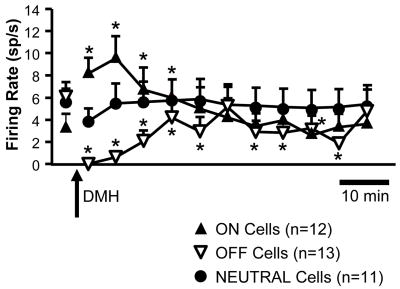
Data for all RVM neurons recorded are summarized in Figure 5, which shows overall ongoing activity in baseline and following activation of DMH. In effect, DMH stimulation shifted the balance of RVM activity, so that ON-cells as a population were more likely to be active, and OFF-cells inactive (Table 1). For ON-cells, the significant increase in overall ongoing activity represents more prolonged periods of activity following DMH stimulation, without increased firing rate when active. The change in OFF-cells complemented the activation of ON-cells, with a shift towards fewer active periods. Firing rate during active periods was also reduced.
Figure 5.
Group data show overall ongoing firing rate of the three different RVM cell classes, ON-cells, OFF-cells and NEUTRAL cells, over time. These cells were held successfully in a subset of the animals shown in Figure 3. Microinjection of bicuculline in DMH (10 pmol/100 nl) at arrow. (*p < 0.05, Friedman’s analysis of variance by ranks with critical value determined for individual comparisons of 9 post-injection time points to mean baseline. ON-cells: Fr(9) = 37.2, p < 0.0001; OFF-cells: Fr (9) = 56.5, p < 0.0001; NEUTRAL cells: Fr (9) = 23.5, p = 0.005)
Table 1. Comparisons of cell parameters in baseline and following DMH stimulation with focal application of bicuculline.
Duration of single active/silent period: single longest active/silent period in baseline compared to the continuous active/silent period beginning within 2 min of completing the injection in DMH. Reflex-related firing is compared in baseline and at 10 min after DMH stimulation, the time of peak change in withdrawal threshold and ongoing activity. Other post-DMH BIC values represent an average over the entire monitoring period. Values are means ± SEM.
| Baseline | Post-DMH BIC | |
|---|---|---|
| ON cells | ||
| Duration of single active period: | 77.3 ± 35.6 s | 661.3 ± 226.7 s* |
| Percent of time active: | 39.3 ± 12.7 % | 59.3 ± 10.0%** |
| Firing rate during active periods: | 7.3 ± 1.3 sp/s | 8.6 ± 1.3/sp/s |
| Reflex-related firing: | 7.1 ± 2.2 sp/s | 10.4 ± 1.6 sp/s* |
| OFF Cells | ||
| Duration of single silent period: | 39.5 ± 13.9 s | 668.3 ± 134.1 s** |
| Percent of time active: | 70.8 ± 7.8 % | 42.5 ± 6.9 %** |
| Firing rate during active periods: | 8.3 ± 1.5 sp/s | 5.6 ± 1.1 sp/s* |
p < 0.05
p < 0.01 compared to baseline, t-test for correlated means
Reflex-related changes in ON- and OFF-cell activity were not generally discernible following DMH stimulation, since ON-cells entered a phase of continuous firing after DMH stimulation and OFF-cells became inactive (see individual paw withdrawal trials in Figure 4, for examples). However, ON-cell firing rate at the time of the reflex generally paralleled the increase in ongoing activity (Table 1).
None of the 11 NEUTRAL cells recorded responded to DMH stimulation (Figure 5). Interestingly, this group included six possible serotonergic NEUTRAL cells, defined by slow (< 2 Hz), regular activity [34].
Control injections of bicuculline in areas surrounding the DMH (Figure 1a) did not result in significant changes in cell firing (11 ON-cells, 7 OFF-cells, 10 NEUTRAL cells, data not shown).
Hyperalgesia evoked by DMH activation is mediated by RVM ON-cells
The correlative data presented above demonstrate that hyperalgesia, tachycardia, and thermogenesis following DMH stimulation are paralleled by changes in firing of ON-cells and OFF-cells, two classes of RVM neurons already known to modulate nociception. In order to determine whether changes in ON- and OFF-cell activity mediate hyperalgesia and/or autonomic changes following DMH activation, we next used the broad-spectrum excitatory amino acid antagonist kynurenate to selectively reduce the excitability of ON-cells. Kynurenate was infused into the RVM using a dose (1 nmol) shown previously to be relevant to the pain-modulating function of this region in producing a selective attenuation of ON-cell firing [22]. A relatively large volume (200 nl) was employed, which is required to influence the broad area over which ON- and OFF-cells are distributed, as seen for example in studies of secondary inflammatory hyperalgesia and opioid analgesia [58, 74]. RVM injection sites are shown in Figure 1b.
