Summary
In vitro organ culture (IVOC) represents a gold standard model to study enteropathogenic E. coli (EPEC) infection of human intestinal mucosa. However, the optimal examination of the bacterial - host cell interaction requires a directional epithelial exposure, without serosal or cut surface stimulation. A polarised IVOC system (pIVOC) was developed in order to overcome such limitations: apical EPEC infection produced negligible bacterial leakage via biopsy edges, resulted in enhanced colonisation compared to standard IVOC, and showed evidence of bacterial detachment, as in natural rabbit EPEC infections. Examination of mucosal innate immune responses in pIVOC showed both interleukin (IL)-8 mRNA and protein levels were significantly increased after apical EPEC infection. Increased IL-8 levels mainly depended on flagellin expression as fliC-negative EPEC did not elicit a significant IL-8 response despite increased mucosal colonisation compared to wild type EPEC. In addition, apical application of purified flagella significantly increased IL-8 protein levels over non-infected controls. Immunofluorescence staining of EPEC-infected small intestinal biopsies revealed apical and basolateral distribution of Toll-like receptor (TLR) 5 on epithelium suggesting that EPEC can trigger mucosal IL-8 responses by apical flagellin/TLR5 interaction ex vivo and does not require access to the basolateral membrane as postulated in cell culture models.
Introduction
Enteropathogenic Escherichia coli (EPEC) were the first E. coli to be associated with human disease and are a major cause of infant diarrhoea in developing countries (Chen and Frankel, 2005;Nataro and Kaper, 1998). Small intestinal EPEC infection results in watery diarrhoea which is due in part to adhering bacteria causing loss of absorptive microvilli. This phenomenon, termed an attaching and effacing (A/E) lesion, is characterised by intimate bacterial attachment to the host cell membrane, microvillous effacement, and actin polymerisation under adhering bacteria (Moon et al., 1983;Ulshen and Rollo, 1980). A/E lesion formation has been studied extensively on epithelial cell lines (Gruenheid et al., 2001;Jerse et al., 1990;Kenny, 1999;Lommel et al., 2004) and has been regarded as a hallmark for pathogenicity (Knutton et al., 1989). However, the use of in vitro human intestinal organ culture (IVOC) has shown that pathways of A/E lesion formation based on in vitro observations do not necessarily apply ex vivo (Bai et al., 2008;Schuller et al., 2007). In parallel with A/E lesion formation work, host response studies have used human intestinal epithelial cell lines (i.e. Caco-2, T84, HT-29) and have identified flagellin (FliC) as the principal inducer of the intestinal inflammatory response against EPEC (Khan et al., 2008;Ruchaud-Sparagano et al., 2007;Sharma et al., 2006;Zhou et al., 2003). FliC is the main component of bacterial flagella and activates innate immune responses via binding to Toll-like receptor (TLR) 5 (Hayashi et al., 2001). Ligation of TLR5 leads to rapid activation of NF-κB mediated by MAP kinases and the expression of a number of proinflammatory cytokines such as the neutrophil chemoattractant interleukin (IL)-8 (Yu et al., 2003). Although there is consensus about a prominent role for EPEC FliC in NF-κB activation and IL-8 induction in intestinal epithelial cell lines, it remains controversial whether FliC is the only stimulus or whether additional bacterial factors are involved. More importantly, it is not known if flagellin activates epithelial cells in vivo when present in the intestinal lumen or whether basolateral epithelial access is required. This is particularly interesting as flagellin is also released by commensal bacteria in the human intestine and aberrant flagellin/TLR5-mediated immune responses have been implicated in inflammatory bowel disease pathogenesis (Lodes et al., 2004). In vitro studies, using polarised intestinal model epithelia, have tried to address this question but results have been inconclusive due to differences in TLR5 distribution patterns between cell lines used (Gewirtz et al., 2001;Miyamoto et al., 2006). To elucidate EPEC-induced inflammatory responses in human small intestinal mucosa, we developed a polarised IVOC (pIVOC) system which allows directional access to the mucosal surface. Using this novel system we show that apical EPEC infection of duodenal mucosa results in increased IL-8 mRNA and protein expression that is mainly, but not wholly, dependent on FliC/TLR5 interaction.
Results
Development of the pIVOC system
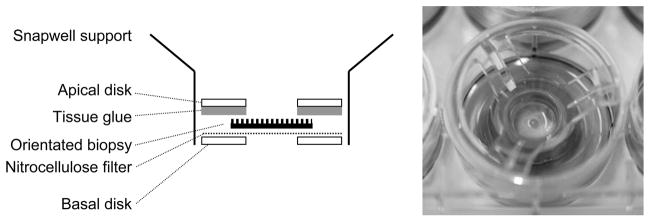
We modified a previously described animal (El Asmar et al., 2002) and human (Raffatellu et al., 2005) Micro-Snapwell intestinal culture system for use with paediatric intestinal biopsies of 2–3 mm diameter. Initial experiments were performed as described previously (Raffatellu et al., 2005) using smaller aperture (2 mm) Perspex disks. Duodenal biopsies were either infected apically with EPEC or left non-infected (NI) and incubated for 5–8 h. At the end of the experiment, tissue integrity was examined by scanning electron microscopy (SEM). Bacterial leakage into the basal compartment was tested by plating serial dilutions on LB agar plates and counting colony forming units (CFU). After 5 h incubation, SEM of NI samples showed extrusion and loss of the surface epithelium (data not shown) and bacterial CFU in the basal chamber ranged from 50–400% of the initial apical inoculum. Therefore, a porous (3 μm) cellulose nitrate filter was introduced to support the biopsy and facilitate optimal orientation and unfolding of the tissue. Histoacryl tissue glue was used to seal the apical disk to the mucosal side of the biopsy to reduce bacterial leakage (Fig. 1). These measures resulted in a drastic reduction of bacterial CFU in the basal compartment. Optimal epithelial survival was achieved by reducing the volume of apical medium after 2 h of incubation to leave a thin film of medium covering the tissue sample. Incubations could be continued for a further 6 h when biopsies showed good tissue morphology within the area of the central aperture (white circle in Fig. 2A) with intact surface epithelium visible at higher magnifications (Fig. 2B).
