Abstract
Objective
Recent data suggest that pretreatment HPV (Human papillomavirus) viral load is useful to predict the severity of intraepithelial lesions of the uterine cervix and formulate a treatment plan. However, the relationship between initial HPV viral load and prognosis of cervical cancer patients has not yet been clearly defined. The objective of this study was to determine whether HPV viral load has prognostic significance in patients with early stage cervical carcinoma treated by surgery.
Methods
A retrospective review of all patients with early stage cervical carcinoma who underwent radical hysterectomy and pelvic lymphadenectomy at our institution from August 2003 to December 2007 was conducted. Patients were included only if they had pretreatment Hybrid Capture II test for HPV DNA detection.
Results
We identified 34 patients who met the inclusion criteria. Two groups were identified: patients who had low HPV viral load (≤100 RLU) versus those who had high viral load (>100 RLU). There were no differences in age, FIGO stage, histology, pathologic risk factors - tumor size, deep stromal invasion, lymph-vascular space invasion, parametrial extensions, vaginal margin involvement, and lymph node metastasis - and adjuvant CCRT. There was no significant difference of disease-free survival regard to pretreatment HPV viral load (p=0.7756).
Conclusion
In our study, survival was not significantly different between early stage cervical cancer patients who had low and high pretreatment HPV viral load. It seems that pretreatment HPV viral load may not be of help to predict disease prognosis.
Keywords: HPV load, Cervical cancer, Prognosis
Introduction
It is well known that some carcinogenic types of human papillomavirus (HPV) infection is associated with the development of cervical cancer and its precursors (cervical intraepithelial neoplasia grade 3, CIN 3).1-3 This infection tend to clear through the host immune system without treatment. Persistent infection with carcinogenic HPV types plays a major role in the development of CIN, and it may particularly be affected with high HPV DNA load as a result of viral replication.4-7
The Hybrid Capture II assay (HC II) detects NA of 13 high-risk HPV types (16,18,31,33,35,39,45,51,52,56,58,59, 68). Previous studies have shown that HPV DNA analysis using semi-quantitative polymerase chain reaction (PCR) may be useful in determining HPV load.8-10 The Food and Drug Administration (FDA) has approved the HC II using a liquid-based cervical smear preparation.
Several studies using HC II have reported that HPV DNA load levels were significantly increased in high-grade cervical lesions (CIN 2 or 3) compared to low-grade lesion11-13 although a few investigators have failed to confirm this relationship.14,15
While the HPV load may be an important predictor of the severity of CIN, the relationship between HPV load and prognosis of patients with cervical carcinoma has not yet been clearly defined. The purpose of this study was to determine whether pretreatment HPV load has prognostic significance in patients with early stage cervical carcinoma treated by surgery.
Materials and Methods
We retrospectively reviewed the medical records of all patients with early stage cervical carcinoma who underwent radical hysterectomy with pelvic lymphadenectomy and para-aortic lymph node sampling at our institution from August 2003 to December 2007. Patients were included only if they had pretreatment HC II for HPV DNA detection, and a total of 34 patients were enrolled in this study.
The Papanicolaou (Pap) smears were taken from exo- and endocervix using a Cytobrush (Digene Cervical Sampler, Digene Corp., Gaithersburg, MD, USA) in all patients. Specimens for HPV DNA testing were stored at -4℃ and sent to the Laboratory Unit for high-risk HPV test using the Hybrid Capture II method.16 Viral load was expressed as relative lights unit (RLU) ratio of specimens. Solutions of high-risk HPV at 10 pg/ml served as positive controls. All RLU measurements of specimens were divided by RLU of appropriate positive controls to provide a ratio. Specimen ratio of ≥1.0 was regarded as positive for HPV DNA, while a ratio <1.0 was regarded as negative.16
We stratified 34 patients positive for HPV DNA into two groups: low HPV load group (≤100 RLU) and high HPV load group (>100 RLU). Two groups were then compared with respect to various clinico-pathological variables - age, histologic types, FIGO stage, tumor size, deep stromal invasion, lymph-vascular space invasion, parametrial invasion, lymph node metastasis, and adjuvant treatment. We also determined the time interval from completion of therapy to recurrence of disease and the relationship between viral loads and recurrence.