We first confirmed that blocking excitatory amino transmission prevented activation of ON-cells following DMH stimulation (Figure 6), without activating OFF-cells or altering the ongoing firing of NEUTRAL cells (not shown), as has been reported previously [22, 74]. The effects of DMH activation on paw withdrawal latency, body temperature and heart rate in animals pretreated with kynurenate are shown in the open bars in Figure 7 (n = 22). Data are shown as change from baseline to aid comparison among the different dependent variables, and the effects of DMH stimulation without RVM pretreatment are replotted from Figure 3 for reference (black bars). By itself, kynurenate did not significantly reduce paw withdrawal latency, heart rate, or body temperature (not shown). In animals pretreated with kynurenate, DMH stimulation failed to evoke a significant thermal hyperalgesia (Figure 7, baseline, post-kynurenate, and subsequent effect of DMH bicuculline microinjection compared using repeated-measures ANOVA, F2,42 = 2.9, p = 0.06). By contrast, DMH-evoked hyperthermia and tachycardia were not prevented by excitatory amino acid receptor block in the RVM (temperature: F2,42 = 65.3, p < 0.0001 followed by Tukey’s post-hoc test; heart rate: F2,42 = 62.5, p < 0.0001 followed by Tukey’s post-hoc test).
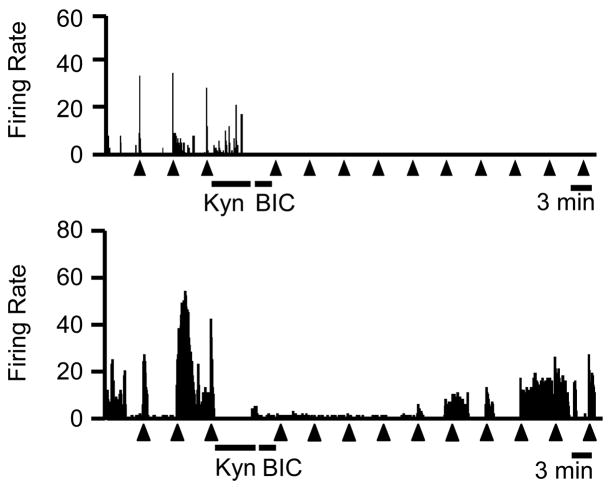
Figure 6.
Ratemeter records illustrate responses of two ON-cells to local administration of kynurenate in the RVM immediately prior to microinjection of bicuculline in the DMH. Firing was profoundly depressed in both cases, although the cell in the lower trace showed significant recovery beginning about 25 min after the kynurenate. 1 s bins, triangles below the trace indicate paw withdrawal trials. KYN: kynurenate microinjection in the RVM, BIC: bicuculline microinjection in the DMH. Neither ON-, OFF- or NEUTRAL cell classes showed a significant increase in activity following DMH disinhibition in kynurenate-pretreated animals (p > 0.05).
Figure 7.
Effects of RVM pretreatment with kynurenate ( to prevent ON-cell activation selectively) or muscimol (to inhibit all RVM neurons) on changes in paw withdrawal, body temperature, and heart rate evoked by activation of the DMH. Mean paw withdrawal latency and heart rate in the period 10–20 min after DMH activation and maximum increase in body temperature after DMH activation are presented as difference from baseline to aid comparison among the different dependent variables. Data for DMH activation alone with no RVM pretreatment are replotted from Figure 3 (black bars) for reference. Kynurenate microinjection in the RVM (open bars) prevented hyperalgesia, but not the DMH-evoked increase in body temperature or heart rate. Muscimol microinjection (diagonal hatching) prevented both hyperthermia and tachycardia. Animals pretreated with muscimol displayed significant hyperalgesia, but muscimol by itself evoked hyperalgesia, as described in the text. For kynurenate group, n = 22, for muscimol group, n = 8. Data in these two groups were analyzed using a repeated-measures ANOVA comparing baseline, post-RVM injection, and after the subsequent DMH bicuculline injection. Tukey’s post-hoc test was used to compare the post-DMH activation timepoint with baseline and post-RVM microinjection. *p < 0.05 compared to baseline. RVM injection sites are shown in Figure 1b.
