Abstract
Tau is a neuronal microtubule-associated protein. Its hyperphosphorylation plays a critical role in Alzheimer disease (AD). Expression and phosphorylation of tau are regulated developmentally, but its dynamic regulation and the responsible kinases or phosphatases remain elusive. Here, we studied the developmental regulation of tau in rats during development from embryonic day 15 through the age of 24 months. We found that tau expression increased sharply during the embryonic stage and then became relatively stable, whereas tau phosphorylation was much higher in developing brain than in mature brain. However, the extent of tau phosphorylation at seven of the 14 sites studied was much less in developing brain than in AD brain. Tau phosphorylation during development matched the period of active neurite outgrowth in general. Tau phosphorylation at various sites had different topographic distributions. Several tau kinases appeared to regulate tau phosphorylation collectively at overlapping sites, and the decrease of overall tau phosphorylation in adult brain might be due to the higher levels of tau phosphatases in mature brain. These studies provide new insight into the developmental regulation of site-specific tau phosphorylation and identify the likely sites required for the abnormal hyperphosphorylation of tau in AD.
Keywords: Alzheimer disease, tau phosphorylation, protein kinases, protein phosphatases, development
Tau is a major microtubule-associated protein (MAP) in the brain. Its main known biological function is to promote microtubule assembly and to stabilize microtubules. In adult human brain, six tau isoforms are expressed as a result of alternative mRNA splicing from a single gene (Goedert et al. 1989b; Goedert et al. 1989a; Kosik et al. 1989). Since the seminal discovery that abnormally hyperphosphorylated tau constitutes paired helical filaments (PHF) of neurofibrillary tangles (NFTs) (Grundke-Iqbal et al. 1986a; Grundke-Iqbal et al. 1986b; Lee et al. 1991; Montejo de Garcini et al. 1986; Ihara et al. 1986), a hallmark brain lesion of Alzheimer disease (AD), extensive studies on the structure, function, and posttranslational modifications of tau as well as its abnormalities in AD have been carried. Both expression and phosphorylation of tau are reported to be regulated developmentally. In fetal human brain, only the smallest isoform of tau is expressed. Compared to adult tau, fetal tau is highly phosphorylated at several phosphorylation sites seen in AD brain (Goedert et al. 1993; Brion et al. 1993; Kenessey and Yen 1993; Morishima-Kawashima et al. 1995). However, fetal tau, though highly phosphorylated, is functional and is not polymerized into NFTs, whereas hyperphosphorylated tau from AD brain inhibits microtubule assembly and polymerizes into NFTs (Alonso et al. 2001; Alonso et al. 2006; Iqbal et al. 1986; Alonso et al. 1994; Yoshida and Ihara 1993). Thus, a detailed comparison between tau in fetal and abnormally hyperphosphorylated tau in AD brain will help elucidate the mechanism underlying the differences between these two states of tau.
Investigation of the mechanisms of abnormal hyperphosphorylation of tau in AD brain has led to identification of major protein kinases and phosphatases that regulate tau phosphorylation. The major tau kinases include glycogen synthase kinase 3β (GSK-3β), cyclin-dependent kinase 5 (CDK5), mitogen-activated protein kinases (MAPKs), cAMP-dependent protein kinase (PKA), calcium/calmodulin-dependent protein kinase II (CaMKII), and dual-specificity tyrosine-regulated kinase 1A (Dyrk1A) (Gong and Iqbal 2008; Ferrer et al. 2005). Among various protein phosphatases, PP1, PP2A, PP2B and PP5 have been shown to dephosphorylate tau (Liu et al. 2005).
In this report, we describe a detailed regulation of tau expression, tau phosphorylation at individual phosphorylation sites, and tau kinases and phosphatases in rat brain during development from embryonic day 15 (E15d) to the age of 24 months.
Materials and methods
Antibodies and reagents
The primary antibodies used in this study are listed in Table 1. Peroxidase-conjugated anti-mouse and anti-rabbit IgG were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The enhanced chemiluminescence (ECL) kit was from Amersham Pharmacia (Piscataway, NJ, USA).
Table 1.
Primary antibodies employed in this study
| Antibody | Type | Specificity | Phosphorylation sites | Reference/Source |
|---|---|---|---|---|
| 92e | Poly- | Tau | (Pei et al. 1998) | |
| pT188 | Poly- | P-tau | Thr181 | Biosource, Camarillo, CA, USA |
| pS199 | Poly- | P-tau | Ser199 | Biosource |
| pS202 | Poly- | P-tau | Ser202 | Biosource |
| pT205 | Poly- | P-tau | Thr205 | Biosource |
| pT212 | Poly- | P-tau | Thr212 | Biosource |
| pS214 | Poly- | P-tau | Ser214 | Biosource |
| pT217 | Poly- | P-tau | Thr217 | Biosource |
| pT231 | Poly- | P-tau | Thr231 | Biosource |
| pS262 | Poly- | P-tau | Ser262 | Biosource |
| pS356 | Poly- | P-tau | Ser356 | Biosource |
| pS396 | Poly- | P-tau | Ser396 | Biosource |
| pS404 | Poly- | P-tau | Ser404 | Biosource |
| pS409 | Poly- | P-tau | Ser409 | Biosource |
| pS422 (R145) | Poly- | P-tau | Ser422 | (Pei et al. 1998) |
| 12E8 | Mono- | P-tau | Ser262/Ser356 | Dr. D. Schenk |
| Anti-MAP1A | Mono- | MAP1A | Sigma-Aldrich, Inc., MO, USA | |
| Anti-MAP1B | Mono- | MAP1B | Sigma-Aldrich | |
| Anti-p-GSK-3β | Poly- | P-GSK-3β | Ser9 | Cell Signaling Technology, MA, USA |
| Anti-p-GSK-3β | Poly- | P-GSK-3β | Tyr216 | Biosource |
| R133d | Poly- | GSK-3β | (Pei et al. 1997) | |
| Anti-p-AKT | Poly- | P-AKT | Ser473 | Cell Signaling Technology |
| Anti-AKT | Poly- | AKT | Cell Signaling Technology | |
| Anti-p-PI3K (85kDa) | Poly- | P-PI3K (85kDa) | Tyr458/Tyr199 | Cell Signaling Technology |
| Anti-PI3K (85kDa) | Poly- | PI3K (85kDa) | Cell Signaling Technology | |
| Anti-PI3K (110kDa) | Poly- | Anti-PI3K (110kDa) | Cell Signaling Technology | |
| Anti-CDK5 | Poly- | CDK5 | Santa Cruz Biotechnology, CA, USA | |
| Anti-P35 | Poly- | P35 | Santa Cruz Biotechnology | |
| Anti-p-MAPK | Poly- | P-ERK1/2 | Thr202/Tyr204 | Cell Signaling Technology |
| Anti-MAPK | Poly- | ERK1/2 | Cell Signaling Technology | |
| Anti-p-JNK | Mono- | P-JNK | Thr183/Tyr185 | Cell Signaling Technology |
| Anti-JNK | Poly- | JNK | Cell Signaling Technology | |
| Anti-PKAα (C-20) | Poly- | PKA-Cα | Santa Cruz Biotechnology | |
| Anti-PKAβ (C-20) | Poly- | PKA-Cβ | Santa Cruz Biotechnology | |
| Anti-PKA-RI | Mono- | PKA-RI | Santa Cruz Biotechnology | |
| Anti-PKA-RIIα | Mono- | PKA-RIIα | BD Biosciences, Palo Alto, CA, USA | |
| Anti-PKA-RIIβ | Mono- | PKA-RIIβ | BD Biosciences | |
| Anti-p-CaMKII | Mono- | P-CaMKII | Thr286 | Cell Signaling Technology |
| Anti-CaMKII | Poly- | CaMKII | EMD Chemicals Inc, NJ, USA | |
| 8D9 | Mono- | Dyrk1A | (Wegiel et al. 2004) | |
| Anti-PP1 | Mono- | PP1 | BD Bioscience | |
| R123d | Poly- | PP2A | (Pei et al. 1998) | |
| R126d | Poly- | PP2B | (Pei et al. 1998) | |
| Anti-PP5 | Poly- | PP5 | (Bahl et al. 2001) |
Animals and human brain tissue
Wistar rats were from Charles River Laboratories, Inc. (Wilmington, MA). Pregnant female rats were sacrificed at 15 (E15d) and 19 (E19d) days of gestation, and the brains of rat fetuses (E15d and E19d) were dissected immediately. Rat brains were also collected from pups on the day of birth (P0), and male rats at post-natal day 5 (P5d), P15d, post-natal month 1 (P1m), P6m, P12m, and P24m. All animal experiments were performed according to the “Principles of Laboratory Animal Care” (NIH Publication 86−23, revised in 1985) and were approved by the Animal Welfare Committee of the New York State Institute for Basic Research in Developmental Disabilities.