Statistical analysis of observed data was performed by utilizing SPSS for Windows (Version 12.0, SPSS Inc, Chicago, Ill, USA). Student T-test and chi-square test were applied for relationship between viral loads and clinical characteristics in two groups. Kaplan-Meier survival curves were compared using the log-rank test for disease-free survival. p values less than 0.05 were considered to be statistically significant.
Results
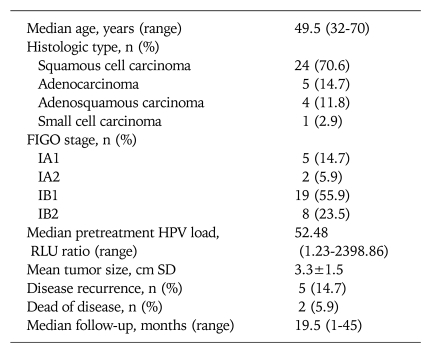
The characteristics of 34 patients enrolled in this study are shown in Table 1. The median age was 49.5 years (range, 32-70). Seven (20.6%) patients had FIGO stage IA and 27 (79.4%) had FIGO stage IB. Histologically, 24 (70.6%) were squamous cell carcinoma, 9 (26.5%) were adenocarcinoma or adenosquamous carcinoma, and 1 (2.9%) was small cell carcinoma. Pretreatment HPV load was 52.48 RLU (range, 1.23-2398.86). The median duration of follow-up for all patients was 19.5 months. Five (14.7%) patients experienced disease recurrence and two (5.9%) patients had died of disease.
Table 1.
Characteristics of 34 patients
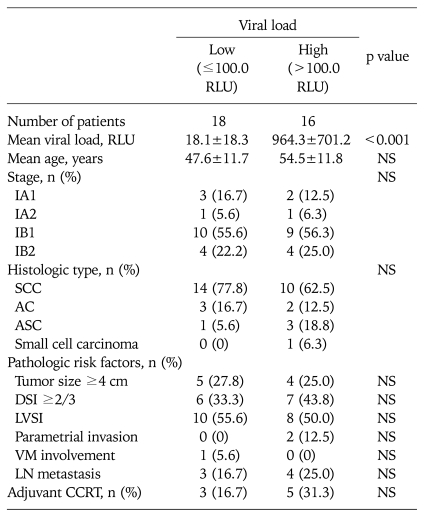
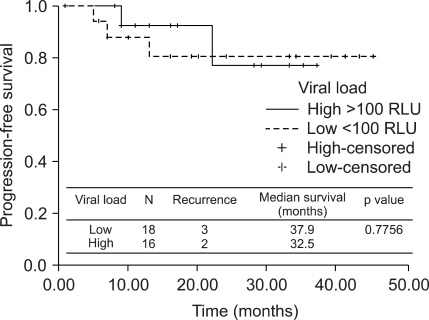
We identified two patient groups according to HPV load: 18 patients with low viral load (≤100 RLU) and 16 with high viral load (>100 RLU). Patients with low viral load had a mean pretreatment HPV load of 18.1 RLU compared with 964.3 RLU for those who showed high viral load (p<0.001). There were no differences among the groups with respect to age, FIGO stage, adjuvant treatment, and pathologic factors such as tumor size, histology, deep stromal invasion, lymph-vascular space invasion, parametrial invasion, vaginal margin involvement, or lymph node metastasis (Table 2). We did not observe any statistically significant difference in disease-free survial between low and high viral load groups (p=0.7756, Fig. 1).
Table 2.