Preferential suppression of ON-cell activation thus prevented hyperalgesia induced by DMH stimulation, but did not block the accompanying increases in heart rate and rectal temperature. As a positive control, we therefore tested the effect of blocking all RVM activity using the GABAA receptor agonist muscimol, since other investigators have reported that the RVM mediates the activation of brown adipose tissue and to some extent tachycardia following DMH stimulation [7, 30, 56] and in a conditioned fear paradigm [69]. In the present experiments, muscimol microinjection in the RVM completely suppressed the firing of ON-cells, OFF-cells and NEUTRAL cells for at least 45 min (6 cells tested in each class, data not shown), thus verifying the inhibitory action of muscimol under the conditions of our experiments.
By contrast with kynurenate pretreatment, which interfered with activation of ON-cells and hyperalgesia, muscimol pretreatment prevented both hyperthermia and tachycardia evoked by DMH activation (n = 8). Muscimol in the RVM did not significantly alter body temperature or heart rate (data not shown), but subsequent activation of DMH failed to evoke a significant increase in either parameter (Figure 7, diagonally hatched bars; baseline, post-kynurenate, and subsequent effect of DMH bicuculline microinjection compared using repeated-measures ANOVA; temperature: F2,14 = 0.94, p = 0.41; heart rate: F2,14 = 0.90, p = 0.43).
RVM muscimol effects on DMH-evoked hyperalgesia were less straightforward. Repeated-measures ANOVA demonstrated a significant change in paw withdrawal latency in this group (F2,14 = 6.6, p = 0.0093). Unlike excitatory amino acid receptor antagonism, muscimol in the RVM by itself produced a significant hyperalgesia (baseline: 4.4 ± 0.3 s, after muscimol: 2.7 ± 0.4 s, p < 0.05 Tukey’s post-hoc test compared to baseline), presumably due to elimination of an ongoing hypoalgesic influence of OFF-cell output [21]. Subsequent disinhibition of the DMH in these animals did not result in a further reduction in paw withdrawal latency (2.6 ± 0.5 s, p < 0.05 compared to baseline, p > 0.05 compared to post-RVM muscimol, Tukey’s post-hoc test). Although this may represent a floor effect, the failure of DMH activation to produce a further decrease in paw withdrawal latency following suppression of RVM activity is consistent with the actions of kynurenate in preventing DMH-evoked hyperalgesia. In addition, post-treatment with RVM muscimol following DMH stimulation significantly attenuated the hyperalgesia produced by DMH disinhibition (data not shown, repeated-measures ANOVA followed by Dunnett’s test for comparison to baseline, p < 0.05, n = 8).
Discussion
Stress-induced hyperalgesia has now been demonstrated using a variety of paradigms, yet the stress parameters that lead to hyperalgesia rather than analgesia have not been defined, and the underlying neural circuits are almost unknown [42]. The present study points to top-down activation of pain-facilitating neurons in the RVM as a possible circuit for stress-induced hyperalgesia. The recruitment of pain-facilitating ON-cells following DMH stimulation thus complements the activation of pain-inhibiting OFF-cells following opioid application in the basolateral nucleus of the amygdala, a circuit-level model of stress-induced analgesia [27, 45], and suggests that differential recruitment of these two classes, possibly by different forebrain pathways, differentiates stress-induced hyperalgesia and analgesia. Additional pronociceptive influences to the RVM may arise from the interpeduncular nucleus and orbital cortex [28, 32].
Activation or disinhibition of the DMH has been increasingly recognized as a model of what is referred to by various authors as “psychological”, “mild”, or “emotional” stress [11, 35, 60, 65]. Stimulation of the DMH gives rise to autonomic, endocrine, and behavioral changes associated with stress in both anesthetized and awake behaving animals. These changes include increased circulating levels of ACTH, increased body temperature (“stress hyperthermia”), increased heart rate and blood pressure, hyperventilation and behavioral activation. Critically, inactivation of the DMH interferes with autonomic and neuroendocrine responses to air-puff stress, validating the physiological relevance of the DMH to organization of these various aspects of the stress response [16, 44, 48, 62, 63]. These findings raised the possibility that the DMH could contribute to stress-induced hyperalgesia via its connections with the RVM, a region with a well-documented role in pain modulation [13, 20]. Whether direct connections from the DMH to the RVM are critical, as has been argued for autonomic components of stress [29, 47, 57, 68], or whether the relevant pathway is multisynaptic is unknown.
