Abstract
Objective
The abnormal expression of fragile histidine triad (FHIT) gene has been frequently reported in a variety of epithelial malignancies including cervical carcinoma. Furthermore, in a recent study it was proposed that transcriptional inactivation of FHIT, as a consequence of aberrant 5'-CpG island methylation, plays an important role in the carcinogenesis of human cervical carcinoma. The authors sought to determine whether abnormal FHIT transcription occurs in human cervical carcinoma, and if so, whether this abnormal expression is associated with aberrant 5'-CpG island methylation. In addition, the clinical significance of FHIT inactivation was investigated in Korean women with cervical cancer.
Methods
To examine for abnormal transcripts of the FHIT gene, quantitative RT-PCR, genomic DNA-PCR and nonisotopic RT-PCR-SSCP analysis were performed using the standard method. The methylation status was determined by methylation specific PCR and bisulfite DNA sequencing.
Results
The FHIT gene was down-regulated in 15 of 58 (25.9%) cervical carcinomas. FHIT promoter hypermethylation was detected in 15 of 15 (100%) abnormally expression in cervical carcinomas. Bisulfite DNA sequencing confirmed these findings and a significant correlation was found between CpG site hypermethylation and low FHIT expression. However, no significant correlation was found between reduced FHIT expression and clinicopathological characteristics.
Conclusion
In this study, FHIT inactivation in cervical cancer was found to be strongly correlated with 5'-CpG island hypermethylation rather than a genetic alteration. Furthermore, no significant relation was found between a lack of FHIT expression and the prognostic factors of cervical cancer in our Korean cohort.
Keywords: Cervical carcinoma, FHIT gene, Hypermethylation, Bisulfite-DNA sequencing
INTRODUCTION
Worldwide, cervical cancer is the leading gynecological malignancy, and in 2002 was found to be the 5th most common malignant disease in Korean women.1 It is well known that human papilloma virus (HPV) is the major causative agent,2 though many patients infected with HPV remain stable. Thus, various other epigenetic events, such as CpG island hypermethylation, are likely to be involved in the genesis of cervical cancer. Furthermore, the hypermethylation of cytosine in normally unmethylated CpG-rich sequences, called CpG islands, is a frequent epigenetic event in cancers of the breast, prostate, liver, thyroid, oral squamous cell carcinoma, and others.3-5
The short arm of human chromosome 3 commonly contains chromosomal abnormalities in cancer patients,6 and chromosomal rearrangements are frequently observed at 3p25, 3p21.3, 3p14.2 and 3p12.7 In particular, the 3p14.2 region is of interest because it contains FRA3B, the most active fragile site in the human genome, which contains the fragile histidine triad (FHIT) gene. Furthermore, FHIT has been identified as a candidate tumor suppressor gene,8 and although its effect on the cell cycle is unclear, it has been reported that reexpression of FHIT in a variety of human cell lines results in growth inhibition and apoptosis induction.9 5'-CpG island methylation of FHIT has been documented in prostate, breast, esophageal, and lung carcinomas, which suggests that it participates in tumorigenesis.10-13 In particular, FHIT was found to be hypermethylated in 40-50% of cervical cancer tissue.14 FHIT abnormalities include loss of heterozygosity, homozygous deletions, and the insertions of other sequences. However, point mutations in FHIT are rare and some homozygous deletions may only affect introns.15,16
In this study, we aimed to elucidate the mechanism that underlies the loss of FHIT expression in cervical carcinoma by measuring 5'-CpG island DNA methylation status in FHIT, and by investigating the nature of the relationship between FHIT hypermethylation and clinicopathological characteristics
MATERIALS AND METHODS
1. Samples and clinical data
Fifty eight primary cervical carcinomas and seven normal cervical epithelial samples were surgically collected during the period 1996-2002 at Kyung Hee University Hospital (Seoul). All normal cervical specimens were obtained from patients with no evidence of cancer during surgery for benign gynecologic conditions. Tissue specimens were snap-frozen immediately in liquid N2 and stored at -70℃ until required. Total cellular RNA was extracted using the single step method and genomic DNA was later extracted from the same cells. Clinical information was retrospectively obtained from medical records, and included patient age, tumor stage, date of diagnosis, histologic subtype, pathologic results, and follow-up data.