Association between HPV load and clinico-pathologic characteristics
SCC, squamous cell carcinoma; AC, adnocarcinoma; ASC, adenosquamous carcinoma; DSI, deep stromal invasion; LVSI, lymph-vascular space invasion; VM, vaginal margin; LN, lymph node; CCRT, concurrent chemoradiation therapy
Fig. 1.
Disease-free survival according to pretreatment HPV load.
Discussion
In this study, we observed that pretreatment HPV load did not have prognostic significance in patients with early stage cervical carcinoma and high viral load did not reflect unfavorable outcome.
Several molecular methods are used to detect and quantify HPV DNA namely, direct probe methods (southern blot), signal amplification (Hybrid Capture II), and target amplification (polymerase chain reaction, PCR) technology). The direct probe techniques offer the least sensitivity for detecting a specific DNA sequence, whereas the HPV DNA Hybrid Capture II (HC) assay is the only HPV test with Food and Drug Administration (FDA) approval at this time that detects a group of 13 cancers associated HPV types, validates its sensitivity, and shows to be an accurate reproducible test.17-19 The sensitivity of HPV testing by HC II for detection of cervical intraepithelial neoplasia (CIN) II and III varied from 62 to 97%, specificity from 41 to 92% and positive predictive value from 6.7 to 13.7%, and is equivocal to PCR assays.19-24
Several studies have evaluated the association between the HPV load and the severity of the cervical lesion.11-13 Hernández-Hernández et al reported that the higher the viral load of HPV DNA infection observed, the higher the probability of being associated with grade of CIN (p<0.001). In their study, the highest association with high viral load and CIN 2 or 3 was observed (OR=365.8). They concluded that a clear association between viral load of HPV DNA was determined by HC II assay and CIN grade.12 Park et al13 reported similar results. They reviewed a total of 259 women who had CIN confirmed by cervical colposcopy-directed biopsy or conization, and showed the odds ratio of the development of CIN 2-3 was 9.0-9.6 in patients with high viral load. Recently, Park et al showed that patients with a pre-conization high-risk HPV load ≥100 RLU had a higher rate of persistent HPV infection and recurrence compared with HPV load <100 RLU.25 These findings support the fact that high HPV load is associated with high grade CIN (CIN 2 or 3) and the use of HPV test as post-treatment surveillance tool is feasible in CIN.
However, to date, there have been few studies that have attempted to evaluate the prognostic impact of HPV load in patients with cervical cancer and the information available is limited. Riou et al reviewed 106 early-stage cervical cancer patients, and they showed that patients with no detectable HPV DNA had a higher risk of disease recurrence (p<0.05) and distant metastasis (p<0.01).26 Niloy et al reported a pilot study on the association between pretreatment HPV titer and survial. They reviewed 21 patients with cervical cancer who were treated by radiation therapy alone. They showed that those with higher pretreatment HPV titiers (>1,000 RLU) had a higher complete response (p=0.022) and better survival (DFS, p=0.005; OS, p=0.012), and concluded that higher pretreatement HPV titers could be considered as a predictor of radiation therapy response and survival in cervical cancer.27
As mentioned above, the prognostic significance of pretreatment HPV load in cervical cancer is still controversial. We analyzed the clinical outcomes of 34 patients with early stage cervical cancer who were treated by radical hysterectomy with pelvic/para-aortic lymphadenectomy. This study demonstrates that pretreatment HPV load did not correlate with pathologic risk factors. The survival analysis did not show any difference in disease-free survival between patients with high and low pretreatment HPV load. Our results thus differs from those of previous studies. It may be presumed that once malignant transformation is established, biologic characters of the tumor itself, such as size or grade, play a pivotal role in determining prognosis, rather than viral load. A potential limitation of our study is the small number of cases and selection bias, which is inherent in any retrospective study.
In summary, our data suggest that pretreatment HPV load is not associated with prognosis in patients with cervical cancer. However, the small number of patients in this study limits our power to demonstrate this. It seems that only a large-scale prospective study may provide clearer answers.