In the present experiments, we took advantage of DMH stimulation as a circuit-level model of stress appropriate for electrophysiological studies. We recorded activity of identified neurons in the RVM, and found that DMH activation shifted the balance between pain-facilitating and pain-inhibiting output channels from this region. ON-cells, known to facilitate nociception, entered a prolonged period of continuous firing. OFF-cells, known to suppress nociception, became less active or inactive. Selective block of the DMH-induced activation of ON-cells using the excitatory amino acid receptor antagonist kynurenate prevented hyperalgesia, showing that the behavioral hyperalgesia observed in these experiments required ON-cell activation, although suppression of OFF-cell firing may also have contributed. Notably, many models of persistent or abnormal pain in which the RVM has been shown to contribute to hyperalgesia involve a positive feedback loop, with convergence of descending facilitation upon sensitized circuits within the dorsal horn [50]. By contrast, the present findings represent an entirely top-down regulation of spinal nociceptive processing, with hyperalgesia despite the absence of a noxious conditioning stimulus. The pattern of ON-cell activation following DMH stimulation nevertheless resembles that seen following cutaneous inflammation [38], with ongoing activity of the ON-cell population predicting behavioral hyperalgesia.
Thermogenesis, and to some extent cardiovascular responses, associated with stress or evoked by activation of the DMH are known to be mediated by a rostral ventromedial medullary region centered on the raphe pallidus, a region that has been equated with the area that we refer to here as the RVM [7, 15, 30, 56, 77]. We confirmed increased body temperature and tachycardia following DMH activation in our lightly anesthetized animals, and showed that these expected autonomic responses were associated with both hyperalgesia and changes in ON- and OFF-cell discharge. This parallel raised the possibility that either or both of these cell classes, which we have considered to be pain-modulating neurons [13, 20], regulate autonomic outflow in addition to nociceptive processing. If so, the early view that neurons of the brainstem reticular core lack specificity of function would be supported (see Brazier [6] for historical review). Indeed, ON- and OFF-cell firing has been reported to be correlated with a number of autonomic parameters [43], and anatomical studies support a role for the RVM as a region in autonomic control [37, 47, 66].
Nevertheless, both functional and anatomical studies suggest that neurons mediating thermogenesis and tachycardia are more concentrated in nucleus raphe pallidus than in the more dorsal aspects of the RVM known to be important in modulating nociception [8, 47, 66, 75, 76]. Consistent with this more specific view of RVM function, the present findings argue for a segregation of pain-modulation and autonomic control within the RVM, differentiated not so much anatomically, but functionally, at the level of ON-, OFF- and NEUTRAL cell classes. Selective block of ON-cells was compared to inhibition of all neurons in the region, and the two approaches resulted in very different effects on DMH-evoked changes in nociceptive threshold, body temperature, and heart rate. Thus, RVM microinjection of the excitatory amino acid receptor antagonist kynurenate, at a concentration that depressed ON-cell firing selectively, blocked the hyperalgesia evoked by DMH stimulation without preventing thermogenesis or heart rate responses. By contrast, infusions of the GABAA receptor agonist muscimol in the RVM, which non-selectively inhibited all neurons in the region, abolished the increases in body temperature and heart rate produced by DMH stimulation. These observations are consistent with and complement the recent report that muscimol, but not kynurenate, in RVM interfered with tachycardia in a conditioned fear paradigm [69]. Further, the fact that muscimol microinjection did not mimic the effect of DMH activation indicates that changes in heart rate and body temperature are not due to the DMH-evoked suppression of OFF-cell firing. Our findings thus indicate that different RVM cell classes play a primary role in pain modulation, cardiovascular control and thermoregulation. The neural basis for thermogenesis and tachycardia remains to be identified physiologically in the RVM.
