Abstract
Biomarkers of Hepatitis B Virus (HBV) infection, aflatoxin B1 (AFB1) exposure and oxidative stress were detected in 71 hepatocellular carcinoma (HCC) patients and 694 controls from southern China. Plasma level of AFB1-Albumin-Adducts (AAA) and protein carbonyl content (PCC) were significantly higher in the 71 HCC cases than in any age/gender matched HBV sero-status groups (P<0.001). HCC patients positive for the p53-249 G-T mutation had a marginally higher level of PCC than those negative for the mutation (p=0.077). HBV infection had a prominent influence on the association between AFB1 exposure and oxidative stress biomarkers in the controls. Our study indicates a significant contribution from HBV infection to oxidative stress in a population with AFB1 exposure which might substantially increase risk for HCC in this region.
Keywords: HBV, Aflatoxin, oxidative stress, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) ranks among the 10 most common malignancies worldwide, accounting for 5.6% of all human cancers [1,2]. The broad characteristics of HCC can be partially explained by the patterns of exposure to the key risk factors in different regions [2]. In Asia and Africa where the majority of cases live, aflatoxin and hepatitis viruses (HBV and HCV) are important factors giving rise to the extraordinarily high incidence rates (24.2∼35.5/100,000) of HCC in these areas [1,2]. HBV-induced chronic active hepatitis and cirrhosis constitute major risk factors in liver carcinogenesis [3]. HBV is associated with 70∼75% of all HCC cases in Asia, the continent with the highest prevalence of HBV [4]. Also, the role of dietary exposure to aflatoxin B1 (AFB1), a category I known human carcinogen and a potent genotoxic agent, in the development of HCC has long been documented in many model systems (reviewed in [5-7]). A synergistic effect of AFB1 and HBV on HCC risk has been reported in many studies (reviewed in [5,8]).
Reactive oxygen species (ROS) are potential carcinogens because of their roles in mutagenesis, tumor promotion, and progression [9]. ROS and oxidative damage have been shown to contribute to the genotoxicity of AFB1 [6]. AFB1 is metabolized by constitutive cellular enzymes, during which there is formation of free radicals [10]. The process results in both lipid peroxidation and covalent adducts with DNA and proteins [6]. A strikingly high frequency of G-T transversions at the third base of codon 249 in p53 was found in Asian and African HCCs with high level exposure to both HBV and AFB1 [5,6]. In vitro studies suggest that the hot-spot mutations may form through preferential binding of AFB1 as well as AFB1-induced oxidation at CpG sites [11,12]. Extensive oxidative DNA damage had also been observed in hepatocytes of HBV-positive, transgenic mice [13] and HBV-infected humans [14,15]. Oxidative stress in the pathogenesis of hepatitis virus might be caused by a combination of chronic inflammation, iron overload, liver damage, and proteins encoded by the virus [16]. This might in consequence, increase sensitivity to chemical carcinogens and is probably relevant in humans exposed to both hepatitis viruses and chemical carcinogens [3]. However, direct evidence linking the two carcinogens to a common damage endpoint is rare, especially in population studies. We hypothesize that if there is a carcinogenic effect of oxidative stress leading in the development of HCC, it would be most evident in a population exposed to environmental hepatocarcinogens where exposure is strong enough to manifest the mutagenic effects. To test this hypothesis, we conducted a multi-center collaborative study in a Chinese population at high-risk for HCC, using multiple biomarkers to profile oxidative status.
Materials and methods
Samples collection
Study subjects came from Guangxi Zhuang Autonomous Region, China, a region of elevated HCC risk and AFB1 exposure and HBV infection [17,18], and Chengdu City, Sichuan province, a region with a relatively low incidence of HCC. Details on recruitment have been described elsewhere and AFB1-Albumin-Adducts and Protein carbonyl content data on a subset of 404 subjects in this analysis has been reported previously [19]. In brief, a total of 695 healthy subjects (530 males, 165 females, age 36.6 ± 15.6 yrs) were recruited from three regions of P.R. China. The local cancer registry revealed a gradient in HCC mortality for males in these regions (Fusui 92-97/100,000, Nanning 32-47/100,000 and Chengdu 21/100,000, respectively) [18]. Samples were collected in the morning before daily work in turn from Chengdu (n=119), Nanning (n=486) and Fusui (n=90) during April to June, 2001. Subjects were screened for hepatitis virus (HBV, HCV, HDV, HEV and HGV) serology and those positive for any hepatitis virus except HBV were excluded from this study. Liver biochemistry (alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), globulin (GLO) and plasma total protein (TP)) was quantified for all subjects as described previously [18]. All liver biochemistries were determined on a Beckman LX20 Chemistry Analyzer (Beckman Coulter, Inc., Brea, CA, USA) in the same laboratory. One subject from Chengdu and one from Nanning City had extraordinarily high concentrations of AAA (5751 fmol/mg albumin) and carbonyl (1.4 nmol/mg protein), respectively. The former was confirmed to be infected with tuberculosis in a later follow up. These two subjects were excluded from the analysis. The 71 HCC subjects were a subset of 561 patients from Guangxi and hospitalized based on national diagnostic criteria for HCC between December, 1998 and July, 2005, and confirmed by pathology of the specimens from biopsy or operation. To compare AAA and PCC levels, they were selected by matching age and gender with 71 controls in each of the four HBV sero-states categories (stated below) from the same region. Plasma was collected right after admission and 37 tumor tissues were collected during surgery. Informed consent was obtained from each participant before sample collection.