Fig. 1.
Mounting of intestinal biopsies for pIVOC. After orientation of the tissue on nitrocellulose filter, the apical Perspex disk was fixed to the mucosal side of the biopsy via tissue glue. The sandwich was then inserted between a Snapwell support.
Fig. 2.
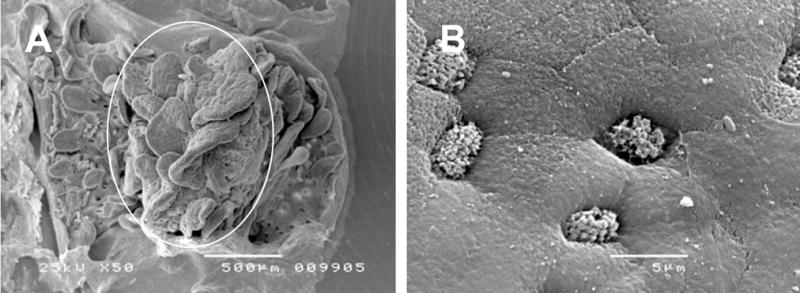
Tissue survival of uninfected duodenal biopsies after 8 h of pIVOC. (A) The white circle shows the area of the biopsy within the central aperture. (B) Intact surface epithelium can be observed at higher magnifications. Bars = 500 μm (A) and 5 μm (B).
EPEC colonisation of duodenal biopsies
SEM showed that EPEC colonisation was enhanced in pIVOC compared to the standard system (biopsy samples with all surfaces exposed to the culture medium) following 5–7 h incubation (Fig. 3). After 5 h infection, several microcolonies of approximately 50 A/E bacteria were observed under standard conditions, whereas most of the epithelial surface was covered by intimately attaching bacteria in pIVOC. Interestingly, bacterial “footprints” were evident in pIVOC indicating bacterial detachment after microvillous effacement (arrowheads in Fig. 3). Determination of CFU after 7 h of infection showed that bacterial leakage into the basal compartment was negligible (<0.01% of the initial inoculum, representing leakage plus bacterial replication in the compartment).
Fig. 3.
Colonisation of duodenal biopsies by EPEC during standard and polarised IVOC conditions. EPEC adherence is increased in pIVOC and bacterial detachment after effacement is seen (arrowheads). Shown are representative images of three separate experiments. Bars = 5 μm.
EPEC infection enhances expression of IL-8 mRNA in duodenal biopsies
pIVOC confines bacterial access to the apical mucosal surface and gives good EPEC colonisation with negligible bacterial leakage into the basal compartment. Thus, it is suitable to study the innate immune response of human intestinal mucosa to EPEC infection which has not been possible so far. Previous studies have shown that EPEC induces production and secretion of IL-8 from human intestinal epithelial cell lines (Savkovic et al., 1996;Sharma et al., 2006). To investigate whether EPEC has a similar effect on ex vivo intestinal epithelium, duodenal pIVOC was performed. Paired biopsies were obtained from different patients and wild type (wt) EPEC or BHI broth (NI control) were applied apically for 5 h, a time period that provides good colonisation with an intact epithelium. At the end of the experiment, total RNA was extracted from the biopsy samples and analysed by real-time RT-PCR. EPEC infection significantly (P=0.004) enhanced duodenal IL-8 mRNA expression relative to matched NI control samples with a median 3-fold increase (Fig. 4A).
Fig. 4.
IL-8 mRNA expression in duodenal biopsies after 5 h pIVOC. (A) EPEC infection significantly enhances IL-8 mRNA expression. Dots represent results from individual patients (n=9). (B) Enhanced IL-8 mRNA expression after EPEC infection is mainly dependent on FliC. Matched duodenal biopsies from individual patients (n=9) were infected with wt EPEC, fliC-negative JG01 or left uninfected. (C) Complementation of flagellin-deficient EPEC with plasmid expressed fliC partially restores IL-8 mRNA induction towards wild type levels. Matched tissue samples from individual patients (n=4) were infected with wt EPEC, fliC-negative AGT01, fliC-complemented AGT02 or left uninfected. IL-8 mRNA expression is shown as fold increase relative to matched NI control samples. Medians are indicated by a line. ** P<0.01, *** P<0.001 vs NI control.
Motility and flagellin production by EPEC mutant strains
Previous studies on intestinal epithelial cell lines have shown that EPEC flagellin is the major inducer of IL-8 expression (Zhou et al., 2003). To determine whether FliC plays a similar role during ex vivo infections of human intestinal mucosa, flagellin-deficient EPEC strain JG01 was constructed for pIVOC experiments. A lack of flagellin production was confirmed by motility assays and Western blotting. When grown in 0.3% LB agar JG01 showed impaired motility and remained restricted to the area of inoculation after 18 h whereas wt EPEC spread throughout the medium (Fig. 8A, supplementary material (SM)). Western blotting of supernatants with H6 antiserum showed FliC production in wt EPEC but none in JG01 (Fig. 8B, SM). FliC-negative EPEC AGT01 and its complemented strain AGT02 were obtained later and showed lack/restoration of FliC production in supernatants, respectively (Fig. 8B, SM) which agrees with previous studies (Giron et al., 2002;Zhou et al., 2003).