In conclusion, stress and fear have long been recognized to give rise to a state of analgesia that can be considered adaptive in allowing the organism to cope with more immediate challenges. There is, however, a growing literature related to stress-induced hyperalgesia. This hyperalgesia may in some situations represent an appropriate increase in vigilance to prevent potential harm. Although the organismal and environmental variables that determine whether a particular stressor is associated with analgesia or hyperalgesia have yet to be determined, the present experiments show that activation of stress-related circuitry in the hypothalamus activates pain-facilitating neurons in the RVM to produce hyperalgesia, suggesting a possible neural mechanism through which stress could facilitate pain.
Acknowledgments
Supported by grants from NINDS (NS 40365) and NIDA (DA 05608). We gratefully acknowledge the assistance of Jennifer Maire with mechanical threshold testing. The authors have no conflict of interest in the work presented.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.al Absi M, Rokke PD. Can anxiety help us tolerate pain? Pain. 1991;46:43–51. doi: 10.1016/0304-3959(91)90032-S. [DOI] [PubMed] [Google Scholar]
- 2.Andre J, Zeau B, Pohl M, Cesselin F, Benoliel JJ, Becker C. Involvement of cholecystokininergic systems in anxiety-induced hyperalgesia in male rats: behavioral and biochemical studies. J Neurosci. 2005;25:7896–904. doi: 10.1523/JNEUROSCI.0743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbaro NM, Heinricher MM, Fields HL. Putative nociceptive modulatory neurons in the rostral ventromedial medulla of the rat display highly correlated firing patterns. Somatosens Mot Res. 1989;6:413–25. doi: 10.3109/08990228909144684. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014–22. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodnar RJ, Kelly DD, Brutus M, Glusman M. Stress-induced analgesia: neural and hormonal determinants. Neurosci Biobehav Rev. 1980;4:87–100. doi: 10.1016/0149-7634(80)90028-7. [DOI] [PubMed] [Google Scholar]
- 6.Brazier MAB, Hobson JA, editors. The reticular formation revisited. New York: Raven Press; 1980. [Google Scholar]
- 7.Cao W-H, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–40. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Cao W-H, Morrison SF. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology. 2006;51:426. doi: 10.1016/j.neuropharm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: Reciprocal neural, endocrine, and immune Interactions. J Pain. 2008;9:122–45. doi: 10.1016/j.jpain.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silva Torres IL, Cucco SNS, Bassani M, Duarte MS, Silveira PP, Vasconcellos AP, Tabajara AS, Dantas G, Fontella FU, Dalmaz C, Ferreira MBC. Long-lasting delayed hyperalgesia after chronic restraint stress in rats--effect of morphine administration. Neurosci Res. 2003;45:277. doi: 10.1016/s0168-0102(02)00232-8. [DOI] [PubMed] [Google Scholar]
- 11.DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–80. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 12.DiMicco JA, Sarkar S, Zaretskaia MV, Zaretsky DV. Stress-induced cardiac stimulation and fever: common hypothalamic origins and brainstem mechanisms. Auton Neurosci. 2006;126–127:106–19. doi: 10.1016/j.autneu.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. 5. London: Elsevier; pp. 125–42. [Google Scholar]
- 14.Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–52. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol. 2001;280:H2891–901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- 16.Furlong T, Carrive P. Neurotoxic lesions centered on the perifornical hypothalamus abolish the cardiovascular and behavioral responses of conditioned fear to context but not of restraint. Brain Res. 2007;1128:107–19. doi: 10.1016/j.brainres.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 17.Gamaro GD, Xavier MH, Denardin JD, Pilger JA, Ely DR, Ferreira MB, Dalmaz C. The effects of acute and repeated restraint stress on the nociceptive response in rats. Physiol Behav. 1998;63:693–7. doi: 10.1016/s0031-9384(97)00520-9. [DOI] [PubMed] [Google Scholar]