Enzyme-linked-immunosorbent-assay (ELISA) for concentrations of AAA and PCC in plasma and AFB1 and 8-OHdG in urine
AFB1-Albumin-Adducts (AAA) and Protein carbonyl content (PCC) were quantified for all 71 HCC cases and 695 controls. AAA concentrations in plasma were quantified as described previously by competitive ELISA using a polyclonal antiserum that recognizes the AFB1-lysine adduct in digested albumin [20]. Values were normalized to the amount of albumin (fmol/mg albumin) and further by albumin concentration in plasma (fmol/ml plasma). The minimum level of AAA among these subjects was 32.6 fmol/mg albumin, above the limit of sensitivity of this assay (10 fmol/mg albumin) and quality control measures were taken as described previously [20]. PCC was determined by a noncompetitive ELISA as described previously that involves derivatization of carbonyl groups with dinitrophenylhydrazine (DNP) followed by detection of the DNP group with a commercial antibody [19]. PCC values were first normalized by TP (nmol/mg TP) and then normalized by TP concentration in plasma (nmol/ml plasma). The range of PCC in these subjects was between 0.11 - 1.41 nmol/mg TP, within the linear range of this assay (0.1 – 2.5 nmol/mg TP) [21], and comparable to the reported level of PCC in human plasma (0.4 – 1.0 nmol/mg TP) [22]. The intra-assay variation was 10.0% (n=22), similar to the mean variation of ±8.8% reported by Buss et al. [21] AFB1 and 8-hydroxydeoxyguanosine (8-OHdG) concentrations in urine among 290 adults in Nanning were quantified by competitive ELISA as described previously using monoclonal antibodies AF8E11 and 1F7, respectively [23,24]. Values were normalized to the urinary concentration of creatinine (fmol AFB1/mg creatinine and ng 8-OHdG /mg creatinine).
P53 gene codon 249 mutation in HCC tumors
Tumor tissues were digested by proteinase K (Qiagen Inc., Valencia CA USA) and DNA was extracted by phenol-chloroform. HaeIII was from New England BioLabs Inc. (Ipswich, MA USA) and the PCR-RFLP assay for the p53 codon 249 mutation was carried on as reported previously [25] and confirmed by sequencing (Alpha Biolaboratory Inc. Burlingame CA, USA).
Statistical analysis
Liver function indices and protein adducts levels were compared among the 71 HCC cases and four age and gender matched groups of HBV-status controls (71 in each category). Associations among biomarkers in plasma (liver function indices, AAA and PCC) and urine (AFB1 and 8-OHdG) were analyzed in controls. A logarithmic transformation of AAA, PCC, urinary AFB1 and 8-OHdG and liver function indices (TP, ALB, ALT and AST) was used for Oneway ANOVA and linear regression analysis. Spearman correlation was used to examine the association among biomarkers and Mann-Whitney Test was used to compared the protein adduct levels between p53 codon 249 mutation carriers and non-carriers. For clarity of presentation, the untransformed adduct levels and SD are presented throughout the text and figures. The relationship of adduct levels to risk factors was examined in a linear regression model or GLM model. All statistical analyses were performed using SPSS 10.0 software (Chicago, USA). A two-tailed p value <0.05 was considered significant.
Results
Plasma biomarkers
HBV serology in HCC cases and controls
25.1% (174/694) of the normal population and 100% (71/71) of the HCC cases were HBV (+), defined as positive for any one of the markers (sAg/eAg/eAb/cAb). The positive percentages for sAg/sAb/eAg/eAb/cAb were 10.6%(75/694), 53.9%(380/694), 1.1%(8/694), 14.0%(99/694), 22.4%(158/694) for the controls and 93.0%(66/71), 1.4%(1/71), 11.3%(8/71), 73.2%(52/71), 98.6%(70/71) for the HCC cases, respectively. Among controls, the 8 subjects positive for eAg were all in the sAg(+) group, and 87.9% (87/99) of the subjects positive for eAb were concomitantly positive for cAb, so the above two markers were combined into the sAg(+) and cAb(+) categories, and the HBV sero-status of the controls were categorized into four groups: null (null for all HBV markers, 30.6%, 212/694), sAg(+) (HBsAg(+) regardless of sAb or cAb status, 10.8%, 75/694), sAb(+) (sAg(-)sAb(+)cAb(-), 46.0%, 319/694), and cAb(+) (sAg(-)cAb(+), 12.5%, 87/694).
Comparison of protein adducts levels among subjects from the three regions
Similar to our previous analysis of a smaller sample size [19], there were significant differences in AAA and PCC levels among the populations from the three different regions, in accordant with their HCC incidences (data not shown).
Comparison of protein adducts levels among 71 HCC cases and age and gender matched HBV-status-controls
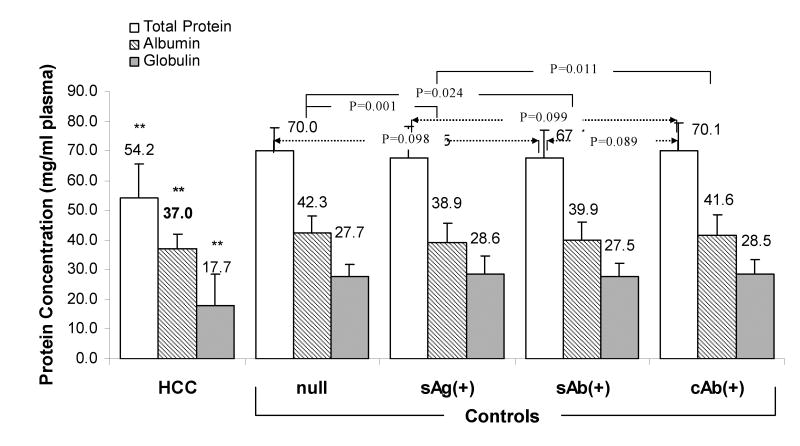
In contrast to all HBV sero-status control groups (71 age and gender matched controls in each category), there were significantly lower levels of TP, ALB and GLO in HCC patients (P<0.01) (Figure 1). Among controls, HBsAg(+) subjects had significantly lower concentrations of albumin (P<0.05) but significantly higher levels of ALT and AST (P<0.001) (data not shown). These results suggested that protein concentration is an important issue to be considered when comparing protein adducts levels among subjects with different HBV infection status.
Figure 1. Comparison of Protein Concentration among age and gender matched HCC and HBV sero-status groups (n=71 per group).
** Levels of TP, ALB and GLO in HCC patients were significantly lower than any HBV sero-status group (P<0.01). Only p values less than 0.1 are indicated. Null (null for all HBV markers), sAg(+) (HBsAg(+) regardless of sAb or cAb status), sAb(+) (sAg(-)sAb(+)cAb(-)), and cAb(+) (sAg(-)cAb(+)).