Tumor stages were assigned according to the International Federation of Gynecology and Obstetrics (FIGO) staging system for cervical cancer. The clinicopathological characteristics of samples are given in Table 1. Four of the tumors were stage IA, 37 stage IB, and 17 stage II. Histologically, 50 of the 58 tumors were squamous cell carcinomas, 4 were adenocarcinomas, and 4 were adenosquamous carcinomas.
Table 1.
Association between abnormal FHIT mRNA expression and clinicopathologic parameters
*LN; lymph node, †LVS; lymphovascular space
2. Quantitative RT-PCR
One microliter of extracted total cellular RNA was converted to cDNA by reverse transcription using random hexamer primers and Moloney murine leukemia virus reverse transcriptase (Life Technologies, Inc., Gaithersburg, MD), 1 : 4 diluted cDNA (12.5 ng/50µl of PCR reaction mix) achieved the logarithmic amplification phase after 24-36 cycles using primers for FHIT (sense: 5'-GAGAAATCCACTGAGAACAGTC-3' (Exon 2), antisense: 5'-ATCAGGACGCAGGTCATGGAAG-3 (Exon 6), PCR product length : 354 bp), and for the GAPDH endogenous standard. Optimal PCR cycling conditions involved 26-32 amplification cycles at 95℃ for 1 min (denaturation), 58-64℃ for 0.5 min (annealing), and 72℃ for 1 min (extension) in a reaction buffer containing 1.5 mM MgCl2 (PCR buffer II, Perkin-Elmer Corp., Norwalk, CT).
3. Quantitative genomic PCR analysis
Genomic DNA was extracted from the same tissue samples after RNA extraction. 200 ng of genomic DNA was to the FHIT amplification using an intron-specific primer pair (Sense: 5'-TCAACTCTCTGGAGTTCAGTGG-3' (Intron 5), Antisense: 5'-GGACAGTAGGGTTGCCCTGCAT-3' (Intron 6), PCR product length : 470 bp).
RT-PCR products were confirmed by Southern hybridization, 10µl of RT- and genomic PCR products were resolved on 2% agarose gels. Quantitation was achieved by densitometric scanning of ethidium bromide stained gels. Absolute area integrations of the curves representing each specimens were compared after adjusting for GAPDH, an endogenous expression standard gene. Integration and analysis were performed using the Molecular Analyst software program (Bio-Rad, Hercules, CA). Quantitative PCR was repeated at least three times per specimen and mean were calculated.
4. Non-isotopic RT-PCR-SSCP analysis
To screen for the presence of somatic mutations in FHIT, RT-PCR-SSCP was performed. The FHIT transcript was amplified with seven sets of primers designed to cover the entire coding region of the gene (sequences of the primers used are available on request). PCR products of more than 300 bp were digested with endonucleases to increase the sensitivity of SSCP. PCR products (20µl) were mixed with 10µl of 0.5 N NaOH, 10 mM EDTA, and 15µl of denaturing loading buffer (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, and 0.05% xylene cyanol). After heating at 95℃ for 5 min, samples were loaded into wells pre-cooled to 4℃ and run using 8% non-denaturating acrylamide gels containing 10% glycerol at 4-8℃ and 18-22℃.
5. Bisulfite DNA sequencing analysis
Genomic DNA (1µg) in a volume of 50µl was denatured with NaOH (final concentration 0.3 M), and 30µl of 10 mM hydroquinone and 520µl of 3M sodium bisulfite (pH 5.0) were added and incubated at 55℃ for 16-20 h. DNA samples were purified using the Wizard DNA clean-up system (Promega Corp., Madison, WI), again treated with NaOH at 37℃ for 15 min, precipitated with ethanol, and resuspended in distilled water. Bisulfite-modified DNA (50 ng) was then subjected to PCR amplification of the FHIT intron 1 region using the following primer sets; FHIT-MS1 (sense; 5'-GGAGGTAAGTTTAAGTGGAA-3') and FHIT-MS2 (antisense; 5'-CCCACCCTAAAACCTCTTTT-3'). The PCR products obtained were cloned into pCRII vectors (Invitrogen, Carlsbad, CA) and 5 clones of each specimen were automatically fluorescence-based DNA sequenced to determine methylation status.