References
- 1.Schiffman MH, Bauer HM, Hoover RN, Glass AG, Cadell DM, Rush BB, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993;85:958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 3.Schlecht NF, Kulaga S, Robitaille J, Ferreira S, Santos M, Miyamura RA, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2001;286:3106–3114. doi: 10.1001/jama.286.24.3106. [DOI] [PubMed] [Google Scholar]
- 4.Londesborough P, Ho L, Terry G, Cuzick J, Wheeler C, Singer A. Human papillomavirus genotypes as a predictor of persistence and development of high-grade lesions in women with minor cervical abnormalities. Int J Cancer. 1996;69:364–368. doi: 10.1002/(SICI)1097-0215(19961021)69:5<364::AID-IJC2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Remmink AJ, Walboomers JM, Helmerhorst TJ, Voorhorst FJ, Rozendaal L, Risse EK, et al. The presence of persistent high-risk HPV genotypes in dysplastic cervical lesions is associated with progressive disease: Natural history up to 36 months. Int J Cancer. 1995;61:306–311. doi: 10.1002/ijc.2910610305. [DOI] [PubMed] [Google Scholar]
- 6.Ho GY, Burk RD, Klein S, Kadish AS, Chang CJ, Palan P, et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365–1371. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- 7.Ho GY, Palan PR, Basu J, Romney SL, Kadish AS, Mikhail M, et al. Viral characteristics of human papillomavirus infection and antioxidant levels as risk factors for cervical dysplasia. Int J Cancer. 1998;78:594–599. doi: 10.1002/(sici)1097-0215(19981123)78:5<594::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Terry G, Ho L, Hollingworth T, Anderson M. Human papillomavirus type 16 in cervical smears as predictor of high-grade cervical intraepithelial neoplasia. Lancet. 1992;339:959–960. doi: 10.1016/0140-6736(92)91532-d. [DOI] [PubMed] [Google Scholar]
- 9.Bavin PJ, Giles JA, Deery A, Crow J, Griffiths PD, Emery VC, et al. Use of semi-quantitative PCR for human papillomavirus DNA type 16 to identify women with high-grade cervical disease in a population presenting with a mild dyskaryotic smear report. Br J Cancer. 1993;67:602–605. doi: 10.1038/bjc.1993.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuzick J, Terry G, Ho L, Hollingworth T, Anderson M. Type-specific human papillomavirus DNA in abnormal scrapings as a predictor of high-grade cervical intraepithelial neoplasia. Br J Cancer. 1994;69:167–171. doi: 10.1038/bjc.1994.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalstein V, Riethmuller D, Prétet JL, Le Bail Carval K, Sautière JL, Carbillet JP, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: A longitudinal French cohort study. Int J Cancer. 2003;106:396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 12.Hernández-Hernández DM, Ornelas-Bernal L, Guido-Jiménez M, Apresa-Garcia T, Alvarado-Cabrero I, Salcedo-Vargas M, et al. Association between high-risk human papillomavirus DNA load and precursor lesions of cervical cancer in Mexican women. Gynecol Oncol. 2003;90:310–317. doi: 10.1016/s0090-8258(03)00320-2. [DOI] [PubMed] [Google Scholar]
- 13.Park JS, Ryu HS, Chang SJ, Kim YM, Chang KH, Lee JP. The association of the cervical intraepithelial neoplasia and human papillomavirus viral Load. Korean J Obstet Gynecol. 2005;48:2888–2895. [Google Scholar]