Enhanced IL-8 mRNA expression after EPEC infection is related to flagellin production
To determine the impact of EPEC flagellin production on mucosal IL-8 response, three matched duodenal biopsies were obtained from individual patients and infected with wt EPEC, JG01 or inoculated with BHI broth (NI control). pIVOC was performed for 5 h and IL-8 mRNA expression was analysed by real-time PCR. Infection with JG01 did not enhance IL-8 mRNA expression (median fold increase versus NI control = 1.8, P>0.05) whereas wt EPEC caused a significant increase (median fold increase = 2.9, P<0.001 versus NI) similar to that observed in the previous experiment (Fig. 4B). To confirm that the attenuated IL-8 mRNA response in JG01 infections was due to loss of flagellin expression, a limited number (n=4) of pIVOCs was performed using four matched biopsies which were infected for 5 h with a different fliC mutant (AGT01) and the complemented strain (AGT02). AGT01, as JG01, failed to elicit significant IL-8 mRNA induction (median fold increase versus NI = 2.0, P>0.05) whereas a significant increase in IL-8 mRNA was seen after infection with wt EPEC (median fold increase = 3.5, P<0.01 versus NI). Complementation of fliC in AGT02 partially restored IL-8 mRNA induction towards wt levels (median fold increase versus NI = 2.9) although this did not reach significance (P>0.05) (Fig. 4C). This agrees with cell line studies where AGT02 has been shown to only partly restore IL-8 secretion (Zhou et al., 2003).
EPEC infection enhances IL-8 protein expression in a FliC-dependent manner
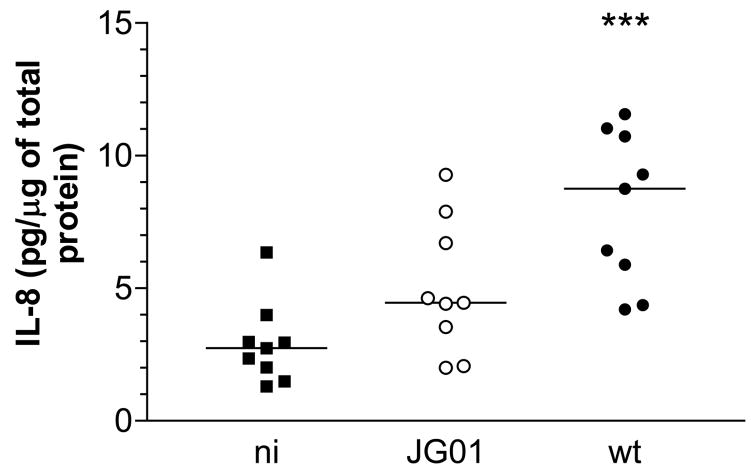
6 h pIVOC was performed to determine whether EPEC infection also enhances duodenal IL-8 protein expression. After incubation, samples were lysed and IL-8 expression was quantified by sandwich ELISA. IL-8 levels in wt EPEC infected samples were increased relative to NI matched samples (median 8.76 versus 2.74 pg/μg total protein respectively, P<0.001). Although JG01 infection also caused increased IL-8 protein levels (median 4.46 pg IL-8/μg total protein) this did not reach significance compared to NI (P>0.05) (Fig. 5).
Fig. 5.
Ex vivo EPEC infection of human duodenal mucosa results in increased IL-8 protein expression which is mainly dependent on FliC. 6 h pIVOC was performed using three matched biopsies from individual patients (n=9). Tissue samples were infected with wt EPEC, fliC-negative JG01 or left uninfected (NI). IL-8 tissue protein levels were determined by ELISA and are expressed relative to amounts of total protein. Medians are indicated by a line. *** P<0.001 vs NI control.
JG01 colonisation of duodenal mucosa ex vivo is enhanced compared to wt EPEC
Impaired flagellin synthesis results in reduced adherence to HeLa cells (Giron et al., 2002). To investigate whether reduced IL-8 induction by JG01 could be explained by less duodenal colonisation, A/E lesion formation was investigated after 6 h pIVOC. After infection, biopsies were studied by SEM for bacterial adhesion or tissue samples were lysed and serial dilutions were plated on LB plates to quantify colonisation. SEM showed typical A/E lesions characterised by intimate bacterial attachment and microvillous elongation around microcolonies for both JG01 and wt EPEC. No bacteria were found on matched NI samples (Fig. 9, SM). Quantification of colonisation showed significantly higher levels for JG01 compared to wt EPEC (12.7 ± 2.8 × 104 versus 7.7 ± 1.9 × 104 CFU/biopsy respectively, mean ± SEM of 7 independent experiments, P<0.05). Therefore, reduced levels of IL-8 production in JG01-infected samples are not related to reduced adherence.