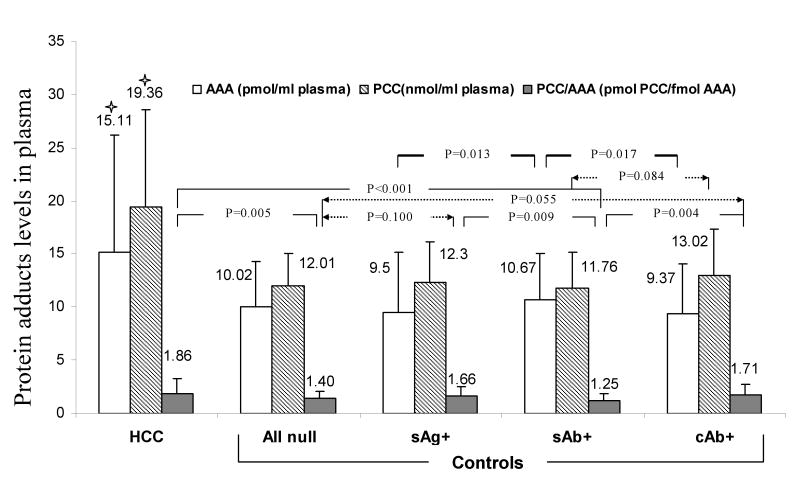
HCC patients had significantly higher levels of AAA and PCC than any matched HBV status group of controls (P<0.001), regardless of plasma protein concentrations. Among the entire control group (n=694), the difference in protein adducts levels was only significant for PCC between the sAb(+) and HBV-null groups (P=0.030), suggesting the presence of HBsAb might be a protective factor, associated with lower PCC levels. Interestingly, the increment in PCC per fmol of AAA was significantly higher in HCC cases, followed by the sAg(+) and cAb(+) control groups, and the sAb(+) control group, suggesting a distinct stress response to AFB1 exposure by HBV status (Figure 2).
Figure 2. Comparison of Protein Adducts Levels among age and gender matched HCC and HBV sero-status groups (n=71 per group).
★ HCC patients had significantly higher levels of AAA and PCC than any HBV group of controls (P<0.001). Only p values less than 0.1 are indicated. Null (null for all HBV markers), sAg(+) (HBsAg(+) regardless of sAb or cAb status), sAb(+) (sAg(-)sAb(+)cAb(-)), and cAb(+) (sAg(-)cAb(+)).
In the 37 HCCs with available tumor DNA, the frequency of p53 codon 249 mutations (all were AGG-AGT transversions) was 64.9% (24/37). HCC patients positive for p53-249 mutations had a marginally higher level of PCC (21.6 nmol/ml plasma) than those negative (15.9 nmol/ml plasma) (P=0.077, Mann-Whitney Test) but no difference in AAA levels (p=0.456).
The present classification may not completely specify HBV infection sero-status. For example, the IgG and IgM forms of cAb were not distinguished in the present study. Among the 87 sAg(-)cAb(+) subjects, those eAb(+) had similar levels of AAA yet significantly (t=3.285, p<0.001) higher level of PCC than those eAb(-), suggesting a different status of oxidative stress in this category (data not shown). If HBV active infection is defined as positive for sAg, eAg, eAb or cAb together with ALT >40 IU/ml and/or AST >45 IU/L, subjects with active HBV infection had a significantly higher level of AAA (12.1±6.1 pmol/ml, n=39) than non-active HBV infectants (9.0±5.8 pmol/ml, n=133, p=0.002) and HBV-free subjects (9.3±6.8 pmol/ml, n=518, p=0.001).
Association between AAA and PCC in controls
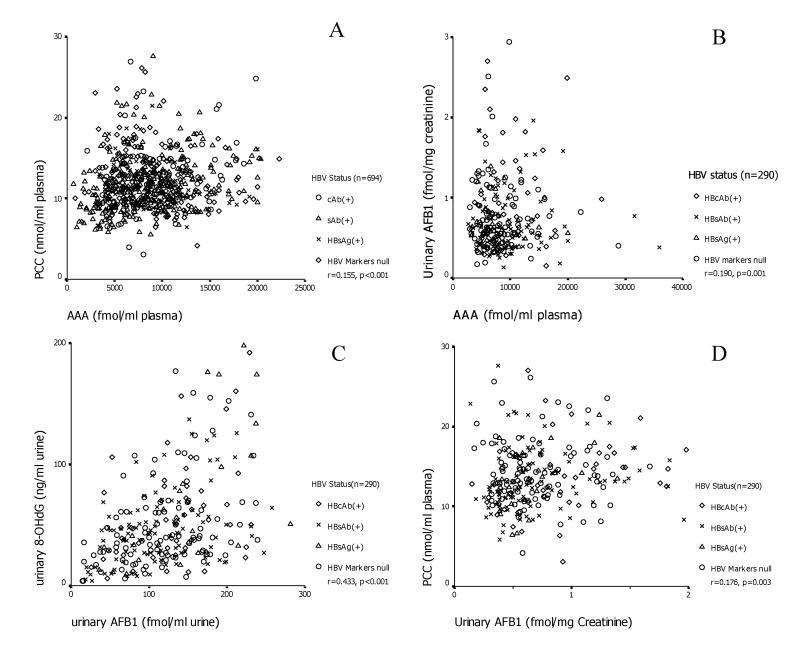
AAA level was correlated with PCC in the 693 controls (r=0.155, P<0.001). In addition, HBV infection status influenced this association: the association between AAA and PCC was significant among sAb(+) (n=319, r=0.309, P<0.001) and cAb(+) (n=87, r=0.310, p=0.004) control groups, marginally significant in the HBV-null (n=212, r=0.133, P=0.055) but not in the sAg(+) control group (n=75, r=0.029, P=0.807). (Figure 3)
Figure 3.
Associations between plasma AAA and PCC (A), plasma AAA and urinary AFB1 (B), urinary AFB1 and 8-OHdG (C), urinary AFB1 and plasma PCC (D).
Association between protein adducts and amino transferases (ALT and AST) in controls
PCC was associated with ALT and AST levels in all HBV sero-status groups (r=0.166∼0.234, P<0.05). AAA was associated with AST levels in only sAg, eAg, eAb, or cAb (+) subjects (r=0.197, P=0.010).