Human brain tissue (frontal cortices) from AD patients (mean age 73.2 ± 10.1 y) and control individuals (mean age 73.6 ± 8.2 y) was obtained from the Harvard Brain Tissue Resource Center (Belmont, MA). The average postmortem delay before tissue collection was 4.7 ± 3.2 h for AD and 5.0 ± 1.2 h for controls. All brain samples were confirmed pathologically and stored at −70°C until used. The use of frozen human brain tissue was in accordance with the National Institutes of Health guidelines and was approved by the Institutional Review Committee of the New York State Institute for Basic Research in Developmental Disabilities.
Western blot analysis
Rat brain tissue was homogenized in buffer consisting of 50 mM Tris-HCl (pH 7.4), 2.0 mM EDTA, 10 mM β-mercaptoethanol, 8.0 μg/ml aprotinin, 100 μg/ml leupeptin, 4.0 μg/ml pepstatin, and 8.5% sucrose. Aliquots of the homogenates were mixed by the same volume of 2× concentrated Laemmeli buffer (125 mM Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 2% β-mercaptoethanol and 0.005% bromophenal blue), followed by heating in boiling water for 5 min. Protein concentrations of the samples were determined by using modified Lowry method (Bensadoun and Weinstein 1976). The levels of specific brain proteins and their phosphorylation were determined by Western blots using 5% (for MAP1A and MAP1B only) or 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The Western blots were developed by using ECL, and the immunoreactivities of the blots were quantified densitometrically.
Immunohistochemistry
Tissue was first fixed in 10% PBS-buffered formalin and embedded in paraffin. Thin (6 μm thick) sections were cut, and immunohistochemical staining was carried out by using avidin-biotin-peroxidase complex system (Vector Labs, Inc., Burlingame, CA) and visualized by diaminobenzidine (DAB) staining.
Results
Developmental regulation of expression and phosphorylation of tau
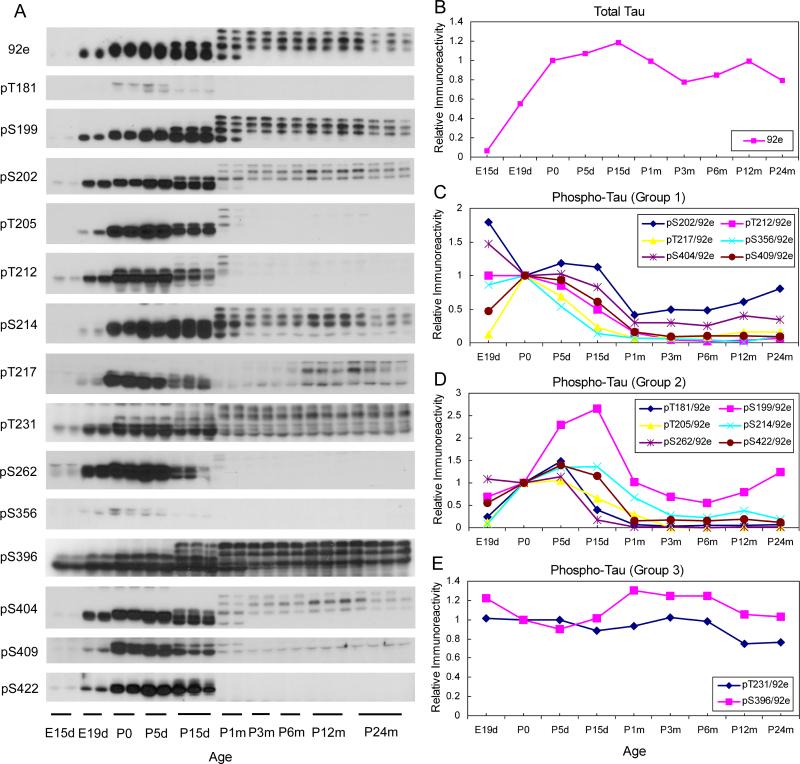
The level and site-specific phosphorylation of tau in the brain homogenates of rats of various ages were determined by Western blots developed with 92e to total tau and phosphorylation-dependent/site-specific tau antibodies, respectively. We found that tau expression increased sharply in the developing brain during the embryonic stage (Fig. 1A, B). After birth, the total level of tau in the brain remained relatively stable. It increased only slightly (∼20%) till the age of 15 days and then decreased by 20% over the life span. The Western blots also demonstrated that during the early embryonic stage, only the shortest tau isoform was expressed in the rat brain, whereas the larger tau isoforms started to show after P5d. The most dramatic change in the expression of various tau isoforms occurred between P15d and P1m. Before P15d, only two major tau bands were seen, whereas by the age of P15m, four tau bands with comparable intensities appeared (Fig. 1A). After rats reached adult age (P3m), the expression of fetal tau disappeared. These observations are consistent with previous reports on the developmental regulation of expression of various tau isoforms (Takuma et al. 2003).
Fig. 1.
Levels of total tau and tau phosphorylated at individual phosphorylation sites during development. (A) Equal amounts of brain homogenates from rats with the indicated ages were analyzed by Western blots developed with antibody 92e to detect total tau or several phosphorylation-dependent and site-specific tau antibodies to detect tau phosphorylation at the individual sites. (B–E) The blots shown in panel A were quantified densitometrically, and the mean values of each time point were plotted against ages. The data represent relative immunoreactivities, those of which at birth (P0) were defined as 1. For quantification of tau phosphorylation, the data had been normalized by the level of total tau, as determined by 92e blots, at each time point. According to the patterns of changes, alterations of site-specific tau phosphorylation were divided into three groups and are presented in (C), (D), and (E).
Tau phosphorylation at a total of 14 phosphorylation sites was studied during development. We found that tau phosphorylation at all these sites were regulated developmentally (Fig. 1A). On the basis of the patterns of changes during development, these phosphorylation sites can be divided into three groups. In group 1 (Ser202, Thr212, Thr217, Ser356, Ser404, and Ser409), tau was found to be highly phosphorylated during development up to the age of P15d, and tau phosphorylation levels were the highest at embryonic stages (Fig. 1C). Tau phosphorylation at group 2 sites (Thr181, Ser199, Thr205, Ser214, Ser262, and Ser422) increased dramatically during the embryonic stage and the first 5 (Thr181, Thr205, Ser262, and Ser422) or 15 days (Ser199 and Ser214) of life (Fig. 1D). After the peak, tau phosphorylation at these sites declined to low levels at the age of 1−3 months old, and then it remained relatively unchanged for the rest of life. In group 3 phosphorylation sites of tau (Thr231 and Ser396), we did not observe any marked alteration of tau phosphorylation from E19d through the age of P24m (Fig. 1E). As demonstrated in Fig. 1A, tau expression at E15d was very low. Thus, this time point was not included for calculation of site-specific tau phosphorylation, because the normalized tau phosphorylation at Thr231 and Ser396 had been extremely high (data not shown), which appeared to be misleading.