Purified EPEC flagella are sufficient to increase mucosal IL-8 protein expression
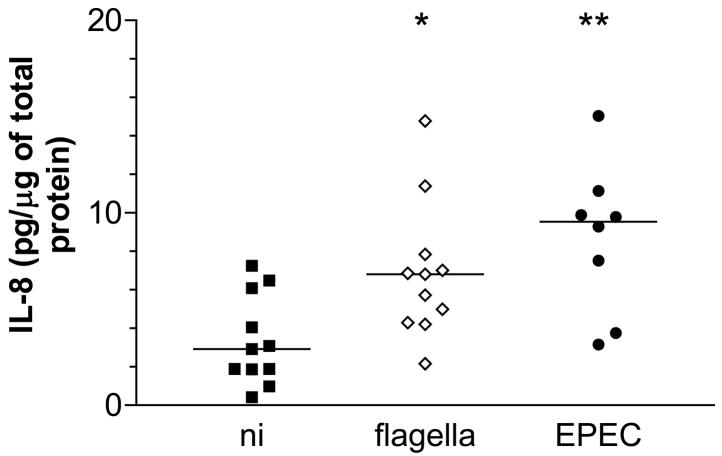
EPEC infection has been demonstrated to diminish epithelial barrier function by redistribution of tight junction proteins (Philpott et al., 1996;Spitz et al., 1995). To exclude the possibility of flagellin leakage to the basolateral side of the epithelium during infection, duodenal biopsies were inoculated with purified EPEC flagella on the apical side and IL-8 production was evaluated after 6 h. As shown in Fig. 6, IL-8 levels in samples treated with purified flagella were increased relative to NI matched samples (median 6.81 versus 2.92 pg/μg total protein respectively, P<0.05). Matched infections with EPEC bacteria resulted in enhanced IL-8 expression relative to NI but not to flagella treated samples (median 9.54 pg/μg total protein, P<0.01 vs NI, P>0.05 vs flagella treated samples). Incubations of duodenal biopsies with flagellin monomers instead of whole flagella produced similar results (data not shown).
Fig. 6.
Apically applied purified EPEC flagella are sufficient to increase IL-8 protein expression in duodenal tissue. Matched biopsies from individual patients (n=11) were inoculated apically with purified EPEC flagella (10 μg/ml), wt EPEC or left uninfected (NI). IL-8 tissue protein levels were determined after 6 h pIVOC and expressed relative to amounts of total protein. Medians are indicated by a line. * P<0.05, ** P<0.01 vs NI control.
TLR5 is distributed apically and basolaterally on EPEC-infected intestinal epithelium
pIVOC has shown that flagellin contributes to the mucosal IL-8 response to apical EPEC infection, raising questions regarding localisation of TLR5, the receptor for bacterial flagellin. Immunostaining of NI duodenal mucosa showed apical and basolateral TLR5 expression in the epithelium with positive staining on scattered cells in the lamina propria (Fig. 7A). TLR5 localisation remained unaltered after EPEC infection and adhering bacteria could be observed in close proximity to apical TLR5 suggesting a direct interaction (Fig. 7B). Specificity of anti-TLR5 was confirmed by staining polarised T84 cells where TLR5 staining was restricted to the basolateral membrane (Fig. 7C) (Gewirtz et al., 2001). No staining was observed with secondary antibody only (data not shown).
Fig. 7.
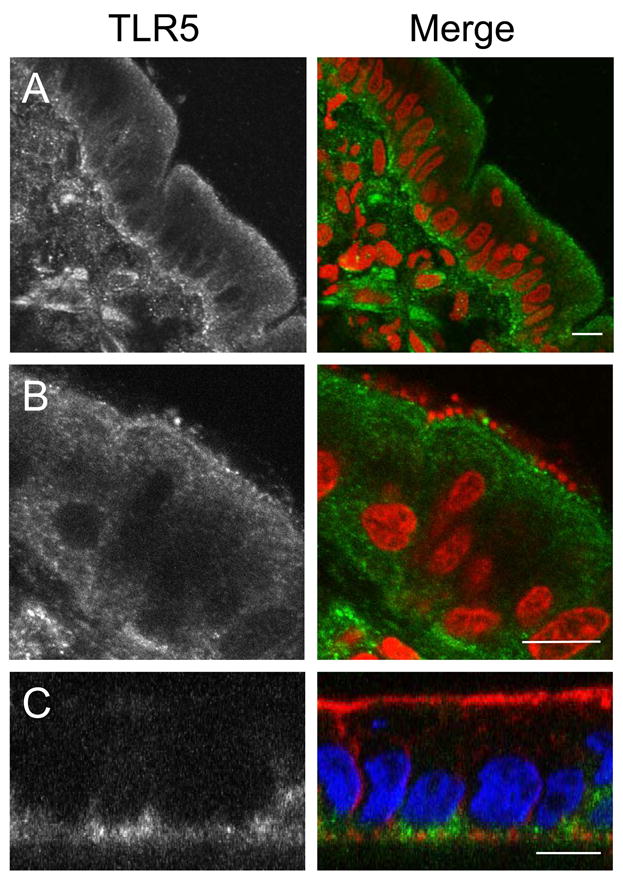
TLR5 is expressed apically and basolaterally on duodenal epithelium. Biopsies were left non-infected (A) or infected with wt EPEC (B) for 8 h. Cryosections from snap-frozen tissue were stained with anti-TLR5 (green) and counterstained with propidium iodide (red) to visualise bacteria and cell nuclei. (C) XZ scan of polarised T84 cells: TLR5 is restricted to the basolateral side. Fixed monolayers were stained with anti-TLR5 (green), TRITC-conjugated phalloidin to label actin (red) and DAPI to label cell nuclei (blue). Shown are separate monochrome images of the green fluorescence channel (TLR5) and merged colour images from all channels. Bars = 10 μm.
