Abstract
A single RNA probe was synthesized and used to detect simultaneously the methanol-soluble heat-stable enterotoxin and heat-labile enterotoxin genes in Escherichia coli strains. The results with the biotinylated or radioactive probe correlated 100% with the biological assay results for both toxins. The RNA probe detected the three known heat-stable enterotoxin A alleles.
Full text
PDF
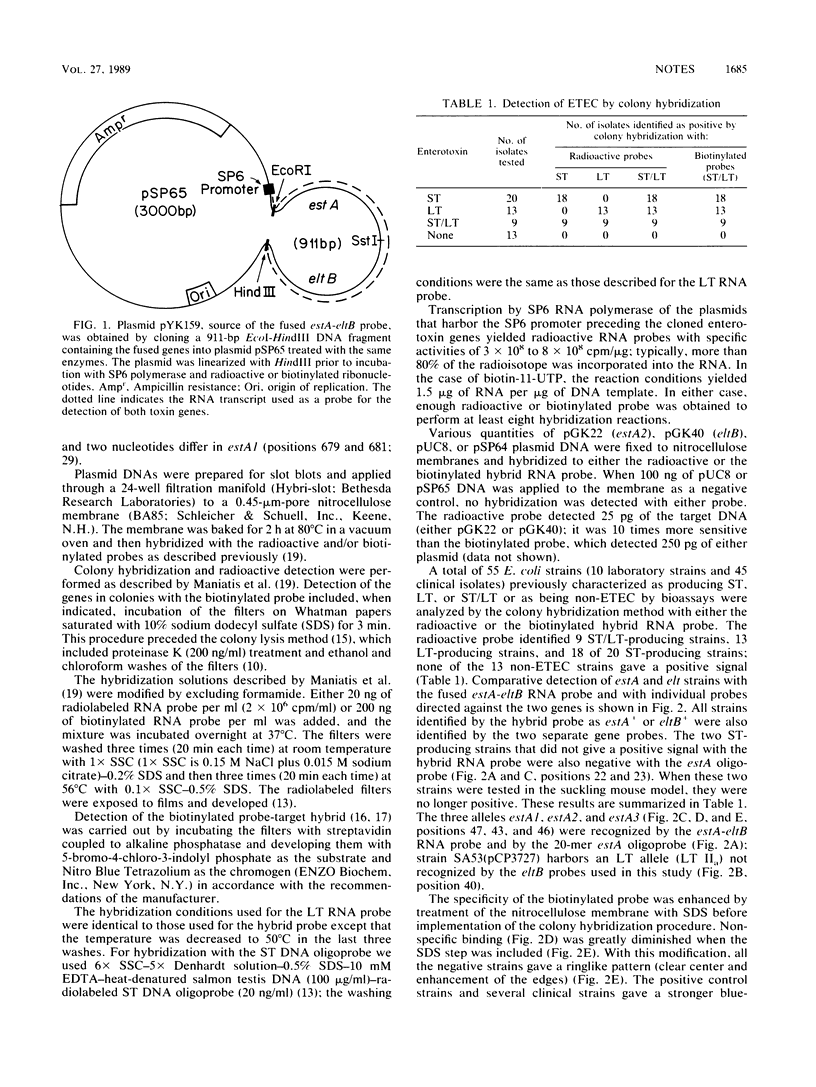
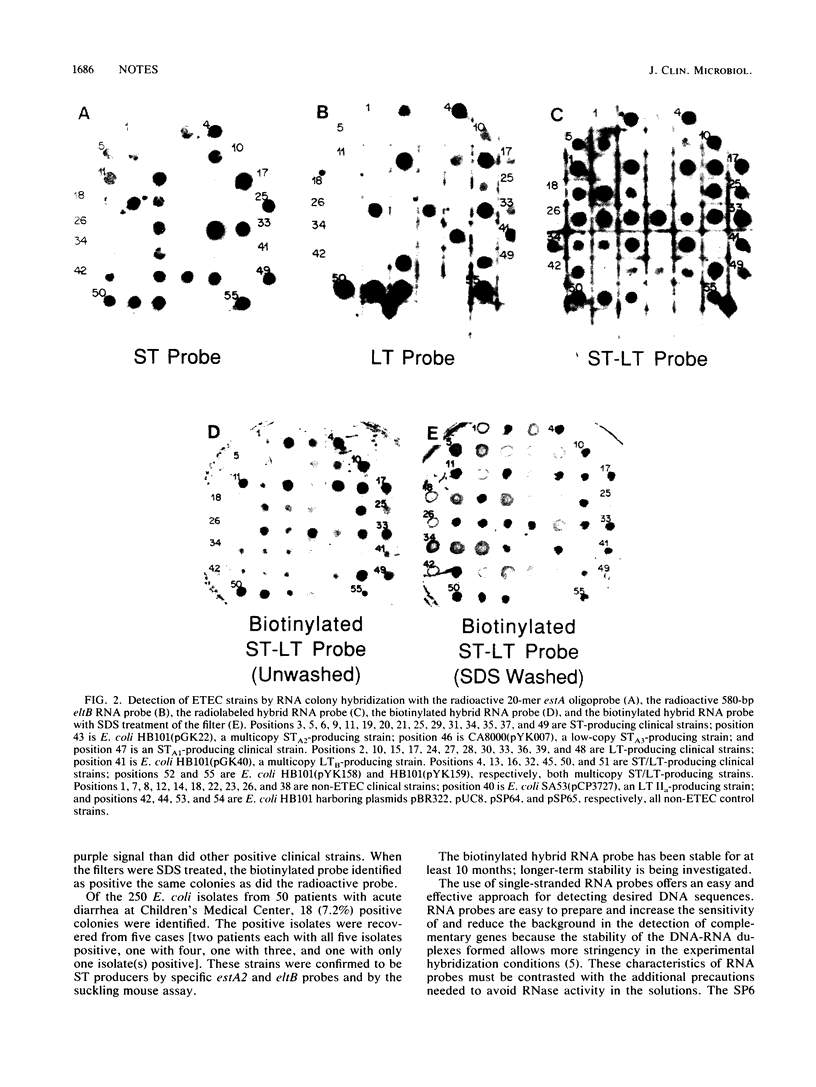


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bialkowska-Hobrzanska H. Detection of enterotoxigenic Escherichia coli by dot blot hybridization with biotinylated DNA probes. J Clin Microbiol. 1987 Feb;25(2):338–343. doi: 10.1128/jcm.25.2.338-343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. E., Merson M. H., Huq I., Alim A. R., Yunus M. Incidence and severity of rotavirus and Escherichia coli diarrhoea in rural Bangladesh. Implications for vaccine development. Lancet. 1981 Jan 17;1(8212):141–143. doi: 10.1016/s0140-6736(81)90719-4. [DOI] [PubMed] [Google Scholar]
- Chityothin O., Sethabutr O., Echeverria P., Taylor D. N., Vongsthongsri U., Tharavanij S. Detection of heat-stable enterotoxigenic Escherichia coli by hybridization with an RNA transcript probe. J Clin Microbiol. 1987 Aug;25(8):1572–1573. doi: 10.1128/jcm.25.8.1572-1573.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Dallas W. S., Gill D. M., Falkow S. Cistrons encoding Escherichia coli heat-labile toxin. J Bacteriol. 1979 Sep;139(3):850–858. doi: 10.1128/jb.139.3.850-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Echeverria P., Taylor D. N., Seriwatana J., Chatkaeomorakot A., Khungvalert V., Sakuldaipeara T., Smith R. D. A comparative study of enterotoxin gene probes and tests for toxin production to detect enterotoxigenic Escherichia coli. J Infect Dis. 1986 Feb;153(2):255–260. doi: 10.1093/infdis/153.2.255. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Pierce N. F. Differences in the response of rabbit small intestine to heat-labile and heat-stable enterotoxins of Escherichia coli. Infect Immun. 1973 Jun;7(6):873–880. doi: 10.1128/iai.7.6.873-880.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L., Schnaitman T. C., Rebhun L. I., Gilman A. G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974 Aug;10(2):320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Kirchhoff L. V., Shields D. S., Nations M. K., Leslie J., de Sousa M. A., Araujo J. G., Correia L. L., Sauer K. T., McClelland K. E. Prospective study of diarrheal illnesses in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. J Infect Dis. 1983 Dec;148(6):986–997. doi: 10.1093/infdis/148.6.986. [DOI] [PubMed] [Google Scholar]