We also observed that, within the groups, different tau isoforms appeared to be phosphorylated differently during development. For example, Ser199 of the three adult tau bands were similarly phosphorylated during adulthood, whereas Ser214 of the upper most band was hardly phosphorylated (Fig. 1A), although both sites were belong to Group 2 and had similar changes in overall phosphorylation. Similarly, Thr231 of the upper two tau bands was more phosphorylated than the lower band, whereas Ser396 of the lower band was also highly phosphorylated. These observations demonstrate the complex dynamic regulation of tau phosphorylation at the levels of both tau isoforms and phosphorylation sites.
Immunohistochemical distribution of tau phosphorylation during development
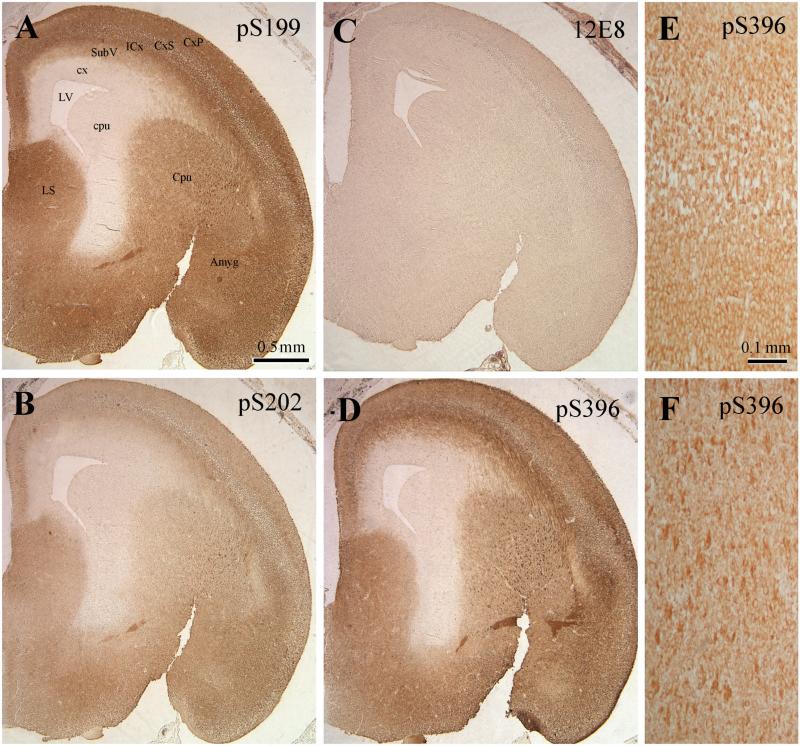
We investigated the topology of tau phosphorylation during development by immunohistochemistry. For these studies, we selected three time points (E19d, P5d, and P6m) to cover the embryonic and postnatal development stages as well as the adult stage. Four antibodies that did not react with any rat brain proteins other than the site-specific phosphorylated tau and covered phosphorylation sites of the three groups (see above) were selected for immunohistochemistry. These antibodies included anti-pS199, anti-pS202, 12E8 (to pS262 and pS356), and anti-pS396. We found that except for 12E8, which stained E19d rat brain very weakly, the three other antibodies had similarly strong staining patterns for E19d rat brains (Fig. 2). The strong staining was seen over the whole brain except for the cortical neuroepithelium (cx) and caudate/putamen neuroepithelium (cpu) that surround the lateral ventricle (LV). A clear laminar staining pattern of the cortical plate (CxP), cortical subplate (CxS), intermediate cortical layer (ICx), and subventricular cortical layer (SubV) was seen. At high magnification, most staining was observed in the cell bodies, whereas the nuclei were nearly free of staining (Fig. 2, E and F).
Fig. 2.
Immunohistochemical staining of E19d rat brains with antibodies against tau phosphorylated at specific individual phosphorylation sites. Paraffin-embedded sections of E19d rat brains were immuno-stained with anti-pS199 (A), anti-pS202 (B), 12E8 (to pS262 and pS356, C), and anti-pS396 (D–F), and the immunoreaction was developed by DAB. Panels E and F are enlarged images in the area of cortex (E) and caudate putamen (F), respectively. Abbreviations: Amyg, amygdala; Cpu, caudate putamen; cpu, caudate/putamen neuroepithelium; cx, neuroepithelium; CxP, cortical plate; CxS, cortical subplate; ICx, intermediate cortical layer; LS, lateral septal nu; LV, lateral ventricle; SubV, subventricular cortical layer.
At postnatal day 5, the staining patterns of phosphorylated tau were different among various phosphorylation sites, and antibody 12E8 still minimally stained rat brain tissue (Table 2). Anti-pS199 and anti-pS396 had the strongest staining in the cerebral cortices. They also strongly stained deeper structures of the brain such as the caudate putamen and globus pallidus, whereas anti-pS202 and 12E8 hardly stained these structures. The hippocampus was moderately stained only by anti-pS396. Within the cerebral cortex, both neuronal cell bodies and cell processes were strongly stained with anti-pS199 and anti-pS396. The staining in different cortical layers appeared to be similar except for a little darker staining in layer II. There was no obvious difference in the staining pattern with various phospho-tau antibodies in the cerebral cortex.
Table 2.
Intensity of immunostaining of rat brains with phospho-tau antibodies
| Age of rats |
5 days |
6 months |
||||||
|---|---|---|---|---|---|---|---|---|
| Antibody | pSer199 | pSer202 | 12E8 | pSer396 | pSer199 | pSer202 | 12E8 | pSer396 |
| Cerebral cortex | +++ | + | −/+ | +++ | ++ | + | −/+ | + |
| Thalamus | + | −/+ | − | +/++ | −/+ | −/+ | − | +/++ |
| Amygdala | + | ++ | − | −/+ | −/+ | −/+ | − | −/+ |
| Caudate putamen | ++ | − | − | ++ | + | −/+ | + | +/++ |
| Hippocampus | + | + | − | +/++ | +++ | ++ | + | ++ |
The intensity of immunostaining was estimated by visual examination and is presented as – (negative), + (weak staining), ++ (moderate staining), and +++ (strong staining).
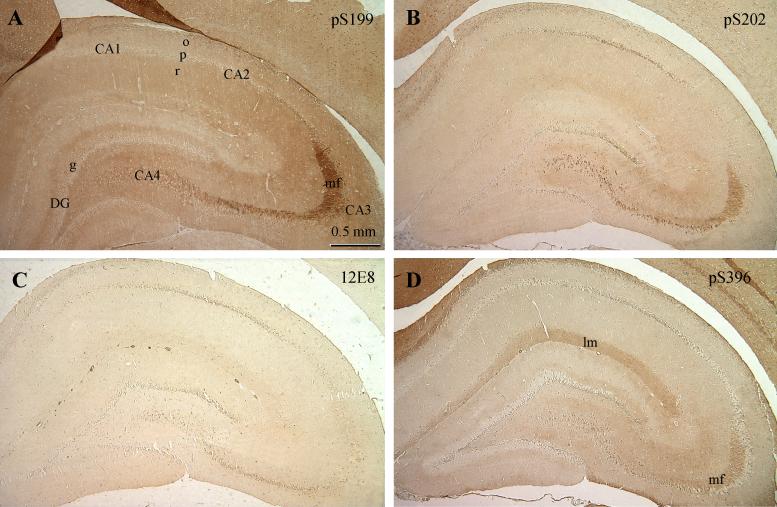
In rats at P6m, immunostaining of brain sections with these phosphor-tau antibodies was found to be generally weak to moderate except with anti-pS199 (Table 2). The topographic distribution of tau phosphorylated at different sites was different, especially in the hippocampus (Fig. 3). Anti-pS199 did not stain cell bodies of most pyramidal neurons (p) of the cornu Ammonis (CA) or granule neurons (g) of the dentate gyrus (DG), but the stratum oriens (o) and stratum radiatum (r) were moderately stained (Fig. 3A). The strongest pS199 staining was observed in the mossy fibers (mf) of the CA3 sector. With anti-pS202, in addition to mossy fibers of the CA3, the pyramidal neurons of CA1/2 and CA4 were also stained, but the neuronal cell bodies at CA3 and DG were not stained (Fig. 3B). Antibody 12E8 only weakly stained pyramidal neurons at CA1/2 and CA4 and some DG neurons (Fig. 3C). Other areas were unstained. Staining of the hippocampus with anti-pS396 was also weak (Fig. 3D). Only weak to moderate staining was seen in the stratium lacumosum/moleculae and mossy fibers.