Discussion
In man, EPEC colonise the small and large intestine with mucosal inflammation and neutrophil infiltration of the lamina propria (Lewis et al., 1987;Rothbaum et al., 1983). Neutrophil recruitment has been attributed to IL-8 production by EPEC-infected intestinal epithelial cells (Savkovic et al., 1996) and EPEC flagellin has been identified as the major inducer of IL-8 secretion (Ruchaud-Sparagano et al., 2007;Sharma et al., 2006;Zhou et al., 2003). To examine whether these findings from in vitro studies could be applied to the ex vivo situation we developed a polarised intestinal IVOC system which restricted bacterial infection to the mucosal surface simulating in vivo EPEC infection in the human gut.
pIVOC showed extensive colonisation of human duodenal mucosa by A/E EPEC. Unlike standard IVOC, “footprint” formation was evident which results from bacterial detachment after microvillous effacement. Interestingly, this is also seen in in vivo REPEC infected ileum in rabbits (Heczko et al., 2001) and may represent a mechanism of bacterial spreading. Enhanced colonisation and footprint formation in pIVOC probably reflect a more advanced colonisation stage compared to standard IVOC due to a higher density of bacteria exposed to the mucosal biopsy surface. In addition, pIVOC also allowed quantification of bacterial adherence. This is not possible in the standard system due to the variable sample size and bacterial binding to submucosal and cut surfaces. In pIVOC, bacterial access is restricted to a defined mucosal surface area (within the central aperture), so that numbers of tissue-associated bacteria can be compared between different samples. When comparing colonisation of duodenal mucosa by wt EPEC to an isogenic fliC mutant, we found that A/E lesions were morphologically similar on SEM but that adherence rates of JG01 were significantly increased. This contrasts with in vitro cell line studies where FliC has been implicated in mediating adherence (Giron et al., 2002), although this has been questioned by others (Cleary et al., 2004).
Once good mucosal colonisation levels with negligible bacterial leakage into the basal compartment was established, we studied IL-8 expression in EPEC-infected mucosa using pIVOC. For the first time, using human intestinal tissue instead of immortalised cell lines, we show that apical EPEC infection results in enhanced IL-8 levels in duodenal mucosa and furthermore, that this increase is mainly, but not entirely, dependent on FliC expression. Up to now it has been controversial whether EPEC-induced IL-8 expression is exclusively mediated by FliC or whether other bacterial factors are involved. Depending on the cell line and its state of differentiation (non-polarised versus polarised), different laboratories have obtained different results. Whereas some studies do not support a role for non-flagellin proteins in IL-8 induction in non-polarised T84 (Zhou et al., 2003) and polarised Caco-2/T84 cells (Ruchaud-Sparagano et al., 2007), other data indicate the existence of a FliC-independent IL-8 response in non-polarised HT-29 (Sharma et al., 2006) and non-polarised Caco-2 cells (Khan et al., 2008). Our results, using human intestinal biopsies, demonstrate a major role for FliC but also support the contribution of additional bacterial factors. Although results did not reach significance, IL-8 protein levels in JG01 infected samples were increased relative to NI controls and it is likely that extended incubation times would result in significant IL-8 induction as demonstrated in vitro (Khan et al., 2008).
Another controversy arising from the use of different in vitro systems concerns the distribution of TLR5 in intestinal epithelial cells. Earlier studies demonstrated that TLR5 expression in polarised T84 cells is restricted to the basolateral surface as flagellin only elicited a pro-inflammatory response when administered basolaterally but not apically (Gewirtz et al., 2001;Ruchaud-Sparagano et al., 2007;Zhou et al., 2003). This led to the notion that polarised TLR5 distribution on intestinal epithelium explains why flagellin of luminal commensal bacteria is tolerated whereas flagellin translocated across the intestinal epithelium (generally produced by pathogens) triggers inflammation. However, it appears that TLR5 expression in other human intestinal epithelial cell lines is not basolaterally restricted. Polarised Caco-2 and HCA-7 cells have been shown to express TLR5 and/or respond to flagellin both apically and basolaterally (Berin et al., 2002;Miyamoto et al., 2006;Ruchaud-Sparagano et al., 2007). In addition, TLR5 immunostaining on normal human colonic and gastric mucosa shows positive staining on both apical and basolateral epithelial surfaces (Cario and Podolsky, 2000;Miyamoto et al., 2006;Schmausser et al., 2004). Consistent with these previous results, we show that TLR5 is expressed both apically and basolaterally on EPEC-infected human duodenal epithelium. Therefore we conclude that basolateral access of flagellin is not required for TLR5 binding and that apical flagellin can stimulate IL-8 production in human intestinal mucosa ex vivo. This agrees with the demonstration that H7 flagellin injected into the lumen of human colon xenografts induced chemokine production and neutrophil recruitment (Miyamoto et al., 2006). Furthermore, Ussing chamber experiments on murine ileal mucosa have shown that apical flagellin from commensal E. coli stimulated basolateral secretion of the murine IL-8 equivalent keratinocyte-derived chemokine ex vivo. As expected, TLR5 was expressed on both sides of the villous epithelium (Bambou et al., 2004). Similar Ussing chamber studies using normal human colonic mucosa have reported opposite results and concluded that the flagellin/TLR5 response is restricted to the basolateral aspect of human colonic mucosa (Rhee et al., 2005). However, in these experiments, flagellin was applied apically for 2–4 h and IL-8 concentrations were determined in apical supernatants. As it appears that the majority of IL-8 is secreted to the basolateral side of the epithelium (Bambou et al., 2004;Ruchaud-Sparagano et al., 2007), it is not surprising that no increase in IL-8 production was detected apically. Furthermore, the authors reported elevated IL-8 levels in basolateral supernatants after basolateral administration of flagellin (Rhee et al., 2005). This might be expected as TLR5 is expressed on human macrophages, dendritic cells, and intestinal microvasculature (Maaser et al., 2004;Means et al., 2003). All these cell types are present in the lamina propria and it appears probable that the observed basolateral IL-8 response resulted from flagellin/TLR5 interaction in these cell populations rather than with the epithelium.