- Guzman-Verduzco L. M., Kupersztoch Y. M. Fusion of Escherichia coli heat-stable enterotoxin and heat-labile enterotoxin B subunit. J Bacteriol. 1987 Nov;169(11):5201–5208. doi: 10.1128/jb.169.11.5201-5208.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Verduzco L. M., Kupersztoch Y. M. Rectification of two Escherichia coli heat-stable enterotoxin allele sequences and lack of biological effect of changing the carboxy-terminal tyrosine to histidine. Infect Immun. 1989 Feb;57(2):645–648. doi: 10.1128/iai.57.2.645-648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Fleming D. J. A simplified lysis method allowing the use of biotinylated probes in colony hybridization. Anal Biochem. 1988 Feb 1;168(2):239–246. doi: 10.1016/0003-2697(88)90313-2. [DOI] [PubMed] [Google Scholar]
- Huovinen S., Huovinen P., Jacoby G. A. Reliability of biotinylated DNA probes in colony hybridization: evaluation of an improved colony lysis method for detection of TEM-1 beta-lactamase. Mol Cell Probes. 1988 Mar;2(1):83–85. doi: 10.1016/0890-8508(88)90047-3. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Medon P. P., Skingle D. C., Lanser J. A., Symons R. H. Enzyme-linked synthetic oligonucleotide probes: non-radioactive detection of enterotoxigenic Escherichia coli in faecal specimens. Nucleic Acids Res. 1987 Jul 10;15(13):5275–5287. doi: 10.1093/nar/15.13.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley S. L., Huq I., Alim A. R., So M., Samadpour-Motalebi M., Falkow S. Detection of enterotoxigenic Escherichia coli by DNA colony hybridization. J Infect Dis. 1980 Dec;142(6):892–898. doi: 10.1093/infdis/142.6.892. [DOI] [PubMed] [Google Scholar]
- Pickett C. L., Weinstein D. L., Holmes R. K. Genetics of type IIa heat-labile enterotoxin of Escherichia coli: operon fusions, nucleotide sequence, and hybridization studies. J Bacteriol. 1987 Nov;169(11):5180–5187. doi: 10.1128/jb.169.11.5180-5187.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B. Human diarrheal disease caused by enterotoxigenic Escherichia coli. Annu Rev Microbiol. 1975;29:333–353. doi: 10.1146/annurev.mi.29.100175.002001. [DOI] [PubMed] [Google Scholar]