- 18.Geerse GJ, van Gurp LC, Wiegant VM, Stam R. Individual reactivity to the open-field predicts the expression of stress-induced behavioural and somatic pain sensitisation. Behav Brain Res. 2006;174:112–8. doi: 10.1016/j.bbr.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Hayes RL, Bennett GJ, Newlon PG, Mayer DJ. Behavioral and physiological studies of non-narcotic analgesia in the rat elicited by certain environmental stimuli. Brain Res. 1978;155:69–90. doi: 10.1016/0006-8993(78)90306-2. [DOI] [PubMed] [Google Scholar]
- 20.Heinricher MM, Ingram SL. The brainstem and nociceptive modulation. In: Bushnell MC, AI Basbaum, editors. The Senses, A Comprehensive Reference, Vol 5, Pain. San Diego: Academic Press; pp. 593–626. [Google Scholar]
- 21.Heinricher MM, Kaplan HJ. GABA-mediated inhibition in rostral ventromedial medulla: role in nociceptive modulation in the lightly anesthetized rat. Pain. 1991;47:105–13. doi: 10.1016/0304-3959(91)90017-R. [DOI] [PubMed] [Google Scholar]
- 22.Heinricher MM, McGaraughty S. Analysis of excitatory amino acid transmission within the rostral ventromedial medulla: Implications for circuitry. Pain. 1998;75:247–55. doi: 10.1016/s0304-3959(97)00226-1. [DOI] [PubMed] [Google Scholar]
- 23.Heinricher MM, McGaraughty S, Farr DA. The role of excitatory amino acid transmission within the rostral ventromedial medulla in the antinociceptive actions of systemically administered morphine. Pain. 1999;81:57–65. doi: 10.1016/s0304-3959(98)00271-1. [DOI] [PubMed] [Google Scholar]
- 24.Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004;92:1982–9. doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- 25.Heinricher MM, Roychowdhury S. Reflex-related activation of putative pain facilitating neurons in rostral ventromedial medulla (RVM) depends upon excitatory amino acid transmission. Neuroscience. 1997;78:1159–65. doi: 10.1016/s0306-4522(96)00683-5. [DOI] [PubMed] [Google Scholar]
- 26.Heinricher MM, Tortorici V. Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience. 1994;63:533–46. doi: 10.1016/0306-4522(94)90548-7. [DOI] [PubMed] [Google Scholar]
- 27.Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108:1005–9. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- 28.Hentall ID, Budhrani VM. The interpeduncular nucleus excites the on-cells and inhibits the off-cells of the nucleus raphe magnus. Brain Res. 1990;522:322–4. doi: 10.1016/0006-8993(90)91476-w. [DOI] [PubMed] [Google Scholar]
- 29.Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 30.Horiuchi J, McAllen RM, Allen AM, Killinger S, Fontes MAP, Dampney RAL. Descending vasomotor pathways from the dorsomedial hypothalamic nucleus: role of medullary raphe and RVLM. Am J Physiol Regul Integr Comp Physiol. 2004;287:R824–32. doi: 10.1152/ajpregu.00221.2004. [DOI] [PubMed] [Google Scholar]
- 31.Horiuchi J, McDowall LM, Dampney RA. Role of 5-HT(1A) receptors in the lower brainstem on the cardiovascular response to dorsomedial hypothalamus activation. Auton Neurosci. 2008 doi: 10.1016/j.autneu.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Hutchison WD, Harfa L, Dostrovsky JO. Ventrolateral orbital cortex and periaqueductal gray stimulation-induced effects on on- and off-cells in the rostral ventromedial medulla in the rat. Neuroscience. 1996;70:391–407. doi: 10.1016/0306-4522(95)00372-x. [DOI] [PubMed] [Google Scholar]
- 33.Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006;11:2179–92. doi: 10.2741/1960. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 35.Johnson PL, Truitt WA, Fitz SD, Lowry CA, Shekhar A. Neural pathways underlying lactate-induced panic. Neuropsychopharmacology. 2008;33:2093–107. doi: 10.1038/sj.npp.1301621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jorum E. Analgesia or hyperalgesia following stress correlates with emotional behavior in rats. Pain. 1988;32:341–8. doi: 10.1016/0304-3959(88)90046-2. [DOI] [PubMed] [Google Scholar]
- 37.Kerman IA. Organization of brain somatomotor-sympathetic circuits. Exp Brain Res. 2008;187:1–16. doi: 10.1007/s00221-008-1337-5. [DOI] [PubMed] [Google Scholar]
- 38.Kincaid W, Neubert MJ, Xu M, Kim CJ, Heinricher MM. Role for medullary pain facilitating neurons in secondary thermal hyperalgesia. J Neurophysiol. 2006;95:33–41. doi: 10.1152/jn.00449.2005. [DOI] [PubMed] [Google Scholar]