In summary, we have developed a polarised IVOC system that allows the ex vivo study of human intestinal mucosal responses to EPEC infection. We show that apical EPEC infection enhances IL-8 expression in human intestinal mucosa and that this is mediated by flagellin/TLR5 interaction on the apical side of the epithelium. The pIVOC system is closer to the in vivo situation and should provide further insights into human intestinal host pathogen interactions.
Experimental procedures
Bacterial strains and flagella
Wild type EPEC E2348/69 and fliC mutant strains AGT01 and AGT02 have been described (Giron et al., 2002;Levine et al., 1985). Flagellin-deficient JG01 was generated from EPEC E2348/69 using the Lambda Red system (Datsenko and Wanner, 2000). Briefly, the chromosomal fliC gene was replaced with a kanamycin resistance cassette amplified from pKD4 using forward primer G72 (5′-AATATAGGATAACGAATCATGGCACAAGTCATTAATACCAACTGTAGGCT GGAGCTGCTTCG-3 ′) and reverse primer G73 (5 ′-TTAATCAGGTTACAACGATTAACCCTGCAGCAGAGACAGAACCATATGAA TATCCTCCTTA-3′). The amplified gene segment was electroporated into bacteria carrying the Lambda Red recombinase plasmid pKD46. Mutants were grown on selective media followed by PCR verification of the fliC mutation utilising primers G94 (5 ′-TCCCAGCGATGAAATACTTGC-3 ′) and G95 (5 ′-GAGTTATCGGCATGATTATCC-3′). For infections, bacteria were grown standing in BHI broth overnight at 37°C. Mutant strains were selected with appropriate antibiotics. H6 flagella from EPEC E2348/69 were purified as described previously (Erdem et al., 2007). To obtain monomeric flagellin, flagella were treated with 1% sodium dodecyl sulphate at 37°C for 30 min and then boiled for 5 min.
Cell culture
Human T84 colon carcinoma cells (CCL-248, ATCC) were grown and polarised on Transwell filter inserts as described previously (Schuller et al., 2004).
Standard IVOC
Paediatric biopsies from the fourth part duodenum were obtained, with fully informed consent and ethical approval, during routine endoscopy of patients (74–210 months old) for intestinal disorders. All biopsies were from grossly normal areas, and intestinal histology was subsequently reported normal. Collected tissue samples were transported to the laboratory in IVOC medium and processed within the next hour. Standard IVOC was performed as described previously (Hicks et al., 1996;Knutton et al., 1987). Briefly, biopsies mounted on a foam support were inoculated with 20 μl bacterial overnight culture (approximately 2 × 107 bacteria) or BHI broth as NI control. Samples were incubated in 95% O2/5% CO2 at 37°C on a rocking platform for 5–8 h.
pIVOC
pIVOC is a modification of a Micro-Snapwell system (El Asmar et al., 2002;Raffatellu et al., 2005) specifically adapted for use with 2–3 mm diameter paediatric intestinal biopsy samples. In this system, the biopsy sample was sandwiched between two 12mm diameter Perspex (acrylic glass) disks with a 2 mm central aperture (manufactured by the Anatomy and Developmental Biology Department workshop, UCL, London) that fits into Snapwell supports (Fig. 1). To mount the sample, a circular piece of cellulose nitrate filter (3 μm pore, Whatman) was soaked in IVOC medium and placed on top of the basal disk. The biopsy was placed centrally on the filter, excess medium was removed and, under a dissection microscope, the tissue was spread out and orientated mucosal side upwards. To minimise bacterial leakage from the apical to the basal compartment, the apical disk was sealed to the mucosal side of the biopsy using Histoacryl tissue glue (Braun Medical Ltd). The sandwich holding the biopsy was then mounted in a Snapwell support (Corning) and inserted into a 6 well culture plate. Apical and basal compartments were filled with IVOC medium (180 μl and 3 ml volume respectively) and 20 μl of bacterial overnight culture, purified flagella (10 μg/ml final concentration) or BHI broth (NI control) was added apically. Biopsies were incubated as described above. To optimise epithelial survival, most of the apical medium was removed at 2 h post-infection leaving a thin film of medium covering the mucosal surface and the tissue was incubated a further 3–6 h. After incubation, biopsies were removed from the Snapwell support, washed in cold PBS to remove mucus and non-adherent bacteria, and processed for further applications.
SEM
Samples were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer, post-fixed in 1% aqueous osmium tetroxide and dehydrated in 2,2 dimethoxy-propane. Specimens were transferred to absolute ethanol, critically point dried using liquid carbon dioxide (Emitech K850 apparatus), mounted on aluminium stubs, sputter-coated with gold-palladium (Polaron E5100 sputter coater), and viewed in a JEOL 5300 SEM.
RNA extraction and real-time PCR analysis
IVOC specimens stored in RNAlater (Sigma) were disrupted and homogenised by passage through 21G needles. Total RNA was extracted using the RNeasy Mini kit (Qiagen) and RNA quality was assessed electrophoretically and by OD260/280 determination. Samples were subjected to DNase treatment using the TURBO DNase-free kit (Ambion). cDNA was transcribed from 1 μg of total RNA using BioScript Reverse Transcriptase (Bioline) in a 20 μl reaction. Real-time PCR was performed using a Rotor-Gene 6000 analyser (Corbett Life Science). Primers were designed using Primer3 software (http://primer3.sourceforge.net/) (GAPDH forward 5′-AGGTCGGAGTCAACGGATTT-3 ′, GAPDH reverse 5 ′-TGGAAGATGGTGATGGGATTT-3 ′, POLR 2A forward 5 ′-GATGGGCAAAAGAGTGGACTT-3′, POLR 2A reverse 5 ′-GGGTACTGACTGTTCCCCCT-3′) and the primer sequence for IL-8 was downloaded from the public RTPrimerDB database (ID: 3079, http://medgen.ugent.be/rtprimerdb/) (IL-8 forward 5 ′-GAACTGAGAGTGATTGAGAGTGGA-3′, IL-8 reverse 5 ′-CTCTTCAAAAACTTCTCCACAACC-3′). cDNA (2 μl of 1:3 dilution) was amplified in a 15 μl reaction containing 0.5 μM of each primer and 7.5 μl of 2× SYBR Green JumpStart Taq ReadyMix (Sigma). PCR product specificity was confirmed by melt curve analysis and agarose gel electrophoresis. The amount of gene product was determined using the comparative quantification method included in the Rotor-Gene 1.7 software. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and RNA polymerase II polypeptide A (POLR2A) were selected as housekeeping genes based on evaluation of six reference genes using geNorm (http://medgen.ugent.be/genorm). The geometric mean of the two housekeepers was used to normalise differences in total cDNA between samples. Fold expression levels of IL-8 mRNA in infected samples were calculated relative to matched NI controls.