Association between protein adducts and HBV sero-status in 693 controls
In a linear regression analysis, if single HBV markers were used, AAA (P=0.002), sAb (P=0.050), eAb (P=0.009) and cAb (P=0.019) significantly contributed to PCC levels. And if HBV sero-status category was applied, AAA (P=0.022), HBV status (P=0.005), gender (P=0.056) and age (P=0.001) were factors significantly contributing to PCC levels.
Urinary biomarkers
Association among plasma and urinary biomarkers of AFB1 and oxidative stress (290 adults in Nanning, China)
Urinary excretion of AFB1 was associated with both the AAA (r=0.190, p=0.001) and PCC (r=0.176, p=0.003) levels in plasma, suggesting an association between recent AFB1 exposure and oxidative protein damage. Urinary excretion of 8-OHdG was also associated with urinary AFB1(r=0.433, p<0.001) and interestingly, in a linear regression, urinary 8-OHdG was associated with plasma AAA level (p=0.011), when urinary creatinine was an independent factor in the model. This suggests an association between recent and chronic AFB1 exposure and oxidative DNA damage.
Furthermore, this association differed by HBV categories. The association between urinary AFB1 and 8-OHdG was consistently significant in all HBV sero-status groups (r=0.305, p=0.031 in HBcAb(+), r>0.400, p<0.001 for the other categories), and the association between urinary AFB1 and plasma PCC was consistently significant in all (r>0.260, p<0.05) except the HBV-null group (p=0.595). This might suggest significant contributions to oxidative DNA and protein damage from recent AFB1 exposure. The association between plasma AAA and urinary 8-OHdG (r=0.446, p=0.020), between plasma PCC and urinary 8-OHdG (r=0.4486, p=0.019), was significant only in sAg(+) subjects, suggesting profound oxidative DNA damage from chronic AFB1 exposure and carbonyl stress among HBV carriers. Finally, the association between plasma AAA and urinary AFB1 was significant in the HBV null group (r=0.256, p=0.011) and marginally significant in the HBsAb(+) group (r=0.163, p=0.088) but not in the sAg(+) and cAb(+) groups, suggesting that HBV infection could interfere with the metabolism and subsequent excretion of AFB1. (Figure 3)
Comparison of urinary excretion of AFB1 and 8-OHdG among HBV status groups (290 adults in Nanning)
The HBcAb(+) group had significantly higher levels of urinary AFB1 and 8-OHdG than the HBV null and sAb(+) groups (p<0.05). There were no significant associations between urinary biomarkers and either ALT or AST, but among subjects with ALT< 41IU/L and AST< 46IU/L, there were significantly (p<0.05) higher levels of urinary AFB1 and 8-OHdG in HBV(+) subjects (n=66) than the HBV(-) (n=181), suggesting higher level of AFB1 exposure and oxidative DNA damage in HBV infected subjects in this sub-set (data not shown). (Figure 4)
Figure 4. Comparison of Urinary AFB1 and 8-OHdG levels among HBV Sero-status groups in 290 adults from Nanning, China.
Only p values less than 0.1 are indicated. Null (null for all HBV markers), sAg(+) (HBsAg(+) regardless of sAb or cAb status), sAb(+) (sAg(-)sAb(+)cAb(-)), and cAb(+) (sAg(-)cAb(+)).
In a GLM model, urinary AFB1 (p=0.002), HBV sero-status (p=0.010), the interaction of HBV and urinary AFB1 (p=0.010) were independent predictors of PCC, whilst urinary AFB1 (p<0.001), age (p=0.004), chronic diseases (p=0.004) and the interaction between PCC and HBV status (p=0.063) were independent predictors of urinary 8-OHdG in this population. Figure 5 summarizes the associations among the oxidative stress, HBV and AFB1 biomarkers in this population.
Figure 5.
Diagram of the association profile among biomarkers for oxidative stress, HBV infection and AFB1 exposure. Only significant associations are indicated. HBV status is specified when the associations are significant in sub-set(s) of HBV serology. Null (null for all HBV markers), sAg(+) (HBsAg(+) regardless of sAb or cAb status), sAb(+) (sAg(-)sAb(+)cAb(-)), and cAb(+) (sAg(-)cAb(+)). * Also significant between eAb(+) and eAb(-) among 87 sAg(-)cAb(+) subjects. ** Also significant between non-active HBV infection and HBV-free subjects.
Discussion
The frequencies of HBsAg(+) in our controls (10.6%) and HCC cases (93.0%) are similar to those found 20 yrs ago in this region [26,27], suggesting that even after decades of nationwide vaccination, HBV infection is still a major risk factor for HCC in this area as well as in the country [28]. It is not surprising to see a significantly lower concentration of plasma proteins in HCC cases and HBsAg(+) control subjects. However, this leads to concerns on protein adduct dosimetry in these special populations [19]. Determining the influence of protein concentration on protein adduct dosimetry in a mal-nourished animal model might provide supportive data addressing this issue.
This study highlights the necessity of using multiple markers rather than a single HBV marker to define individual infection status. The host immunoresponse to HBV is a dynamic process [29]. Our data revealed that HBV status, defined by either a combination of HBV sero-markers and/or amino transferases, had a profound influence on host response to AFB1 in terms of AFB1 metabolism and oxidative damage to protein and DNA. In fact, HBsAg plus aminotransferase or the pattern of HBV sero-transversion has been used to differentiate active or past HBV infection, and to explore the association between active HBV infection and AAA levels [30,31]. One limitation of the present study is the IgM form of cAb, a marker of recent HBV infection [29], was not determined. Among the 87 sAg(-)cAb(+) subjects, those positive for eAb had similar levels of AAA yet significantly higher level of PCC than those negative, suggesting a subset in this category possessed higher oxidative stress relevant to HBV replication. The different redox-status among HBV-null, sAg(-)cAb(+) and HBsAb(+) individuals we observed might be one explanation for the etiology of HBsAg(-) HCC but the mechanism remains unknown [3]. To address this limitation in our ongoing study, HBV DNA copy number and quantified/dynamic HBV sero-markers are being measured to distinguish a possible subset of HBV infectants susceptible to oxidative damage.