6. Methylation-specific PCR analysis
PCR was performed with the methylation-specific primers FHIT-MS-1 (sense, 5'-TGGGGCGCGGGTTTGGGTTTTTACGC-3') and FHIT-MS-2 (antisense, 5'-CGTAAACGACGCCGACCCCACTA-3') and the unmethylation-specific primers FHIT-UMS-1 (sense, 5'-TTGGGGTGTGGGTTTGGGTTTTTATG-3') and FHIT-UMS-2 (antisense, 5'-CATAAACAACACCAACCCCACTA-3') using 200 ng of the bisulfite-modified genomic DNA as a template for 38 cycles of 95℃ for 1 min, 60-63℃ for 1 min, and 72℃ for 1 min. PCR products (10µl) were then resolved on 2% agarose gels.
RESULTS
1. Loss of FHIT mRNA expression in cervical carcinoma
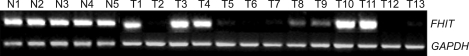
The mRNA expression of FHIT was examined in 58 human cervical carcinomas and 7 noncancerous cervical tissues. As shown in Fig. 1, FHIT transcripts were easily detected in noncancerous cervical tissue samples, at levels of 0.72-0.92 (mean; 0.82±0.07). In contrast, the mRNA expression statuses of FHIT in cervical carcinomas were significantly lower (range; 0.18-0.92, mean; 0.58±0.22, p-value<0.05). Levels of less than half the normal mean (i.e., <0.41) were defined as abnormally low. Accordingly, 25.9% (15 of 58) of primary tumors were deemed to express FHIT at abnormally low levels. Nevertheless, FHIT expression showed no statistical correlation with clinicopathologic parameters as previously described17 (Table 1).
Fig. 1.
Reduced expression of FHIT mRNA in human cervical cancer tissues (quantitative RT-PCR analysis). N; normal cervix tissue, T; cervical cancer tissue.
2. Absence of allelic deletion of the FHIT gene in cervical carcinoma
To elicit whether loss of or reduced FHIT mRNA expression in cervical carcinomas is associated with the allelic deletion of FHIT, we examined FHIT levels using quantitative genomic PCR. None of the tumors, including the 15 with abnormally low expression, showed a detectable reduction in FHIT level, which suggests that genomic deletion of FHIT is infrequent and not associated with the abnormal downregulation of FHIT mRNA in human cervical carcinoma.
3. Tumor-specific downregulation of FHIT by the hypermethylation of promoter CpG sites
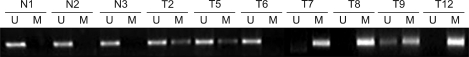
Methylation-specific PCR (MSP) of FHIT promoter sequences was used to determine the overall frequency of FHIT hypermethylation in tumors. Methylation-specific primers (FHIT-MS-1 and FHIT-MS-2) and unmethylation-specific primers (FHIT-UMS-1 and FHIT-UMS-2) were designed, and methylation was detected in all 15 tumor cell lines with no or diminished FHIT expression. Moreover, it was not found in any of the seven noncancerous cell lines that expressed FHIT at normal levels (Fig. 2).
Fig. 2.
FHIT 5'-CpG island hypermethylation in human cervical cancer (methylation-specific PCR analysis). N; normal cervix tissue, T; cervical cancer tissue, U; unmethylation-specific PCR, M; methylation-specific PCR.
4. Aberrant hypermethylation at CpG sites in FHIT promoter
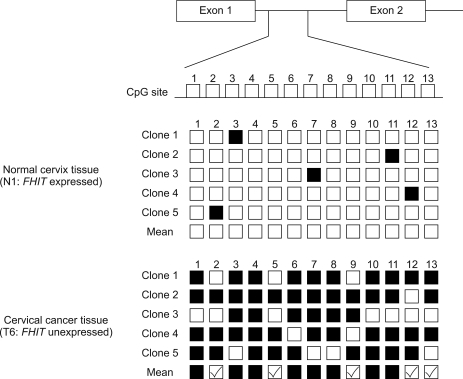
To explore the relationship between aberrant CpG methylation and gene silencing, we performed bisulfite DNA sequencing analysis of FHIT promoter and analyzed the methylation status 13 CpGs located in its 5' proximal region. The sequence (intron1) spanning these 13 CpG sites was amplified by PCR using sodium bisulfite-modified DNA as a template, and 5 PCR clones were sequenced to determine methylation frequencies at individual CpG sites (4-5 clones; complete methylation, 2-3 clones; partial methylation, 0-1 clones; unmethylation) (Fig. 3).