IL-8 ELISA
To determine IL-8 protein levels after IVOC, biopsies were washed in cold DPBS and homogenised on ice in lysis buffer (1% Triton X-100, 1 mM PMSF, and 1 μl/200 μl protease inhibitor cocktail (Sigma) in DPBS) using a 21G needle and syringe. After removal of Triton insoluble proteins by centrifugation, IL-8 concentrations in the soluble fractions were determined using a human IL-8 ELISA kit (PeproTech). IL-8 levels were normalised against total tissue protein, quantified by DC protein assay (BioRad).
Quantification of bacterial adherence in pIVOC
Biopsies were removed from Snapwell inserts at the end of the experiment and washed three times in cold PBS to remove mucus and non-adherent bacteria. Tissue was lysed in 0.1% Triton X-100 for 15 min and serial dilutions were plated out on LB agar plates. CFU were determined after overnight incubation at 37°C.
Immunofluorescence staining
IVOC samples were embedded in OCT compound (Sakura), snap-frozen in liquid nitrogen and stored at −70°C until use. 8 μm serial sections were cut with an MTE cryostat (SLEE Technik), picked up on poly-L-lysine-coated slides and air-dried. Tissue sections and polarised T84 monolayers were fixed in formalin for 10 min and blocked/permeabilised with 0.1% Triton X-100, 0.5% BSA, and 2% normal goat serum in PBS for 20 min at room temperature. Samples were incubated with rabbit polyclonal anti-TLR5 (Zymed) overnight at 4°C, washed and incubated in Alexa Fluor 488-conjugated goat anti-rabbit IgG (Molecular Probes) for 30 min. Bacteria and cell nuclei were counterstained with propidium iodide (Sigma) or DAPI (Roche). Actin filaments were labelled with TRITC-conjugated phalloidin (Sigma). Specimens were analysed with a Zeiss LSM 510 Meta confocal laser scanning microscope.
Statistics
Statistical analysis was performed using GraphPad Prism software. Wilcoxon signed rank test was used to determine significant differences between two groups; Friedman test with Dunn’s multiple comparison post test was used for multiple groups. P<0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank R Heuschkel, F Torrente and C Salvestrini (Royal Free Hampstead NHS Trust, London, UK) for endoscopic biopsy samples and SB Fang (UCL Medical School, Royal Free Campus, London, UK) for digital photography. This project was supported by the NIH (Grant R37AI21657 and R01DK58957 to JBK), the Peter Samuel Royal Free Fund (Grant 922 to SS) and by Crohn’s and Colitis in Childhood.
References
- Bai L, Schuller S, Whale A, Mousnier A, Marches O, Wang L, et al. Enteropathogenic Escherichia coli O125:H6 triggers attaching and effacing lesions on human intestinal biopsy specimens independently of Nck and TccP/TccP2. Infect Immun. 2008;76:361–368. doi: 10.1128/IAI.01199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambou JC, Giraud A, Menard S, Begue B, Rakotobe S, Heyman M, et al. In vitro and ex vivo activation of the TLR5 signaling pathway in intestinal epithelial cells by a commensal Escherichia coli strain. J Biol Chem. 2004;279:42984–42992. doi: 10.1074/jbc.M405410200. [DOI] [PubMed] [Google Scholar]
- Berin MC, Darfeuille-Michaud A, Egan LJ, Miyamoto Y, Kagnoff MF. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-kappaB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell Microbiol. 2002;4:635–648. doi: 10.1046/j.1462-5822.2002.00218.x. [DOI] [PubMed] [Google Scholar]
- Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HD, Frankel G. Enteropathogenic Escherichia coli: unravelling pathogenesis. FEMS Microbiol Rev. 2005;29:83–98. doi: 10.1016/j.femsre.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Cleary J, Lai LC, Shaw RK, Straatman-Iwanowska A, Donnenberg MS, Frankel G, et al. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology. 2004;150:527–538. doi: 10.1099/mic.0.26740-0. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterol. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- Erdem AL, Avelino F, Xicohtencatl-Cortes J, Giron JA. Host protein binding and adhesive properties of H6 and H7 flagella of attaching and effacing Escherichia coli. J Bacteriol. 2007;189:7426–7435. doi: 10.1128/JB.00464-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- Giron JA, Torres AG, Freer E, Kaper JB. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol Microbiol. 2002;44:361–379. doi: 10.1046/j.1365-2958.2002.02899.x. [DOI] [PubMed] [Google Scholar]
- Gruenheid S, DeVinney R, Bladt F, Goosney D, Gelkop S, Gish GD, et al. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat Cell Biol. 2001;3:856–859. doi: 10.1038/ncb0901-856. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Heczko U, Carthy CM, O’Brien BA, Finlay BB. Decreased apoptosis in the ileum and ileal Peyer’s patches: a feature after infection with rabbit enteropathogenic Escherichia coli O103. Infect Immun. 2001;69:4580–4589. doi: 10.1128/IAI.69.7.4580-4589.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks S, Candy DC, Phillips AD. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun. 1996;64:4751–4760. doi: 10.1128/iai.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse AE, Yu J, Tall BD, Kaper JB. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny B. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol Microbiol. 1999;31:1229–1241. doi: 10.1046/j.1365-2958.1999.01265.x. [DOI] [PubMed] [Google Scholar]