Fig. 3.
Immunohistochemical staining of P6m rat brains with antibodies against tau phosphorylated at specific individual phosphorylation sites. Paraffin-embedded sections of P6m rat brains were immuno-stained with anti-pS199 (A), anti-pS202 (B), 12E8 (to pS262 and pS356, C), and anti-pS396 (D), and the immunoreaction was developed with DAB. Abbreviations: CA, cornu Ammonis; DG, dentate gyrus; g, granule neurons; lm, stratium lacumosum/moleculae; mf, mossy fibers; o, stratum oriens; p, pyramidal neurons; r, stratum radiatum.
Comparison of tau phosphorylation during development with AD abnormal tau phosphorylation
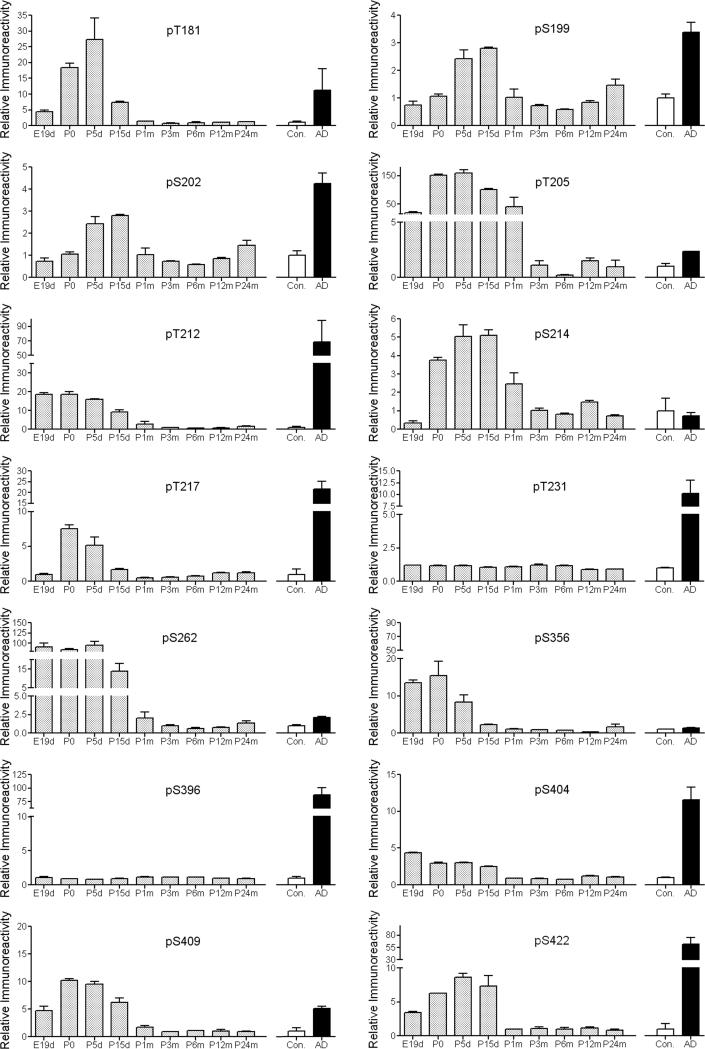
Although tau both in normal developing brain and in AD brain is highly phosphorylated at multiple sites, only the latter inhibits its activity to stimulate microtubule assembly and polymerizes into NFTs. The functional change and NFT formation of tau are believed to result mainly from its abnormal hyperphosphorylation (Del et al. 2008; Gong et al. 2006). To investigate what makes the functional differences between normal tau in the immature brain and tau in AD brain, we compared the extents of tau phosphorylation at individual phosphorylation sites between tau in the developing rat brain and pathological tau from AD brain. We found that, as compared with adult tau, tau was hyperphosphorylated at different sites to various extents during different developmental stages (Fig. 4). When we compared tau between AD brains and control brains, 12 of the 14 examined sites were found to be dramatically hyperphosphorylated in AD. Among these 14 sites, the phosphorylation level of seven sites (Ser202, Thr212, Thr217, Thr231, Ser396, Ser404, and Ser422) was much higher in AD brain than in developing rat brain, whereas the phosphorylation level of the other seven sites (Thr181, Ser199, Thr205, Ser214, Ser262, Ser356, and Ser409) in AD brain were similar to or lower than tau in the developing rat brain. These results suggest that the different phosphorylation levels in the selective sites between tau in the developing brain and tau in AD brain might play a key role in converting tau from a molecule that is biologically active into one that inhibits microtubule assembly and polymerizes into NFTs.
Fig. 4.
Quantitative comparison of phosphorylation levels between tau in immature brains and tau in AD brain. Site-specific phosphorylation of tau in brains of rats (shaded bars) with various ages from E19d to P24m, and of tau in AD (black bars) and age-matched normal human brains (open bars) was determined in brain homogenates by quantitative Western blots. All data had been normalized with the amounts of total tau level. For rat brain tau, data (mean ± SEM) are presented as relative immunoreactivities (phosphorylation levels), where the mean immunoreactivities of tau in adult rat brains (P3m to P24m) were defined as 1. For human brain tau, data are presented as relative immunoreactivities (phosphorylation levels), where the mean immunoreactivities of tau in control brains were defined as 1.
Correlation of tau phosphorylation to the period of intense neurite outgrowth during development
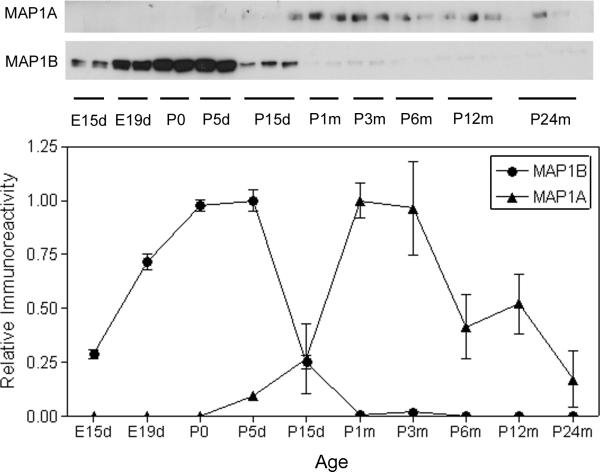
Establishment of neuronal connections in developing brain can be divided into two periods. The first period is characterized by active neurite outgrowth during the embryonic and early postnatal days, which is marked by MAP1B expression (Riederer et al. 1986; Schoenfeld et al. 1989). The second period is involved mainly in neurite stabilization and synapse formation and is marked by the appearance of MAP1A expression (Riederer and Innocenti 1991; Schoenfeld et al. 1989). To study if tau phosphorylation correlates to neuronal development, we detected the expression of MAP1B and MAP1A in rat brain during development. As expected, MAP1B expression was found to increase dramatically during early development and reached its peak at P0–P5d (Fig. 5). The MAP1B expression then decreased rapidly and became undetectable after P1m. In contrast, MAP1A started to express after P5d, and the expression reached its peak at P1–3m. When the dynamic changes of MAP1B and MAP1A were compared with those of site-specific tau phosphorylation, we found that tau phosphorylation at the sites of groups 1 and 2 generally correlated with the increase in MAP1B expression (compare Fig. 5 with Fig. 1). Among these tau phosphorylation sites, the patterns of tau phosphorylation at Thr181, Thr205, Thr217, and Ser404 matched with the change of MAP1B expression best during development. Tau phosphorylation at some other sites, such as Ser202, Thr212, Ser356, and Ser404, was not increased or even decreased during the period of E19-P0, whereas the MAP1B expression was markedly increased during the same period, suggesting that tau phosphorylation at these sites might not be related to neurite outgrowth. Taken together, these observations suggest that tau phosphorylation at some sites might regulate the dynamic changes of microtubule assembly that, in turn, play a critical role in neurite outgrowth.