In this population, sAb(+) subjects had significantly lower levels of plasma AAA and PCC and urinary AFB1 and 8-OHdG than those of cAb(+), suggesting a protective effect from HBsAb in reducing oxidative damage from AFB1 exposure. This supports the role of HBV vaccination in preventing HCC in HBV endemic areas [32]. Strikingly, if AFB1 exposure was balanced, the order (from high to low) in the increment of PCC per fmol of AAA in age and gender matched groups was HCC > cAb/sAg > null > sAb (Figure 3), suggesting that HBV infection alone significantly increased oxidative stress. Oxidative damage to proteins may be a critical pathological event because enzyme inactivation can have rapid effects, by nature of their catalytic functions [33]. PCC (aldehyde or ketone) is a widely used marker for the presence of oxidative stress in physiological and pathological conditions [34]. It is hypothesized that oxygen uptake changes due to the viral infection process is responsible for the increase of protein oxidation and the death of virally infected cells [35]. This is supported by the observation of a higher risk of aminotransferase flare-up among symptom-free HCV carriers with impaired redox state [36], but seems not in accordance with the consistent associations we observed between PCC and ALT and AST levels in all HBV sero-status groups.
High level of dietary aflatoxins in Guangxi, an HCC endemic area in China, was first reported in the 1980s [27]. We found in HCC patients from this region extraordinarily high levels of AAA, a biomarker of chronic AFB1 exposure [20], and a high frequency (64.9%) of p53 codon 249 mutations, a molecular fingerprint of AFB1 contamination in tumor tissues [5,6]. The significant correlation between PCC and both plasma AAA and urinary AFB1, to the best of our knowledge, has not been reported previously. Also, the AAA and PCC levels in people from the three regions were in accordance with the corresponding HCC incidences. This is consistent with previous reports [37,38] and a recent investigation on food hygiene in this area [39], supporting the role of AFB1 exposure as an independent risk factor for HCC in this area [37]. Moreover, this suggests that even after decades' long implementation of a primary prevention program for food hygiene improvement, AFB1 contamination is still a prominent risk factor for HCC in this region.
In contrast to AAA which reflects exposure over a period of weeks/months because of the relatively long half-life of albumin (∼20 days) in humans [40], urinary excretion of aflatoxin metabolites is very rapid and hence is dependent on the consumption of aflatoxin in the proceeding 24 hrs [41]. The significant associations between urinary AFB1 and urinary 8-OHdG, and between urinary AFB1 and PCC in this study suggests that urinary excretion of 8-OHdG as a biomarker of DNA damage, and PCC as a biomarker of protein damage, may partly result from the recent exposure to AFB1. This is a strong evidence supporting the reported association between levels of urinary AFB1 metabolites and HCC risk [23,42-44]. 8-OHdG is widely studied and considered a key biomarker of oxidative DNA damage [45,46]. We found significant associations between urinary 8-OHdG and urinary AFB1 and plasma AAA in a linear regression model, suggesting associations between recent and chronic AFB1 exposure and oxidative DNA damage. Shimoda et al. reported a significant correlation between the 8-OHdG content in noncancerous liver tissues with individual serum ALT concentration [47]. However, we saw no direct association between the urinary 8-OHdG and aminotransferase levels. Given the uncertainty of in vivo 8-OHdG turnover, and the multi-source contribution to the urinary excretion of 8-OHdG, to what extent its level represents events occurring inside the liver, the target organ for both HBV and AFB1, remains unclear.
A synergistic interaction between AFB1 exposure and HBV infection on HCC risk has been proposed in epidemiological [23,40] and animal studies [48-50]. However, elevated levels of AAA in HBV(+) adolescents and children had been reported in our study conducted in Taiwan [51] and three other studies in Gambia [31,52,53] but not in adults [30,54]. Kew summarized a number of possible mechanisms for this synergistic effect, mainly focused on the devastated effect from HBV infection to the AFB1 exposed subjects (review in [8]). However, new evidence suggests that the interaction between HBV and AFB1 might not always be in one direction: carcinogen-induced transcription factors may influence viral carcinogenesis and initiate HCC [55]. AFB1-induced immunosuppression might increase the risk of secondary infection [56]. Further, recent DNA damage sites can serve as sites for hepadnaviral DNA integration, and increasing the number of DNA damage sites dramatically increases viral integration frequency [57,58].
The present data highlights a synergistic effect of HBV and AFB1 in inducing oxidative stress and subsequently increasing risk for HCC in this region. First, there was interference from HBV infection on the metabolism and subsequent excretion of AFB1 (plasma AAA vs. urinary AFB1). Second, there was a protective effect from HBsAb in reducing oxidative damage from AFB1 exposure whilst HBV infection altered individuals' response to external and internal carcinogens: HBV infection enhanced the effects of exposure to AFB1 (active HBV infection vs. AAA), enhanced AFB1-relevant damage to parenchyma cells in liver (AAA vs. AST), and enhanced oxidative response to AFB1 exposure (PCC/AAA vs. HBV, AAA vs. 8-OHdG, and PCC vs. 8-OHdG). Third, HCC patients positive for p53 codon 249 mutations had a similar level of AAA but a higher level of PCC which supports the mutagenic role of ROS [59]. And finally, the interaction of HBV and urinary AFB1 was an independent predictor of PCC in a multivariate model. These observations suggest an essential role for oxidative stress in hepatocarcinogenesis with connections to both HBV and AFB1. An antioxidant-based chemoprevention strategy might be appropriate for individuals presently at risk in this area [60]. In fact, chemoprevention trials conducted in this region have shown promising effects: Oltiplaz inhibited phase 1 activation while increasing phase 2 conjugation of aflatoxin [61], and an intervention by green tea polyphenols significantly decreased urinary 8-OHdG levels [62].
In summary, we evaluated a biomarker profile of oxidative stress in a population exposed to environmental carcinogens, highlighting the contribution from HBV and aflatoxins, and especially the synergistic effect of both on macromolecular damage and risk for HCC in the region. The clear associations between oxidative biomarkers and exposure to HBV and AFB1 in this population suggest a core role of oxidative damage to macromolecules in HBV and AFB1 relevant hepatocarcinogenesis [6].