Fig. 3.
FHIT 5'-CpG island hypermethylation in human cervical cancer (sodium bisulfite DNA sequencing analysis). PCR products were cloned, and 5 plasmid clones were sequenced (■ methylated CpG; □ unmethylated CpG). methylation status of 13 CpGs in FHIT promoter. mean methylation status was determined from the number of alleles containing a methylated CpG at each position (■ complete methylation; 4-5 clones, partial methylation; 2-3 clones, □ unmethylation; 0-1 clone).
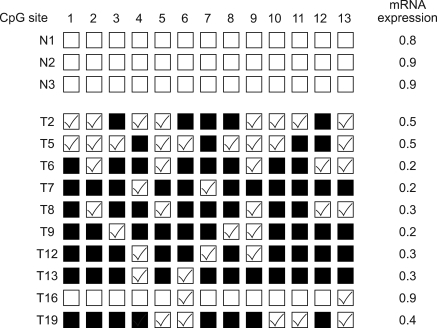
A tight correlation was observed between gene silencing and methylation of the 13 CpGs. All 13 CpGs were partially or completely methylated in the 15 carcinoma tissues showing abnormal expression, whereas none of the CpGs were methylated in the 7 normal cervical tissues. Furthermore, in carcinoma tissues showing normal expression, these 13 CpGs were partially methylated or non-methylated. In addition, completely methylated CpG sites were more frequently observed when mRNA expressions were lowest (Fig. 4), which indicated that methylation extent correlates with mRNA expressional status.
Fig. 4.
Methylation status of the FHIT CpG Island in cervical cancer (correlation between CpG site hypermethylation with low FHIT expression). N; normal cervical tissue, T; cervical cancer tissue.
5. Absence of FHIT mutations in cervical cancer
RT-PCR-SSCP was performed on all 58 primary carcinomas to evaluate the mutational statuses of FHIT. The entire coding regions of FHIT transcripts were amplified using seven sets of exon-specific primers. To improve mutation detection sensitivity, the same RT-PCR products were digested using a different restriction endonucleases and SSCP was performed using two different running conditions. However, no mutations leading to an amino acid substitutions or frameshift were found in the FHIT transcripts expressed, thus indicating that somatic mutations of FHIT are infrequent in cervical cancer.
DISCUSSION
Promoter methylation is the primary epigenetic alteration found in the human genome, and is believed to play an important role in tumorigenesis. It has been well demonstrated that hypermethylations of CpG-rich promoter or exonic regions are strongly associated with transcriptional silencing, and that CpG islands are more methylated in cancerous tissues than in non-CpG island regions. Furthermore, hypermethylation at CpG islands in transcription regulatory regions are known to lead to the epigenetic inactivations of tumor suppressor genes during tumorigenesis in man.18
FHIT is a tissue-specific tumor suppressor gene,19 and its inactivity correlates with the occurrence and development of cancer, especially epithelial cancer.8,19 In addition to genetic alterations, promoter methylation of CpG islands has been reported to silence FHIT.20 In previous reports, we suggested that FHIT is inactivated in cervical carcinoma by epigenetic (promoter methylation) alterations rather than genetic mutations (deletions or chromosomal rearrangements).17,21
To determine the reason for diminished FHIT expression in cervical carcinoma, we investigated the methylation status of the FHIT 5'-CpG island using methylation-specific PCR and bisulfate DNA sequencing. Several studies have demonstrated that only the region situated between nucleotides 195 and 283 (GenBank, Accession No. U76263) of the 5'-CpG island of FHIT is highly methylated in primary tumors.12,22 Therefore, we used primers specific for methylated and unmethylated DNA to amplify the FHIT 5'-CpG island region using methylation-specific PCR.
To investigate the connection between hypermethylation of the FHIT 5'-CpG Island and loss of FHIT expression we analyzed levels of FHIT expression in selected samples with methylated and unmethylated FHIT 5'-CpG islands. High levels of FHIT expression were observed in all normal tissues and in tumor tissues with a unmethylated FHIT 5'-CpG island. Conversely, FHIT expression was significantly depressed in all tumors with a hypermethylated FHIT 5'-CpG Island. These findings demonstrate that FHIT 5'-CpG island hypermethylation and reduced FHIT expression are related. Furthermore, our findings suggest that FHIT 5'-CpG island hypermethylation is an important mechanism of FHIT inactivation in cervical carcinoma.