- 14.Farthing A, Masterson P, Mason WP, Vousden KH. Human papillomavirus detection by hybrid capture and its possible clinical use. J Clin Pathol. 1994;47:649–652. doi: 10.1136/jcp.47.7.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun XW, Ferenczy A, Johnson D, Koulos JP, Lungu O, Richard RM, et al. Evaluation of the Hybrid Capture human papillomavirus deoxyribonucleic acid detection test. Am J Obstet Gynecol. 1995;173:1432–1437. doi: 10.1016/0002-9378(95)90629-0. [DOI] [PubMed] [Google Scholar]
- 16.Sailors J, Gander R, Saboorian MH, Berkley P, Foster B, Ashfaq R. Stability of PreservCyt for Hybrid Capture (HC II) HPV test. Diagn Cytopathol. 2005;32:260–263. doi: 10.1002/dc.20232. [DOI] [PubMed] [Google Scholar]
- 17.Hubbard RA. Human Papillomavirus testing methods. Arch Pathol Lab Med. 2003;127:940–945. doi: 10.5858/2003-127-940-HPTM. [DOI] [PubMed] [Google Scholar]
- 18.Bolick DR, Bolick RE, Coates F, Daniels CM, Juretich MB, Lin KK, et al. Laboratory implementation of human papillomavirus testing. Arch Pathol Lab Med. 2003;127:984–990. doi: 10.5858/2003-127-984-LIOHPT. [DOI] [PubMed] [Google Scholar]
- 19.Castle PE, Lorincz AT, Mielzynska-Lohnas I, Scott DR, Glass AG, Sherman ME, et al. Results of human papillomavirus DNA testing with the hybrid capture 2 assay are reproducible. J Clin Microbiol. 2002;40:1088–1090. doi: 10.1128/JCM.40.3.1088-1090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belinson J, Qiao YL, Pretorius R, Zhang WH, Elson P, Li L, et al. Shanxi province cervical cancer screening study: A cross sectional comparativetrial of multiple techniques to detect cervical neoplasia. Gynecol Oncol. 2001;83:439–444. doi: 10.1006/gyno.2001.6370. [DOI] [PubMed] [Google Scholar]
- 21.Wright TC, Jr, Denny L, Kuhn L, Pollack A, Lorincz A. HPV DNA testing of self collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA. 2000;283:81–86. doi: 10.1001/jama.283.1.81. [DOI] [PubMed] [Google Scholar]
- 22.Schiffman M, Herrero R, Hildesheim A, Sherman ME, Bratti M, Wacholder S, et al. HPV DNA testing in cervical cancer screening: Results from women in a high risk province of Costa Rica. JAMA. 2000;283:87–93. doi: 10.1001/jama.283.1.87. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn L, Denny L, Pollack A, Lorincz A, Richart RM, Wright TC, et al. Human papillomavirus DNA testing for cervical cancer screening in low resource settings. J Natl Cancer Inst. 2000;92:818–825. doi: 10.1093/jnci/92.10.818. [DOI] [PubMed] [Google Scholar]
- 24.Bozzetti M, Nonnenmacher B, Mielzinska II, Villa L, Lorincz A, Breitenbach VV, et al. Comparison between hybrid capture II and polymerase chain reaction results among women at low risk for cervical cancer. Ann Epidemiol. 2000;10:466. doi: 10.1016/s1047-2797(00)00147-2. [DOI] [PubMed] [Google Scholar]
- 25.Park JY, Lee KH, Dong SM, Kang S, Park SY, Seo SS. The association of pre-conization high-risk HPV load and the persistence of HPV infection and persistence/recurrence of cervical intraepithelial neoplasia after conization. Gynecol Oncol. 2008;108:549–554. doi: 10.1016/j.ygyno.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Riou G, Favre M, Jeannel D, Bourhis J, Le Doussal V, Ortho G. Association between poor prognosis in early stage invasive cervical carcinoma and non-detection of HPV DNA. Lancet. 1990;335:1171–1174. doi: 10.1016/0140-6736(90)92693-c. [DOI] [PubMed] [Google Scholar]
- 27.Datta NR, Kumar P, Singh S, Gupta D, Srivastava A, Dhole TN. Does pretreatment human papillomavirus titers predict radiation response and survival outcomes in cancer cervix? Gynecol Oncol. 2006;103:100–105. doi: 10.1016/j.ygyno.2006.01.058. [DOI] [PubMed] [Google Scholar]