Fig. 5.
Developmental changes of MAP1A and MAP1B expression. Levels of MAP1A and MAP1B in brain homogenates of rats during development from embryonic day 15 (E15d) to the age of 24-months were determined by Western blots. The blots were quantified densitometrically, and the relative immunoreactivities (mean ± SEM) are shown.
Developmental regulation of tau protein kinases
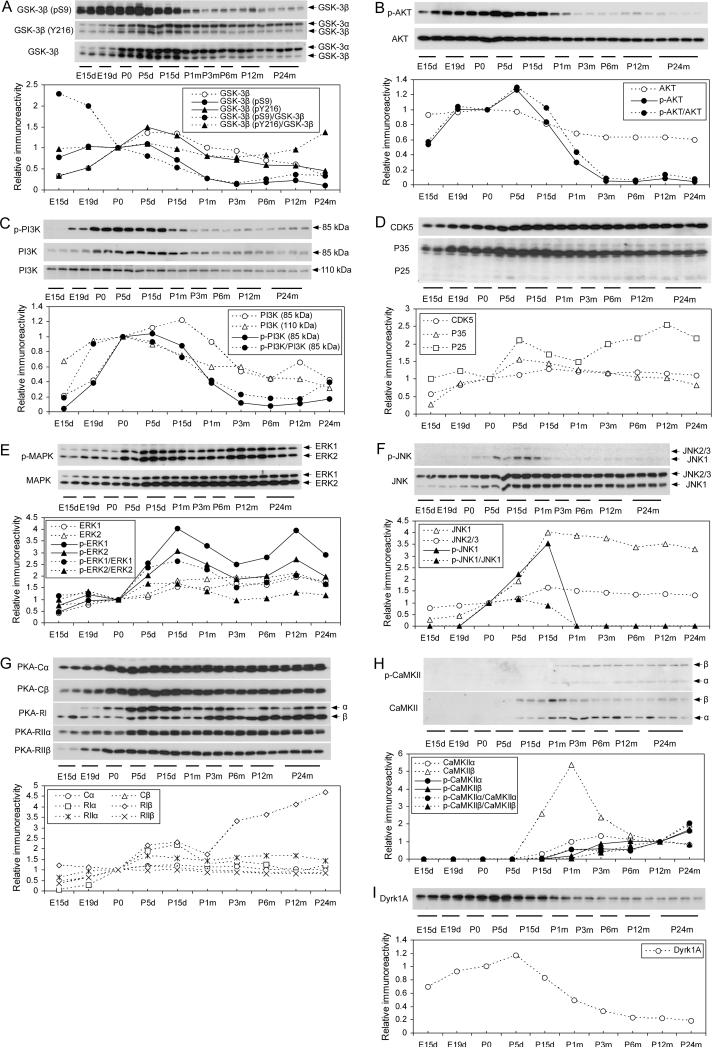
Since tau phosphorylation at various sites is catalyzed by specific tau kinases, we investigated several tau kinases during development. GSK-3β is the tau kinase most often implicated and phosphorylates tau at several phosphorylation sites (Ferrer et al. 2005; Takashima 2006; Aghdam and Barger 2007). We found that the expression of GSK-3β was regulated developmentally with dynamics similar to that of tau phosphorylation at group 2 phosphorylation sites, which increased during early development and peaked at P5d to P15d and then declined during the rest of life (compare Fig. 6A with Fig. 1D). It is well established that GSK-3β activity requires its phosphorylation at Tyr216, and the kinase is inactivated by phosphorylation at Ser9, which is the major regulating mechanism of GSK-3β activity (Plyte et al. 1992; Hughes et al. 1993). Thus, we also determined the level of GSK-3β phosphorylated at Tyr216 or Ser9 by using phosphorylation-dependent and site-specific GSK-3β antibodies. We found that the pTyr216 profile was similar to the total GSK-3β profile (Fig. 6A), indicating that this site is not an active regulator of GSK-3β activity, which is consistent with the general belief that Tyr216 is constitutively phosphorylated and plays a minor role in regulating the kinase activity (Shaw et al. 1997). In contrast, Ser9 phosphorylation of GSK-3β was found to be high during early development and declined markedly during late development (P5d–P1m). This change appeared to be much more dramatic after the data were normalized with the total GSK-3β level. Considering both the expression level and the Ser9 phosphorylation of GSK-3β, we speculate that the kinase activity was higher during P5d–P15d than other periods of development.
Fig. 6.
Developmental regulation of the levels and activation of tau kinases. The total levels of protein kinases and their phosphorylated form, which represent their activation or inactivation, in brain homogenates of rats of various ages were determined by Western blots. The blots were also quantified densitometrically, and the data are shown under the corresponding blots. Open symbols with dotted lines represent the total levels of kinases or regulators. Closed symbols represent the levels of the indicated phosphorylated kinases quantified either without (solid lines), or after being normalized with, the total level of the corresponding kinases (dotted lines).
The activity of GSK-3β is regulated mainly via phosphorylation at Ser9 by AKT, which, in turn, is phosphorylated and activated by PI3K (Cross et al. 1995). Thus, we also determined the levels and phosphorylation/activation of these two upstream kinases of GSK-3β. We found that AKT expression was relatively stable during early development and slowly declined during late development (P0–P1m) to the level equal to 60−70% of the embryonic level (Fig. 6B). The phosphorylation of AKT at Ser473, which represents its activation, increased during early development and peaked at P5d. Then, it declined to a very low level at P3m. PI3K is composed of two subunits: the catalytic subunit p110 and the regulatory p85. The expression of both subunits was found to increase during embryonic stages and then decreased slowly after birth (p110) or after P15d (p85) (Fig. 6C). The developmental changes of phosphorylation of PI3K p85 at Tyr458, which activates the kinase, were found to resemble those of AKT (compare Fig. 6C with Fig. 6B), consistent with the fact that AKT is phosphorylated and activated by PI3K activation. However, the activation of these two kinases did not match Ser9 phosphorylation of GSK-3β completely. These results suggest that PI3K-AKT is not the sole upstream regulator of GSK-3β.
CDK5 is another important tau kinase, which is activated when associated with its activators, P35 or P25 (Lee and Tsai 2003; Ferrer et al. 2005). The expression of CDK5 increased during development and peaked at the age of P15d. Then, it maintained unchanged till P24m (Fig. 6D). The levels of both P35 and P25 increased during early development, but the former slightly declined after P5d and the latter slightly declined during P5d and P30d and then increased again afterwards.
ERKs and JNKs are the major brain mitogen-activated protein kinases (MAPKs) and play important roles in neuronal differentiation and development as well as in phosphorylation of tau, especially under stress condition (Reynolds et al. 1997; Petersen et al. 2007; Zhu et al. 2002; Pei et al. 2002). We found that the expression of ERK1/2 and, especially, the phosphorylation at Thr202/Tyr204, which represents their activation, increased during development and peaked at P5–15d (Fig. 6E). The developmental regulation of JNK levels resembled those of ERKs, but the activation of JNK1, as evidenced by its phosphorylation at Thr183/Tyr185, was detectable only during P0–P15d (Fig. 6F). This JNK activation window matched well to phosphorylation of tau at group 2 sites (compare Fig. 6F with Fig. 1D).
PKA, another important tau kinase (Jicha et al. 1999; Liu et al. 2004; Gong et al. 2006; Wang et al. 2007), is a tetrameric holoenzyme consisting of two catalytic (C) and two regulatory (R) subunits. We studied the developmental regulation of expression of the major brain isoforms of PKA subunits. We found that their levels all increased during development before P5d, after which they remained stable, except for RIβ, which kept increasing during adulthood (Fig. 6G). Both RIα and RIβ also had a small peak between P5d and P15d.