In the present study, FHIT expression was downregulated in 25.9% (15/58) of invasive cervical carcinomas, and promoter hypermethylation was detected in all 15 cervical carcinomas showing abnormally low FHIT expression. Whereas other studies have reported FHIT downregulation in 43% to 71% of cervical carcinomas.23,24
Several studies which examined different tumor types have shown that FHIT expressional loss and FHIT 5'-CpG island hypermethylation are associated with an advanced tumor stage, poorer overall survival and prognosis, and with tumor recurrence.25-29 A study of cervical cancer revealed that low FHIT expression is associated with a poorer prognosis in advanced cervical carcinoma,30,31 and another study on cervical carcinoma found that FHIT downregulation is correlated with lymph node metastasis and tumor invasion.32 As cancer stage and histologic grade increases, so does the rate of methylation. By using this as a potential diagnostic and treatment tool, cervical cancer may be detected more readily and treated more efficiently.33
In the present study, we were not able to determine whether a statistically significant correlation exists between FHIT 5'-CpG island methylation status and tumor stage or tumor histological grade, because of the size and make-up of our patient group (no precancerous lesions were included). A more detailed study on a cohort with a broader disease spectrum is required to resolve this issue. Although we did not find any significant correlation between FHIT methylation status and clinicopathological characteristics of the cervical carcinoma patients, a perfect correlation was found between FHIT mRNA expression and hypermethylation status. Assays for hypermethylation status is representative biomarker of FHIT expression. However, we suggest caution in its use as a functionally relevant biomarker for cervical carcinoma.
In summary, our results shown that all tumors found to have a methylated FHIT 5'-CpG island showed a reduction in FHIT expression, and that FHIT expression levels were normal in tumors with a unmethylated FHIT 5'-CpG island and in normal tissues. These results suggest that FHIT 5'-CpG island hypermethylation underlies FHIT inactivation in cervical carcinoma.
References
- 1.Shin HR, Jung KW, Won YJ, Park JG. 2002 Annual Report of the Korea Central Cancer Registry: Based on registered data from 139 hospitals. Cancer Res Treat. 2004;36:103–114. doi: 10.4143/crt.2004.36.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waggoner SE. Cervical cancer. Lancet. 2003;361:2217–2225. doi: 10.1016/S0140-6736(03)13778-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, Lipkina G, Song Q, Kramer RH. Promoter methylation regulates cadherin switching in squamous cell carcinoma. Biochem Biophys Res Commun. 2004;315:850–856. doi: 10.1016/j.bbrc.2004.01.143. [DOI] [PubMed] [Google Scholar]
- 4.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: A fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- 5.Costello JF, Fruhwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, et al. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nat Genet. 2000;24:132–138. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- 6.Naylor SL, Johnson BE, Minna JD, Sakaguchi AY. Loss of heterozygosity of chromosome 3p markers in small-cell lung cancer. Nature. 1987;329:451–454. doi: 10.1038/329451a0. [DOI] [PubMed] [Google Scholar]
- 7.Hibi K, Takahashi T, Yamakawa K, Ueda R, Sekido Y, Ariyoshi Y, et al. Three distinct regions involved in 3p deletion in human lung cancer. Oncogene. 1992;7:445–449. [PubMed] [Google Scholar]
- 8.Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, et al. The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t (3;8) breakpoint, is abnormal in digestive tract cancers. Cell. 1996;84:587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- 9.Dumon KR, Ishii H, Vecchione A, Trapasso F, Baldassarre G, Chakrani F, et al. Fragile histidine triad expression delays tumor development and induces apoptosis in human pancreatic cancer. Cancer Res. 2001;61:4827–4836. [PubMed] [Google Scholar]