Supplementary Material
Acknowledgments
This work was supported in part by the National Nature Science Foundation of China (NSFC 30460143, 30560133), China Postdoctoral Science Foundation (2004035617), Guangxi Nature Sciences Grant (GKJ 0236030, 0632007-1E, 0640101), Guangxi Health Ministry Medicine Grant (Z2001087), Singapore Science Grant (No.R-186-000-044-213) and the USA National Institutes of Health Grants (ES05116 and ES09089). We thank Ms. Gail Garbowski (Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, USA), Mr. Ong Her Yam (COFM, NUS, Singapore), Mr. Li Jia Quan (Laboratory Center, GMU, China) and Dr. Chen Zhuo (Nanning CDC, China) for their laboratory assistance. We thank Dr. Cheryl Ann Winkler (Laboratory of Genomic Diversity, National Cancer Institute at Frederick, U.S.A.) for critical review of the manuscript.
Abbreviations
- HBV
Hepatitis B Virus
- AFB1
aflatoxin B1
- AAA
AFB1-albumin adduct
- PCC
Protein carbonyl content
- HCC
hepatocellular carcinoma
- ROS
Reactive oxygen species
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ALB
albumin
- GLO
globulin
- TP
total protein
- ELISA
enzyme-linked-immunosorbent-assay
- 8-OHdG
8-hydroxydeoxyguanosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seeff LB, Hoofnagle JH. Epidemiology of hepatocellular carcinoma in areas of low hepatitis B and hepatitis C endemicity. Oncogene. 2006;25:3771–3777. doi: 10.1038/sj.onc.1209560. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes R, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Kremsdorf K, Soussan P, Paterlini-Brechot P, Brechot C. Hepatitis B virus-related hepatocellular carcinoma: paradigms for viral-related human carcinogenesis. Oncogene. 2006;25:3823–3833. doi: 10.1038/sj.onc.1209559. [DOI] [PubMed] [Google Scholar]
- 4.Huy TT, Abe K. Molecular epidemiology of hepatitis B and C virus infections in Asia. Pediatr Int. 2004;46:223–230. doi: 10.1046/j.1442-200x.2004.01867.x. [DOI] [PubMed] [Google Scholar]
- 5.Groopman JD, Kensler TW. Role of metabolism and viruses in aflatoxin-induced liver cancer. Toxicol Appl Pharmacol. 2005;206:131–137. doi: 10.1016/j.taap.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Shen HM, Ong CN. Mutations of the p53 tumor suppressor gene and ras oncogenes in aflatoxin hepatocarcinogenesis. Mutat Res. 1996;366:23–44. doi: 10.1016/s0165-1110(96)90005-6. [DOI] [PubMed] [Google Scholar]
- 7.Wang JS, Groopman JD. DNA damage by mycotoxins. Mutat Res. 1999;424:167–181. doi: 10.1016/s0027-5107(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 8.Kew MC. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 2003;23:405–409. doi: 10.1111/j.1478-3231.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 9.Droge W. Oxidative stress and aging. Adv Exp Med Biol. 2003;543:191–200. doi: 10.1007/978-1-4419-8997-0_14. [DOI] [PubMed] [Google Scholar]
- 10.Kodama M, Inoue F, Akao M. Enzymatic and non-enzymatic formation of free radicals from aflatoxin B1. Free Radic Res Commun. 1990;10:137–142. doi: 10.3109/10715769009149882. [DOI] [PubMed] [Google Scholar]
- 11.Chen JX, Zheng Y, West M, Tang MS. Carcinogens preferentially bind at methylated CpG in the p53 mutational hot spots. Cancer Res. 1998;58:2070–2075. [PubMed] [Google Scholar]
- 12.Hussain SP, Aguilar F, Amstad P, Cerutti P. Oxy-radical induced mutagenesis of hotspot codons 248 and 249 of the human p53 gene. Oncogene. 1994;9:2277–2281. [PubMed] [Google Scholar]
- 13.Hagen TM, Huang S, Curnutte J, Fowler P, Martinez V, Wehr CM, Ames BN, Chisari FV. Extensive oxidative DNA damage in hepatocytes of transgenic mice with chronic active hepatitis destined to develop hepatocellular carcinoma. Proc Natl Acad Sci USA. 1994;91:12808–12812. doi: 10.1073/pnas.91.26.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitada T, Seki S, Iwai S, Yamada T, Sakaguchi H, Wakasa K. In situ detection of oxidative DNA damage, 8-hydroxydeoxyguanosine, in chronic human liver disease. JHepatol. 2001;35:613–618. doi: 10.1016/s0168-8278(01)00171-4. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh YH, Su IJ, Wang HC, Chang WW, Lei HY, Lai MD, Chang WT, Huang W. Pre-S mutant surface antigens in chronic hepatitis B virus infection induce oxidative stress and DNA damage. Carcinogenesis. 2004;25:2023–2032. doi: 10.1093/carcin/bgh207. [DOI] [PubMed] [Google Scholar]
- 16.Choi J, Ou JH. Mechanisms of liver injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. Am J Physiol Gastrointest Liver Physiol. 2006;290:G847–G851. doi: 10.1152/ajpgi.00522.2005. [DOI] [PubMed] [Google Scholar]
- 17.Gan LS, Skipper PL, Peng XC, Groopman JD, Chen JS, Wogan GN, Tannenbaum SR. Serum albumin adducts in the molecular epidemiology of aflatoxin carcinogenesis: correlation with aflatoxin B1 intake and urinary excretion of aflatoxin M1. Carcinogenesis. 1988;9:1323–1325. doi: 10.1093/carcin/9.7.1323. [DOI] [PubMed] [Google Scholar]
- 18.Tao P, Zhi-Ming L, Tang-Wei L, Le Qun L, Min-Hao P, Xue Q, Lu-Nam Y, Ren-Xiang L, Zong-Liang W, Lian-Wen W, Qiao W, Han-Ming S, Choon-Nam O, Santella RM. Associated factors in modulating aflatoxin B1-albumin adduct level in three Chinese populations. Dig Dis Sci. 2005;50:525–532. doi: 10.1007/s10620-005-2468-1. [DOI] [PubMed] [Google Scholar]