CaMKII phosphorylates a broad range of substrate proteins including tau (Lindwall and Cole 1984; Baudier and Cole 1987). Neither of the two isoforms, CaMKIIα or CaMKIIβ, was detectable before P5d (Fig. 6H). Expression of CaMKIIα was observed after P15d and reached the highest level at P3m and then slightly declined. Expression of CaMKIIβ peaked sharply at P1m and also rapidly declined to a lower level for the rest of adulthood. Because CaMKII can be activated by autophosphorylation at Thr286 (Giese et al. 1998), we studied its activation by detecting the phosphorylated CaMKII at this site. We found that CaMKII activity gradually increased after P15d.
Dyrk1A phosphorylates tau and also primes tau phosphorylation by GSK-3β (Liu et al. 2008). We observed that Dyrk1A expression increased during development and peaked at P5d. Then, it declined to a much lower level at P6m (Fig. 6I).
Developmental regulation of tau protein phosphatases
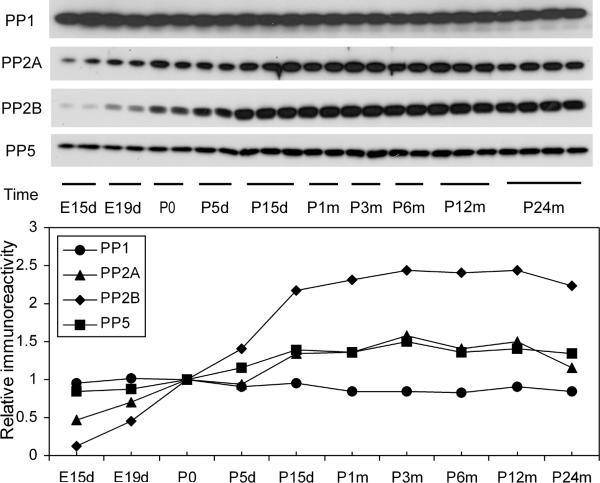
In addition to protein kinases, activities of tau protein phosphatases also regulate the level of tau phosphorylation. Among many protein phosphatases, PP1, PP2A, PP2B, and PP5 have been implicated in the regulation of tau phosphorylation (Tian and Wang 2002; Liu et al. 2005). We thus studied the levels of the catalytic subunits of these phosphatases during development. We found that except for PP1, whose level remained stable over the life span, the levels of PP2A, PP2B, and PP5 increased during development and reached stable, high levels after P15d (Fig. 7). The developmental regulation of PP2B level was more remarkable than that of PP2A and PP5 levels.
Fig. 7.
Developmental regulation of the levels of major tau phosphatases. The levels of the catalytic subunits of PP1, PP2A, PP2B, and PP5 in brain homogenates of rats of various ages were determined by Western blots. The blots were also quantified densitometrically, and the relative immunoreactivities (mean values), with those at P0 defined as 1, are shown in the graph.
Discussion
In general, phosphorylation regulates the biological activity of tau to stimulate microtubule assembly negatively, and AD-like abnormal hyperphosphorylation makes it to inhibit microtubule assembly and to polymerize into PHFs/NFTs (Del et al. 2008; Gong and Iqbal 2008; Avila 2006). Fetal tau is also highly phosphorylated at many phosphorylation sites, as seen in PHF-tau (Brion et al. 1993; Goedert 1993; Kenessey and Yen 1993; Morishima-Kawashima et al. 1995), but there is no evidence of the lack of microtubule assembly or PHF/NFT formation in the fetal brain. Recent studies suggest that the functional impacts of tau phosphorylation depend on the specific phosphorylation sites and the extent of phosphorylation (Liu et al. 2007; Wang et al. 2007). To learn what determines the functional differences between fetal tau and AD pathological tau, we compared the levels of tau phosphorylation at several individual phosphorylation sites. Among 14 tau phosphorylation sites studied, we found that tau in the developing rat brain was phosphorylated to a similar or higher level at Thr181, Ser199, Thr205, Ser214, Ser262, Ser356, and Ser409, as compared to tau in AD brain. These results suggest that the phosphorylation of tau at these seven sites is not sufficient to induce neurofibrillary pathology. It is worthy to note that two of these sites, Ser262 and Ser356, are located in the microtubule-binding domains of tau and that phosphorylation of tau at these sites inhibits or diminishes tau's biological activity in vitro (Sengupta et al. 1998; Biernat et al. 1993; Drewes et al. 1995; Xie et al. 1998; Liu et al. 2007; Singh et al. 1996). As the phosphorylation level of these two sites was much higher in fetal brain than in AD brain, it is possible that tau in the developing brain has a smaller microtubule-binding activity than tau in the mature brain. A smaller tau activity might be required in the developing brain, in which microtubules are more dynamic and the intraneuronal tubulin concentration is much higher than the mature brain (Charriere-Bertrand and Nunez 1992; Bhattacharya and Sarkar 1991).
Although tau was found to be abnormally hyperphosphorylated in AD brain two decades ago, the present study is the first to compare quantitatively the levels of tau phosphorylation at multiple individual phosphorylation sites between tau in AD brain and tau in age-matched control brains. We found that the phosphorylation levels of tau at 12 of the 14 studied sites, except Ser214 and Ser356, were much higher in AD brain than in control brain. At some phosphorylation sites, such as Thr212, Thr217, Thr231, Ser396, Ser404, and Ser422, phosphorylation was very low in control brain, and the increase in AD brain reached 10- to 100-fold. It appears that the high level of tau phosphorylation at a combination of multiple sites distinguishes tau in AD brain from tau in fetal brain and contributes to the loss of its biological activity and to its polymerization into NFTs. This conclusion is consistent with our recent in vitro study showing that tau phosphorylation only at multiple regions maximally diminishes its activity and disrupts microtubules (Liu et al. 2007). It should be noted that Ser396, Ser404, and Ser422 at the C-terminal end of tau are among those sites that were very highly phosphorylated in AD brain but were only slightly to moderately phosphorylated in developing brain. Therefore, a high level of hyperphosphorylation of tau at the C-terminus may be responsible for tau's self-aggregation into NFTs. Consistent with our findings, in vitro phosphorylation of tau at the C-terminus by GSK-3β promotes its self-aggregation (Liu et al. 2007). Mutations of Ser396, Ser404, and Ser422 of tau into glutamate to mimic phosphorylation make it more fibrillogenic than the wild-type tau (Abraha et al. 2000; Haase et al. 2004), and mutation of Ser422 to alanine prevents β-amyloid–induced tau aggregation (Ferrari et al. 2003).
As compared with in the mature brain, tau was found to be highly phosphorylated at many sites in the developing brain from E19d to P5d or P15d. These sites include Ser262 and Ser356, which are located within the microtubule-binding domains and are believed to be critical to tau's biological activity (Biernat et al. 1993; Drewes et al. 1995; Xie et al. 1998; Liu et al. 2007). Considering that a very low level of tau is expressed at the early embryonic stage, our results suggest that tau's function might not be required at this early stage. Consistent with this conclusion, no developmental abnormality is observed in the brain of tau knockout mice (Harada et al. 1994). The window of tau phosphorylation at several sites matched well with the period of active neurite outgrowth during brain development, as marked by MAP1B expression. It is possible that tau phosphorylation at these sites might be required to keep the high dynamics of microtubule assembly and disassembly. Tau phosphorylation at AT8 sites (Ser202 and Thr205), which belongs to our group 1 and group 2 sites, has been reported during this active neurite outgrowth period (Brion et al. 1994).
A high level of tau phosphorylation was also seen immunohistochemically during development. We also found that the topographic distributions of tau phosphorylated at the various sites examined were identical on E15d, while these distributions became site-specific at P5d and P6m. It is possible that tau is functionally regulated by phosphorylation at different sites, along with the development of various brain structures to fit their structure/region-specific functions.