- 39.King CD, Devine DP, Vierck CJ, Mauderli A, Yezierski RP. Opioid modulation of reflex versus operant responses following stress in the rat. Neuroscience. 2007;147:174–82. doi: 10.1016/j.neuroscience.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Leistad RB, Nilsen KB, Stovner LJ, Westgaard RH, Ro M, Sand T. Similarities in stress physiology among patients with chronic pain and headache disorders: evidence for a common pathophysiological mechanism? J Headache Pain. 2008;9:165–75. doi: 10.1007/s10194-008-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis JW, Cannon JT, Liebeskind JC. Opioid and nonopioid mechanisms of stress analgesia. Science. 1980;208:623–5. doi: 10.1126/science.7367889. [DOI] [PubMed] [Google Scholar]
- 42.Lovick TA. Pro-nociceptive action of cholecystokinin in the periaqueductal grey: A role in neuropathic and anxiety-induced hyperalgesic states. Neurosci Biobehav Rev. 2008;32:852–62. doi: 10.1016/j.neubiorev.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Mason P. Deconstructing endogenous pain modulations. J Neurophysiol. 2005;94:1659–63. doi: 10.1152/jn.00249.2005. [DOI] [PubMed] [Google Scholar]
- 44.McDougall SJ, Widdop RE, Lawrence AJ. Medial prefrontal cortical integration of psychological stress in rats. Eur J Neurosci. 2004;20:2430–40. doi: 10.1111/j.1460-9568.2004.03707.x. [DOI] [PubMed] [Google Scholar]
- 45.McGaraughty S, Heinricher MM. Microinjection of morphine into various amygdaloid nuclei differentially affects nociceptive responsiveness and RVM neuronal activity. Pain. 2002;96:153–62. doi: 10.1016/s0304-3959(01)00440-7. [DOI] [PubMed] [Google Scholar]
- 46.Meagher MW, Ferguson AR, Crown ED, McLemore S, King TE, Sieve AN, Grau JW. Shock-induced hyperalgesia: IV. Generality. J Exp Psychol Anim Behav Process. 2001;27:219–38. [PubMed] [Google Scholar]
- 47.Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–80. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nalivaiko E, Ootsuka Y, Blessing WW. Activation of 5-HT1A receptors in the medullary raphe reduces cardiovascular changes elicited by acute psychological and inflammatory stresses in rabbits. Am J Physiol Regul Integr Comp Physiol. 2005;289:R596–R604. doi: 10.1152/ajpregu.00845.2004. [DOI] [PubMed] [Google Scholar]
- 49.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1997. [Google Scholar]
- 50.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–25. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 51.Quintero L, Cuesta MC, Silva JA, Arcaya JL, Pinerua-Suhaibar L, Maixner W, Suarez-Roca H. Repeated swim stress increases pain-induced expression of c-Fos in the rat lumbar cord. Brain Res. 2003;965:259–68. doi: 10.1016/s0006-8993(02)04224-5. [DOI] [PubMed] [Google Scholar]
- 52.Rhudy JL, Dubbert PM, Parker JD, Burke RS, Williams AE. Affective modulation of pain in substance-dependent veterans. Pain Med. 2006;7:483–500. doi: 10.1111/j.1526-4637.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 53.Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84:65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- 54.Rivat C, Laboureyras E, Laulin JP, Le Roy C, Richebe P, Simonnet G. Non-nociceptive environmental stress induces hyperalgesia, not analgesia, in pain and opioid-experienced rats. Neuropsychopharmacology. 2007;32:2217–28. doi: 10.1038/sj.npp.1301340. [DOI] [PubMed] [Google Scholar]
- 55.Robbins MT, DeBerry J, Ness TJ. Chronic psychological stress enhances nociceptive processing in the urinary bladder in high-anxiety rats. Physiol Behav. 2007;91:544–50. doi: 10.1016/j.physbeh.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samuels BC, Zaretsky DV, DiMicco JA. Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J Physiol. 2002;538:941–6. doi: 10.1113/jphysiol.2001.013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol. 2004;287:R472–8. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- 58.Sandkühler J, Gebhart GF. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Res. 1984;305:77–87. doi: 10.1016/0006-8993(84)91121-1. [DOI] [PubMed] [Google Scholar]
- 59.Sarkar S, Zaretskaia MV, Zaretsky DV, Moreno M, DiMicco JA. Stress- and lipopolysaccharide-induced c-fos expression and nNOS in hypothalamic neurons projecting to medullary raphe in rats: a triple immunofluorescent labeling study. Eur J Neurosci. 2007;26:2228–38. doi: 10.1111/j.1460-9568.2007.05843.x. [DOI] [PubMed] [Google Scholar]