- 19.Peng T, Li Q, Peng MH, Liu ZM, Liu TW, Yan LN, Shen HM, Wang L, Wang Q, Wang KB, Liang RX, Wei ZL, Ong CN, Santella RM. Is correction for protein concentration appropriate for protein adduct dosimetry? Hypothesis and clues from an aflatoxin B1-exposed population. Cancer Sci. 2007;98:140–146. doi: 10.1111/j.1349-7006.2006.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CJ, Wang LY, Lu SN, Wu MH, You SL, Zhang YJ, Wang LW, Santella RM. Elevated aflatoxin exposure and increased risk of hepatocellular carcinoma. Hepatology. 1996;24:38–42. doi: 10.1002/hep.510240108. [DOI] [PubMed] [Google Scholar]
- 21.Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 22.Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic Res. 1996;25:57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- 23.Wang LY, Hatch M, Chen CJ, Levin B, You SL, Lu SN, Wu MH, Wu WP, Wang LW, Wang Q, Huang GT, Yang PM, Lee HS, Santella RM. Aflatoxin exposure and risk of hepatocellular carcinoma in Taiwan. Int J Cancer. 1996;67:620–625. doi: 10.1002/(SICI)1097-0215(19960904)67:5<620::AID-IJC5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 24.Yin B, Whyatt RM, Perera FP, Randall MC, Cooper TB, Santella RM. Determination of 8-hydroxydeoxyguanosine by an immunoaffinity chromatography-monoclonal antibody-based ELISA. Free Radic Biol Med. 1995;18:1023–1032. doi: 10.1016/0891-5849(95)00003-g. [DOI] [PubMed] [Google Scholar]
- 25.Peng Tao, Li Le Qun, Lin jin Ling, Lu Yun Fei, Wu San, Liang Sui Ting, Xiao Qiang, L QH. p53 gene 249codon mutation in recurrent hepatocellular carcinoma from Guangxi Province. Chinese Journal of General Surgery. 2000;15:17–18. [Google Scholar]
- 26.Yeh FS, Mo CC, Yen RC. Risk factors for hepatocellular carcinoma in Guangxi, People's Republic of China. Natl Cancer Inst Monogr. 1985;69:47–48. [PubMed] [Google Scholar]
- 27.Yeh FS, Yu MC, Mo CC, Luo S, Tong MJ, Henderson BE. Hepatitis B virus, aflatoxins, and hepatocellular carcinoma in southern Guangxi, China. Cancer Res. 1989;49:2506–2509. [PubMed] [Google Scholar]
- 28.Cheng Margaret Harris. Hepatitis B vaccination in China. The Lancet Oncology. 2006;6:548. [Google Scholar]
- 29.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 30.Wild CP, Yin F, Turner PC, Chemin I, Chapot B, Mendy M, Whittle H, Kirk GD, Hall AJ. Environmental and genetic determinants of aflatoxin-albumin adducts in the Gambia. Int J Cancer. 2000;86:1–7. doi: 10.1002/(sici)1097-0215(20000401)86:1<1::aid-ijc1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 31.Turner PC, Mendy M, Whittle H, Fortuin M, Hall AJ, Wild CP. Hepatitis B infection and aflatoxin biomarker levels in Gambian children. Trop Med Int Health. 2000;5:837–841. doi: 10.1046/j.1365-3156.2000.00664.x. [DOI] [PubMed] [Google Scholar]
- 32.Chang MH, Shau WY, Chen CJ, Wu TC, Kong MS, Liang DC, Hsu HM, Chen HL, Hsu HY, Chen DS. Hepatitis B vaccination and hepatocellular carcinoma rates in boys and girls. JAMA. 2000;284:3040–3042. doi: 10.1001/jama.284.23.3040. [DOI] [PubMed] [Google Scholar]
- 33.Dean RT, Fu S, Stocker R, Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 1997;324(Pt 1):1–18. doi: 10.1042/bj3240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chevion M, Berenshtein E, Stadtman ER. Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radic Res. 2000;33(Suppl):S99–108. [PubMed] [Google Scholar]
- 35.Saarinen MA, Murhammer DW. The response of virally infected insect cells to dissolved oxygen concentration: recombinant protein production and oxidative damage. Biotechnol Bioeng. 2003;81:106–114. doi: 10.1002/bit.10460. [DOI] [PubMed] [Google Scholar]
- 36.Vendemiale G, Grattagliano I, Portincasa P, Serviddio G, Palasciamo G, Altomare E. Oxidative stress in symptom-free HCV carriers: relation with ALT flare-up. Eur J Clin Invest. 2001;31:54–63. doi: 10.1046/j.1365-2362.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang JS, Huang T, Su J, Liang F, Wei Z, Liang Y, Luo H, Kuang SY, Qian GS, Sun G, He X, Kensler TW, Groopman JD. Hepatocellular carcinoma and aflatoxin exposure in Zhuqing Village, Fusui County, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2001;10:143–146. [PubMed] [Google Scholar]
- 38.Stern MC, Umbach DM, Yu MC, London SJ, Zhang ZQ, Taylor JA. Hepatitis B, aflatoxin B(1), p53 codon 249 mutation in hepatocellular carcinomas from Guangxi, People's Republic of China, and a meta-analysis of existing studies. Cancer Epidemiol Biomarkers Prev. 2001;10:617–625. [PubMed] [Google Scholar]
- 39.Li FQ, Yoshizawa T, Kawamura O, Luo XY, Li YW. Aflatoxins and fumonisins in corn from the high-incidence area for human hepatocellular carcinoma in Guangxi, China. J Agric Food Chem. 2001;49:4122–4126. doi: 10.1021/jf010143k. [DOI] [PubMed] [Google Scholar]
- 40.Wild CP, Jiang YZ, Sabbioni G, Chapot B, Montesano R. Evaluation of methods for quantitation of aflatoxin-albumin adducts and their application to human exposure assessment. Cancer Res. 1990;50:245–251. [PubMed] [Google Scholar]
- 41.Wild CP, Hudson GJ, Sabbioni G, Chapot B, Hall AJ, Wogan GN, Whittle H, Montesano R, Groopman JD. Dietary intake of aflatoxins and the level of albumin-bound aflatoxin in peripheral blood in The Gambia, West Africa. Cancer Epidemiol Biomarkers Prev. 1992;1:229–234. [PubMed] [Google Scholar]