Multiple protein kinases can phosphorylate tau, and each of them favors different phosphorylation sites of tau. We, thus, studied the developmental regulation of the major known tau kinases, including GSK-3β, CDK5, ERK, JNK, PKA, CaMKII, and Dyrk1A. We found that these kinases had different profiles of expression and activation during development. Among the kinases studied, the expression/activation of GSK-3β, JNK1, and Dyrk1A peaked postnatal 5−15 days, suggesting that these kinases contribute to tau phosphorylation at group 2 sites (Thr181, Ser199, Thr205, Ser214, Ser262, and Ser422) that also peak at the same periods. This notion is consistent with previous reports showing phosphorylation of tau at these sites by these three kinases (Yoshida et al. 2004; Reynolds et al. 2000; Liu et al. 2007; Liu et al. 2008). Tau phosphorylation patterns at group 1 sites did not match with the level/activation of any kinases studied during development. It is, therefore, impossible to suggest which kinases play major roles in the regulation of tau phosphorylation at which phosphorylation sites of these groups on the basis of these data. On the other hand, we found that the levels of PP2A, PP2B, and PP5 were increased during early development. Because these phosphatases have very broad site specificities, it is likely that the overall developmental regulation of tau phosphorylation is attributed mainly to the developmental regulation of tau phosphatases.
In summary, we have studied the detailed dynamic changes of expression and phosphorylation of tau at 14 individual phosphorylation sites, and expression and activation of the major tau kinases and tau phosphatases in rat brain during development, ranging from embryonic day 15 through the age of 24 months. We have found that while tau level steadily increased during the embryonic stage, tau phosphorylation level was higher in the developing brain than in the adult brain. However, tau phosphorylation at half of the phosphorylation sites studied in the developing brain was much lower than that in AD brain, and at the remaining sites, the tau phosphorylation was the opposite. Developmental regulation of tau phosphorylation varied at different sites. The group 1 sites peaked at embryonic day 19 to birth, and the group 2 sites peaked at postnatal day 5 to day 15. Tau phosphorylation in the developing brain matched the period of active neurite outgrowth. Tau phosphorylation at various sites had different topographic distributions. Studies of developmental regulation of major tau kinases and tau phosphatases suggest that several tau kinases might regulate tau phosphorylation at overlapping sites collectively and that the decrease of overall tau phosphorylation in the adult brain might be due to the higher tau phosphatases in the adult brain than in the developing brain. These studies provide new insight into the developmental regulation of site-specific tau phosphorylation and identify the likely sites required for the abnormal hyperphosphorylation of tau in AD.
Acknowledgements
We thank Dr. Bin Li of our department for his help in immunohistochemistry, Ms. J. Murphy for secretarial assistance, and Ms. M. Marlow for editorial suggestions. This work was supported in part by the New York State Office of Mental Retardation and Developmental Disabilities, the National Institutes of Health (R01 AG027429 and R01 AG019158), the Alzheimer's Association (IIRG-05−13095), The National Science Foundation of China (Grant 30500475), and the Li Foundation, NY.
Abbreviations used
- AD
Alzheimer's disease
- CaMKII
calcium/calmodulin-dependent protein kinase II
- CDK5
cyclin-dependent kinase 5
- Dyrk1A
dual-specificity tyrosine-regulated kinases 1A
- ERK
Extracellular signal-regulated kinases
- GSK-3β
glycogen synthase kinase 3β
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinases
- NFTs
neurofibrillary tangles
- PAGE
polyacrylamide gel electrophoresis
- PHF
paired helical filaments
- PKA
protein kinase A
- PP
protein phosphatase
References
- Abraha A, Ghoshal N, Gamblin TC, Cryns V, Berry RW, Kuret J, Binder LI. C-terminal inhibition of tau assembly in vitro and in Alzheimer's disease. J Cell Sci. 2000;113(Pt 21):3737–3745. doi: 10.1242/jcs.113.21.3737. [DOI] [PubMed] [Google Scholar]
- Aghdam SY, Barger SW. Glycogen synthase kinase-3 in neurodegeneration and neuroprotection: lessons from lithium. Curr Alzheimer Res. 2007;4:21–31. doi: 10.2174/156720507779939832. [DOI] [PubMed] [Google Scholar]
- Alonso AD, Li B, Grundke-Iqbal I, Iqbal K. Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc. Natl. Acad. Sci USA. 2006;23:8864–8869. doi: 10.1073/pnas.0603214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso AD, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso AD, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J. Tau phosphorylation and aggregation in Alzheimer's disease pathology. FEBS Lett. 2006;580:2922–2927. doi: 10.1016/j.febslet.2006.02.067. [DOI] [PubMed] [Google Scholar]
- Bahl R, Bradley KC, Thompson KJ, Swain RA, Rossie S, Meisel RL. Localization of protein Ser/Thr phosphatase 5 in rat brain. Brain Res Mol Brain Res. 2001;90:101–109. doi: 10.1016/s0169-328x(01)00089-4. [DOI] [PubMed] [Google Scholar]
- Baudier J, Cole RD. Phosphorylation of tau proteins to a state like that in Alzheimer's brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem. 1987;262:17577–17583. [PubMed] [Google Scholar]
- Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bhattacharya B, Sarkar PK. Tubulin gene expression during synaptogenesis in rat, mouse and chick brain. Int J Dev Neurosci. 1991;9:89–99. doi: 10.1016/0736-5748(91)90076-x. [DOI] [PubMed] [Google Scholar]
- Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993;11:153–163. doi: 10.1016/0896-6273(93)90279-z. [DOI] [PubMed] [Google Scholar]
- Brion JP, Octave JN, Couck AM. Distribution of the phosphorylated microtubule-associated protein tau in developing cortical neurons. Neuroscience. 1994;63:895–909. doi: 10.1016/0306-4522(94)90533-9. [DOI] [PubMed] [Google Scholar]
- Brion JP, Smith C, Couck AM, Gallo JM, Anderton BH. Developmental changes in tau phosphorylation: fetal tau is transiently phosphorylated in a manner similar to paired helical filament-tau characteristic of Alzheimer's disease. J Neurochem. 1993;61:2071–2080. doi: 10.1111/j.1471-4159.1993.tb07444.x. [DOI] [PubMed] [Google Scholar]
- Charriere-Bertrand C, Nunez J. Regulation of tubulin, Tau and microtubule associated protein 2 expression during mouse brain development. Neurochem Int. 1992;21:535–541. doi: 10.1016/0197-0186(92)90085-6. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Del CAA, Li B, Grundke-Iqbal I, Iqbal K. Mechanism of tau-induced neurodegeneration in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2008;5:375–384. doi: 10.2174/156720508785132307. [DOI] [PubMed] [Google Scholar]
- Drewes G, Trinczek B, Illenberger S, Biernat J, Schmitt-Ulms G, Meyer HE, Mandelkow EM, Mandelkow E. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem. 1995;270:7679–7688. doi: 10.1074/jbc.270.13.7679. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Hoerndli F, Baechi T, Nitsch RM, Gotz J. beta-Amyloid induces paired helical filament-like tau filaments in tissue culture. J Biol Chem. 2003;278:40162–40168. doi: 10.1074/jbc.M308243200. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribe E, Dalfo E, Avila J. Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies. Curr Alzheimer Res. 2005;2:3–18. doi: 10.2174/1567205052772713. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer's disease. Trends Neurosci. 1993;16:460–465. doi: 10.1016/0166-2236(93)90078-z. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Crowther RA, Six J, Lubke U, Vandermeeren M, Cras P, Trojanowski JQ, Lee VM. The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci U S A. 1993;90:5066–5070. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989a;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. Embo J. 1989b;8:393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX, Iqbal K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr Med Chem. 2008 doi: 10.2174/092986708785909111. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Dysregulation of protein phosphorylation/dephosphorylation in Alzheimer's disease: a therapeutic target. J Biomed Biotechnol. 