- Schenborn E. T., Mierendorf R. C., Jr A novel transcription property of SP6 and T7 RNA polymerases: dependence on template structure. Nucleic Acids Res. 1985 Sep 11;13(17):6223–6236. doi: 10.1093/nar/13.17.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seriwatana J., Echeverria P., Escamilla J., Glass R., Huq I., Rockhill R., Stoll B. J. Identification of enterotoxigenic Escherichia coli in patients with diarrhea in Asia with three enterotoxin gene probes. Infect Immun. 1983 Oct;42(1):152–155. doi: 10.1128/iai.42.1.152-155.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The relationship between two apparently different enterotoxins produced by enteropathogenic strains of Escherichia coli of porcine origin. J Med Microbiol. 1970 Aug;3(3):387–401. doi: 10.1099/00222615-3-3-387. [DOI] [PubMed] [Google Scholar]
- Sommerfelt H., Svennerholm A. M., Kalland K. H., Haukanes B. I., Bjorvatn B. Comparative study of colony hybridization with synthetic oligonucleotide probes and enzyme-linked immunosorbent assay for identification of enterotoxigenic Escherichia coli. J Clin Microbiol. 1988 Mar;26(3):530–534. doi: 10.1128/jcm.26.3.530-534.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz H., Cervantes L., Robledo R., Fonseca R., Covarrubias L., Bolivar F., Kupersztoch Y. M. Cloning, sequencing, and expression in Ficoll-generated minicells of an Escherichia coli heat-stable enterotoxin gene. Plasmid. 1988 Jul;20(1):42–53. doi: 10.1016/0147-619x(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Stieglitz H., Fonseca R., Olarte J., Kupersztoch-Portnoy Y. M. Linkage of heat-stable enterotoxin activity and ampicillin resistance in a plasmid isolated from an Escherichia coli strain of human origin. Infect Immun. 1980 Nov;30(2):617–620. doi: 10.1128/iai.30.2.617-620.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Wikström M., Lindblad M., Holmgren J. Monoclonal antibodies against Escherichia coli heat-stable toxin (STa) and their use in a diagnostic ST ganglioside GM1-enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Oct;24(4):585–590. doi: 10.1128/jcm.24.4.585-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Greenberg H. B., Merson M. H., Sack R. B., Kapikian A. Z. Enzyme-linked immunosorbent assay for detection of Escherichia coli heat-labile enterotoxin. J Clin Microbiol. 1977 Nov;6(5):439–444. doi: 10.1128/jcm.6.5.439-444.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]