- 42.Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, Groopman JD, Gao YT, Henderson BE. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339:943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- 43.Zhu JQ, Zhang LS, Hu X, Xiao Y, Chen JS, Xu YC, Fremy J, Chu FS. Correlation of dietary aflatoxin B1 levels with excretion of aflatoxin M1 in human urine. Cancer Res. 1987;47:1848–1852. [PubMed] [Google Scholar]
- 44.Groopman JD, Zhu JQ, Donahue PR, Pikul A, Zhang LS, Chen JS, Wogan GN. Molecular dosimetry of urinary aflatoxin-DNA adducts in people living in Guangxi Autonomous Region, People's Republic of China. Cancer Res. 1992;52:45–52. [PubMed] [Google Scholar]
- 45.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res. 1997;387:147–163. doi: 10.1016/s1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 46.Hazra TK, Hill JW, Izumi T, Mitra S. Multiple DNA glycosylases for repair of 8-oxoguanine and their potential in vivo functions. Prog Nucleic Acid Res Mol Biol. 2001;68:193–205. doi: 10.1016/s0079-6603(01)68100-5. [DOI] [PubMed] [Google Scholar]
- 47.Shimoda R, Nagashima M, Sakamoto M, Yamaguchi N, Hirohashi S, Yokota J, Kasai H. Increased formation of oxidative DNA damage, 8-hydroxydeoxyguanosine, in human livers with chronic hepatitis. Cancer Res. 1994;54:3171–3172. [PubMed] [Google Scholar]
- 48.Cova L, Wild CP, Mehrotra R, Turusov V, Shirai T, Lambert V, Jacquet C, Tomatis L, Trepo C, Montesano R. Contribution of aflatoxin B1 and hepatitis B virus infection in the induction of liver tumors in ducks. Cancer Res. 1990;50:2156–2163. [PubMed] [Google Scholar]
- 49.Bannasch P, Khoshkhou NI, Hacker HJ, Radaeva S, Mrozek M, Zillmann U, Kopp-Schneider A, Haberkorn U, Elgas M, Tolle T. Synergistic hepatocarcinogenic effect of hepadnaviral infection and dietary aflatoxin B1 in woodchucks. Cancer Res. 1995;55:3318–3330. [PubMed] [Google Scholar]
- 50.Sell S, Hunt JM, Dunsford HA, Chisari FV. Synergy between hepatitis B virus expression and chemical hepatocarcinogens in transgenic mice. Cancer Res. 1991;51:1278–1285. [PubMed] [Google Scholar]
- 51.Chen SY, Chen CJ, Chou SR, Hsieh LL, Wang LY, Tsai WY, Ahsan H, Santella RM. Association of aflatoxin B(1)-albumin adduct levels with hepatitis B surface antigen status among adolescents in Taiwan. Cancer Epidemiol Biomarkers Prev. 2001;10:1223–1226. [PubMed] [Google Scholar]
- 52.Allen SJ, Wild CP, Wheeler JG, Riley EM, Montesano R, Bennett S, Whittle HC, Hall AJ, Greenwood BM. Aflatoxin exposure, malaria and hepatitis B infection in rural Gambian children. Trans R Soc Trop Med Hyg. 1992;86:426–430. doi: 10.1016/0035-9203(92)90253-9. [DOI] [PubMed] [Google Scholar]
- 53.Wild CP, Fortuin M, Donato F, Whittle HC, Hall AJ, Wolf CR, Montesano R. Aflatoxin, liver enzymes, and hepatitis B virus infection in Gambian children. Cancer Epidemiol Biomarkers Prev. 1993;2:555–561. [PubMed] [Google Scholar]
- 54.Wang JS, Qian GS, Zarba A, He X, Zhu YR, Zhang BC, Jacobson L, Gange SJ, Munoz A, Kensler TW. Temporal patterns of aflatoxin-albumin adducts in hepatitis B surface antigen-positive and antigen-negative residents of Daxin, Qidong County, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 1996;5:253–261. [PubMed] [Google Scholar]
- 55.Banerjee R, Caruccio L, Zhang YJ, McKercher S, Santella RM. Effects of carcinogen-induced transcription factors on the activation of hepatitis B virus expression in human hepatoblastoma HepG2 cells and its implication on hepatocellular carcinomas. Hepatology. 2000;32:367–374. doi: 10.1053/jhep.2000.9197. [DOI] [PubMed] [Google Scholar]
- 56.Bondy GS, Pestka JJ. Immunomodulation by fungal toxins. J Toxicol Environ Health B Crit Rev. 2000;3:109–143. doi: 10.1080/109374000281113. [DOI] [PubMed] [Google Scholar]
- 57.Dandri M, Burda MR, Burkle A, Zuckerman DM, Will H, Rogler CE, Greten H, Petersen J. Increase in de novo HBV DNA integrations in response to oxidative DNA damage or inhibition of poly(ADP-ribosyl)ation. Hepatology. 2002;35:217–223. doi: 10.1053/jhep.2002.30203. [DOI] [PubMed] [Google Scholar]
- 58.Petersen J, Dandri M, Burkle A, Zhang L, Rogler CE. Increase in the frequency of hepadnavirus DNA integrations by oxidative DNA damage and inhibition of DNA repair. J Virol. 1997;71:5455–5463. doi: 10.1128/jvi.71.7.5455-5463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 60.Kensler W, Qian GS, Chen JG, Groopman JD. Translational strategies for cancer prevention in liver. Nat Rev Cancer. 2003;3:321–329. doi: 10.1038/nrc1076. [DOI] [PubMed] [Google Scholar]
- 61.Wang JS, Shen X, He X, Zhu YR, Zhang BC, Wang JB, Qian GS, Kuang SY, Zarba A, Egner PA, Jacobson LP, Munoz A, Helzlsouer KJ, Groopman JD, Kensler TW. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People's Republic of China. J Natl Cancer Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- 62.Luo H, Tang L, Tang M, Billam M, Huang T, Yu J, Wei Z, Liang Y, Wang K, Zhang ZQ, Zhang L, Wang JS. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27:262–268. doi: 10.1093/carcin/bgi147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.