2006;2006:31825. doi: 10.1155/JBB/2006/31825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986a;261:6084–6089. [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986b;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase C, Stieler JT, Arendt T, Holzer M. Pseudophosphorylation of tau protein alters its ability for self-aggregation. J Neurochem. 2004;88:1509–1520. doi: 10.1046/j.1471-4159.2003.02287.x. [DOI] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, et al. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y, Nukina N, Miura R, Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer's disease. J Biochem (Tokyo) 1986;99:1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I, Zaidi T, Merz PA, Wen GY, Shaikh SS, Wisniewski HM, Alafuzoff I, Winblad B. Defective brain microtubule assembly in Alzheimer's disease. Lancet. 1986;2:421–426. doi: 10.1016/s0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Weaver C, Lane E, Vianna C, Kress Y, Rockwood J, Davies P. cAMP-dependent protein kinase phosphorylations on tau in Alzheimer's disease. J Neurosci. 1999;19:7486–7494. doi: 10.1523/JNEUROSCI.19-17-07486.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenessey A, Yen SH. The extent of phosphorylation of fetal tau is comparable to that of PHF-tau from Alzheimer paired helical filaments. Brain Res. 1993;629:40–46. doi: 10.1016/0006-8993(93)90478-6. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Orecchio LD, Bakalis S, Neve RL. Developmentally regulated expression of specific tau sequences. Neuron. 1989;2:1389–1397. doi: 10.1016/0896-6273(89)90077-9. [DOI] [PubMed] [Google Scholar]
- Lee MS, Tsai LH. Cdk5: one of the links between senile plaques and neurofibrillary tangles? J Alzheimers Dis. 2003;5:127–137. doi: 10.3233/jad-2003-5207. [DOI] [PubMed] [Google Scholar]
- Lee VM, Balin BJ, Otvos L, Jr., Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lindwall G, Cole RD. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984;259:5301–5305. [PubMed] [Google Scholar]
- Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Li B, Tung EJ, Grundke-Iqbal I, Iqbal K, Gong CX. Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. Eur J Neurosci. 2007;26:3429–3436. doi: 10.1111/j.1460-9568.2007.05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Liang Z, Wegiel J, Hwang YW, Iqbal K, Grundke-Iqbal I, Ramakrishna N, Gong CX. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. FASEB J. 2008 doi: 10.1096/fj.07-104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zhang JY, Li HL, et al. Tau becomes a more favorable substrate for GSK-3 when it is prephosphorylated by PKA in rat brain. J Biol Chem. 2004;279:50078–50088. doi: 10.1074/jbc.M406109200. [DOI] [PubMed] [Google Scholar]
- Montejo de Garcini E, Serrano L, Avila J. Self assembly of microtubule associated protein tau into filaments resembling those found in Alzheimer disease. Biochem Biophys Res Commun. 1986;141:790–796. doi: 10.1016/s0006-291x(86)80242-x. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Watanabe A, Titani K, Ihara Y. Hyperphosphorylation of tau in PHF. Neurobiol Aging. 1995;16:365–371. doi: 10.1016/0197-4580(95)00027-c. discussion 371−380. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Braak H, An WL, Winblad B, Cowburn RF, Iqbal K, Grundke-Iqbal I. Up-regulation of mitogen-activated protein kinases ERK1/2 and MEK1/2 is associated with the progression of neurofibrillary degeneration in Alzheimer's disease. Brain Res Mol Brain Res. 2002;109:45–55. doi: 10.1016/s0169-328x(02)00488-6. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Gong CX, Iqbal K, Grundke-Iqbal I, Wu QL, Winblad B, Cowburn RF. Subcellular distribution of protein phosphatases and abnormally phosphorylated tau in the temporal cortex from Alzheimer's disease and control brains. J Neural Transm. 1998;105:69–83. doi: 10.1007/s007020050039. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Tanaka T, Tung YC, Braak E, Iqbal K, Grundke-Iqbal I. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol. 1997;56:70–78. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Petersen RB, Nunomura A, Lee HG, Casadesus G, Perry G, Smith MA, Zhu X. Signal transduction cascades associated with oxidative stress in Alzheimer's disease. J Alzheimers Dis. 2007;11:143–152. doi: 10.3233/jad-2007-11202. [DOI] [PubMed] [Google Scholar]
- Plyte SE, Hughes K, Nikolakaki E, Pulverer BJ, Woodgett JR. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim Biophys Acta. 1992;1114:147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. J Neurochem. 2000;74:1587–1595. doi: 10.1046/j.1471-4159.2000.0741587.x. [DOI] [PubMed] [Google Scholar]
- Reynolds CH, Utton MA, Gibb GM, Yates A, Anderton BH. Stress-activated protein kinase/c-jun N-terminal kinase phosphorylates tau protein. J Neurochem. 1997;68:1736–1744. doi: 10.1046/j.1471-4159.1997.68041736.x. [DOI] [PubMed] [Google Scholar]
- Riederer B, Cohen R, Matus A. MAP5: a novel brain microtubule-associated protein under strong developmental regulation. J Neurocytol. 1986;15:763–775. doi: 10.1007/BF01625193. [DOI] [PubMed] [Google Scholar]
- Riederer BM, Innocenti GM. Differential Distribution of Tau Proteins in Developing Cat Cerebral Cortex and Corpus Callosum. Eur J Neurosci. 1991;3:1134–1145. doi: 10.1111/j.1460-9568.1991.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, McKerracher L, Obar R, Vallee RB. MAP 1A and MAP 1B are structurally related microtubule associated proteins with distinct developmental patterns in the CNS. J Neurosci. 1989;9:1712–1730. doi: 10.1523/JNEUROSCI.09-05-01712.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Kabat J, Novak M, Wu Q, Grundke-Iqbal I, Iqbal K. Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch Biochem Biophys. 1998;357:299–309. doi: 10.1006/abbi.1998.0813. [DOI] [PubMed] [Google Scholar]
- Shaw M, Cohen P, Alessi DR. Further evidence that the inhibition of glycogen synthase kinase-3beta by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett. 1997;416:307–311. doi: 10.1016/s0014-5793(97)01235-0. [DOI] [PubMed] [Google Scholar]
- Singh TJ, Wang JZ, Novak M, Kontzekova E, Grundke-Iqbal I, Iqbal K. Calcium/calmodulin-dependent protein kinase II phosphorylates tau at Ser-262 but only partially inhibits its binding to microtubules. FEBS Lett. 1996;387:145–148. doi: 10.1016/0014-5793(96)00485-1. [DOI] [PubMed] [Google Scholar]
- Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer's disease. J Alzheimers Dis. 2006;9:309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- Takuma H, Arawaka S, Mori H. Isoforms changes of tau protein during development in various species. Brain Res Dev Brain Res. 2003;142:121–127. doi: 10.1016/s0165-3806(03)00056-7. [DOI] [PubMed] [Google Scholar]
- Tian Q, Wang J. Role of serine/threonine protein phosphatase in Alzheimer's disease. Neurosignals. 2002;11:262–269. doi: 10.1159/000067425. [DOI] [PubMed] [Google Scholar]
- Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Kuchna I, Nowicki K, et al. Cell type- and brain structure-specific patterns of distribution of minibrain kinase in human brain. Brain Res. 2004;1010:69–80. doi: 10.1016/j.brainres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Xie H, Litersky JM, Hartigan JA, Jope RS, Johnson GV. The interrelationship between selective tau phosphorylation and microtubule association. Brain Res. 1998;798:173–183. doi: 10.1016/s0006-8993(98)00407-7. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hastie CJ, McLauchlan H, Cohen P, Goedert M. Phosphorylation of microtubule-associated protein tau by isoforms of c-Jun N-terminal kinase (JNK). J Neurochem. 2004;90:352–358. doi: 10.1111/j.1471-4159.2004.02479.x. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Ihara Y. Tau in paired helical filaments is functionally distinct from fetal tau: assembly incompetence of paired helical filament-tau. J Neurochem. 1993;61:1183–1186. doi: 10.1111/j.1471-4159.1993.tb03642.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee HG, Raina AK, Perry G, Smith MA. The role of mitogen-activated protein kinase pathways in Alzheimer's disease. Neurosignals. 2002;11:270–281. doi: 10.1159/000067426. [DOI] [PubMed] [Google Scholar]