- Khan MA, Bouzari S, Ma C, Rosenberger CM, Bergstrom KS, Gibson DL, et al. Flagellin-dependent and -independent inflammatory responses following infection by enteropathogenic Escherichia coli (EPEC) and Citrobacter rodentium. Infect Immun. 2008 doi: 10.1128/IAI.01141-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S, Baldwin T, Williams PH, McNeish AS. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S, Lloyd DR, McNeish AS. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987;55:69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM, Nataro JP, Karch H, Baldini MM, Kaper JB, Black RE, et al. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985;152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- Lewis DC, Walker-Smith JA, Phillips AD. Polymorphonuclear neutrophil leucocytes in childhood Crohn’s disease: a morphological study. J Pediatr Gastroenterol Nutr. 1987;6:430–438. doi: 10.1097/00005176-198705000-00021. [DOI] [PubMed] [Google Scholar]
- Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommel S, Benesch S, Rohde M, Wehland J, Rottner K. Enterohaemorrhagic and enteropathogenic Escherichia coli use different mechanisms for actin pedestal formation that converge on N-WASP. Cell Microbiol. 2004;6:243–254. doi: 10.1111/j.1462-5822.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- Maaser C, Heidemann J, von EC, Lugering A, Spahn TW, Binion DG, et al. Human intestinal microvascular endothelial cells express Toll-like receptor 5: a binding partner for bacterial flagellin. J Immunol. 2004;172:5056–5062. doi: 10.4049/jimmunol.172.8.5056. [DOI] [PubMed] [Google Scholar]
- Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Iimura M, Kaper JB, Torres AG, Kagnoff MF. Role of Shiga toxin versus H7 flagellin in enterohaemorrhagic Escherichia coli signalling of human colon epithelium in vivo. Cell Microbiol. 2006;8:869–879. doi: 10.1111/j.1462-5822.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott DJ, McKay DM, Sherman PM, Perdue MH. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:634–645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- Raffatellu M, Chessa D, Wilson RP, Dusold R, Rubino S, Baumler AJ. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect Immun. 2005;73:3367–3374. doi: 10.1128/IAI.73.6.3367-3374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Im E, Riegler M, Kokkotou E, O’brien M, Pothoulakis C. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci U S A. 2005;102:13610–13615. doi: 10.1073/pnas.0502174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum RJ, Partin JC, Saalfield K, McAdams AJ. An ultrastructural study of enteropathogenic Escherichia coli infection in human infants. Ultrastruct Pathol. 1983;4:291–304. doi: 10.3109/01913128309140582. [DOI] [PubMed] [Google Scholar]
- Ruchaud-Sparagano MH, Maresca M, Kenny B. Enteropathogenic Escherichia coli (EPEC) inactivate innate immune responses prior to compromising epithelial barrier function. Cell Microbiol. 2007;9:1909–1921. doi: 10.1111/j.1462-5822.2007.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savkovic SD, Koutsouris A, Hecht G. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect Immun. 1996;64:4480–4487. doi: 10.1128/iai.64.11.4480-4487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Muller-Hermelink HK, et al. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol. 2004;136:521–526. doi: 10.1111/j.1365-2249.2004.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüller S, Chong Y, Lewin J, Kenny B, Frankel G, Phillips AD. Tir phosphorylation and Nck/N-WASP recruitment by enteropathogenic and enterohaemorrhagic Escherichia coli during ex vivo colonization of human intestinal mucosa is different to cell culture models. Cell Microbiol. 2007;9:1352–1364. doi: 10.1111/j.1462-5822.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- Schüller S, Frankel G, Phillips AD. Interaction of Shiga toxin from Escherichia coli with human intestinal epithelial cell lines and explants: Stx2 induces epithelial damage in organ culture. Cell Microbiol. 2004;6:289–301. doi: 10.1046/j.1462-5822.2004.00370.x. [DOI] [PubMed] [Google Scholar]
- Sharma R, Tesfay S, Tomson FL, Kanteti RP, Viswanathan VK, Hecht G. Balance of bacterial pro- and anti-inflammatory mediators dictates net effect of enteropathogenic Escherichia coli on intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G685–G694. doi: 10.1152/ajpgi.00404.2005. [DOI] [PubMed] [Google Scholar]
- Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374–379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- Ulshen MH, Rollo JL. Pathogenesis of Escherichia coli gastroenteritis in man - another mechanism. N Eng J Med. 1980;302:99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zeng H, Lyons S, Carlson A, Merlin D, Neish AS, et al. TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via posttranscriptional mechanism. Am J Physiol Gastrointest Liver Physiol. 2003;285:G282–G290. doi: 10.1152/ajpgi.00503.2002. [DOI] [PubMed] [Google Scholar]
- Zhou X, Giron JA, Torres AG, Crawford JA, Negrete E, Vogel SN, et al. Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in T84 cells. Infect Immun. 2003;71:2120–2129. doi: 10.1128/IAI.71.4.2120-2129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.