- 60.Sarkar S, Zaretskaia MV, Zaretsky DV, Moreno M, DiMicco JA. Stress- and lipopolysaccharide-induced c-fos expression and nNOS in hypothalamic neurons projecting to medullary raphe in rats: a triple immunofluorescent labeling study. Eur J Neurosci. 2007;26:2228–38. doi: 10.1111/j.1460-9568.2007.05843.x. [DOI] [PubMed] [Google Scholar]
- 61.Siegel S, Castellan NJ. Nonparametric statistics for the behavioral sciences. 2. New York: McGraw Hill; 1988. [Google Scholar]
- 62.Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res. 1996;742:219–24. doi: 10.1016/s0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- 63.Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J Neurosci. 1996;16:1173–9. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suarez-Roca H, Quintero L, Arcaya JL, Maixner W, Rao SG. Stress-induced muscle and cutaneous hyperalgesia: differential effect of milnacipran. Physiol Behav. 2006;88:82–7. doi: 10.1016/j.physbeh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka M, McAllen RM. Functional topography of the dorsomedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2008;294:R477–86. doi: 10.1152/ajpregu.00633.2007. [DOI] [PubMed] [Google Scholar]
- 66.Ter Horst GJ, Hautvast RW, De Jongste MJ, Korf J. Neuroanatomy of cardiac activity-regulating circuitry: a transneuronal retrograde viral labelling study in the rat. Eur J Neurosci. 1996;8:2029–41. doi: 10.1111/j.1460-9568.1996.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 67.Thompson T, Keogh E, French CC, Davis R. Anxiety sensitivity and pain: generalisability across noxious stimuli. Pain. 2008;134:187–96. doi: 10.1016/j.pain.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 68.Verner TA, Pilowsky PM, Goodchild AK. Retrograde projections to a discrete apneic site in the midline medulla oblongata of the rat. Brain Res. 2008;1208:128–36. doi: 10.1016/j.brainres.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 69.Vianna DM, Allen C, Carrive P. Cardiovascular and behavioral responses to conditioned fear after medullary raphe neuronal blockade. Neuroscience. 2008;153:1344–53. doi: 10.1016/j.neuroscience.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 70.Vidal C, Jacob JJ. Stress hyperalgesia in rats: an experimental animal model of anxiogenic hyperalgesia in human. Life Sci. 1982;31:1241–4. doi: 10.1016/0024-3205(82)90352-6. [DOI] [PubMed] [Google Scholar]
- 71.Vierck CJ., Jr Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia) Pain. 2006;124:242–63. doi: 10.1016/j.pain.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 72.Watkins LR, Mayer DJ. Organization of endogenous opiate and nonopiate pain control systems. Science. 1982;216:1185–92. doi: 10.1126/science.6281891. [DOI] [PubMed] [Google Scholar]
- 73.Williams AE, Rhudy JL. The influence of conditioned fear on human pain thresholds: does preparedness play a role? J Pain. 2007;8:598–606. doi: 10.1016/j.jpain.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Xu M, Kim CJ, Neubert MJ, Heinricher MM. NMDA receptor-mediated activation of medullary pronociceptive neurons is required for secondary thermal hyperalgesia. Pain. 2007;127:253–62. doi: 10.1016/j.pain.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaretskaia MV, Zaretsky DV, Sarkar S, Shekhar A, Dimicco JA. Induction of Fos-immunoreactivity in the rat brain following disinhibition of the dorsomedial hypothalamus. Brain Res. 2008;1200:39–50. doi: 10.1016/j.brainres.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaretsky DV, Zaretskaia MV, DiMicco JA. Stimulation and blockade of GABA(A) receptors in the raphe pallidus: effects on body temperature, heart rate, and blood pressure in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R110–6. doi: 10.1152/ajpregu.00016.2003. [DOI] [PubMed] [Google Scholar]
- 77.Zaretsky DV, Zaretskaia MV, Samuels BC, Cluxton LK, DiMicco JA. Microinjection of muscimol into raphe pallidus suppresses tachycardia associated with air stress in conscious rats. J Physiol. 2003;546:243–50. doi: 10.1113/jphysiol.2002.032201. [DOI] [PMC free article] [PubMed] [Google Scholar]