- 10.Maruyama R, Toyooka S, Toyooka KO, Virmani AK, Zochbauer-Muller S, Farinas AJ, et al. Aberrant promoter methylation profile of prostate cancers and its relationship to clinicopathological features. Clin Cancer Res. 2002;8:514–519. [PubMed] [Google Scholar]
- 11.Zochbauer-Muller S, Fong KM, Maitra A, Lam S, Geradts J, Ashfaq R, et al. 5' CpG island methylation of the FHIT gene is correlated with loss of gene expression in lung and breast cancer. Cancer Res. 2001;61:3581–3585. [PubMed] [Google Scholar]
- 12.Tanaka H, Shimada Y, Harada H, Shinoda M, Hatooka S, Imamura M, et al. Methylation of the 5' CpG island of the FHIT gene is closely associated with transcriptional inactivation in esophageal squamous cell carcinomas. Cancer Res. 1998;58:3429–3434. [PubMed] [Google Scholar]
- 13.Pekarsky Y, Palamarchuk A, Huebner K, Croce CM. FHIT as tumor suppressor: mechanisms and therapeutic opportunities. Cancer Biol Ther. 2002;1:232–236. doi: 10.4161/cbt.73. [DOI] [PubMed] [Google Scholar]
- 14.Shi HR, Wu QH, Suo ZH, Nesland JM. Correlation between methylation of 5'-CpG islands and inactivation of FHIT gene in cervical cancer. Ai Zheng. 2005;24:7–11. [PubMed] [Google Scholar]
- 15.Helland A, Kraggerud SM, Kristensen GB, Holm R, Abeler VM, Huebner K, et al. Primary cervical carcinomas show 2 common regions of deletion at 3P, 1 within the FHIT gene: Evaluation of allelic imbalance at FHIT, RB1 and TP53 in relation to survival. Int J Cancer. 2000;88:217–222. doi: 10.1002/1097-0215(20001015)88:2<217::aid-ijc11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Chu TY, Shen CY, Chiou YS, Lu JJ, Perng CL, Yu MS, et al. HPV-associated cervical cancers show frequent allelic loss at 3p14 but no apparent aberration of FHIT mRNA. Int J Cancer. 1998;75:199–204. doi: 10.1002/(sici)1097-0215(19980119)75:2<199::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Tong SY, Lee SK, Chi SG. Abnormal fragile histidine triad (FHIT) expression in cervical carcinomas. Korean J Obstet Gynecol. 2004;47:1093–1099. [Google Scholar]
- 18.Ehrlich M, Jiang G, Fiala E, Dome JS, Yu MC, Long TI, et al. Hypomethylation and hypermethylation of DNA in Wilms tumors. Oncogene. 2002;21:6694–6702. doi: 10.1038/sj.onc.1205890. [DOI] [PubMed] [Google Scholar]
- 19.Barnes LD, Garrison PN, Siprashvili Z, Guranowski A, Robinson AK, Ingram SW, et al. FHIT, a putative tumor suppressor in humans, is a dinucleoside 5',5'''-P1,P3-triphosphate hydrolase. Biochemistry. 1996;35:11529–11535. doi: 10.1021/bi961415t. [DOI] [PubMed] [Google Scholar]
- 20.Velickovic M, Delahunt B, Grebe SK. Loss of heterozygosity at 3p14.2 in clear cell renal cell carcinoma is an early event and is highly localized to the FHIT gene locus. Cancer Res. 1999;59:1323–1326. [PubMed] [Google Scholar]
- 21.Choi CH, Lee KM, Choi JJ, Kim TJ, Kim WY, Lee JW, et al. Hypermethylation and loss of heterozygosity of tumor suppressor genes on chromosome 3p in cervical cancer. Cancer Lett. 2007;255:26–33. doi: 10.1016/j.canlet.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birrer MJ, Hendricks D, Farley J, Sundborg MJ, Bonome T, Walts MJ, et al. Abnormal FHIT expression in malignant and premalignant lesions of the cervix. Cancer Res. 1999;59:5270–5274. [PubMed] [Google Scholar]
- 24.Nakagawa S, Yoshikawa H, Kimura M, Kawana K, Matsumoto K, Onda T, et al. A possible involvement of aberrant expression of the FHIT gene in the carcinogenesis of squamous cell carcinoma of the uterine cervix. Br J Cancer. 1999;79:589–594. doi: 10.1038/sj.bjc.6690093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capuzzi D, Santoro E, Hauck WW, Kovatich AJ, Rosato FE, Baffa R, et al. FHIT expression in gastric adenocarcinoma: Correlation with disease stage and survival. Cancer. 2000;88:24–34. [PubMed] [Google Scholar]
- 26.Lee JI, Soria JC, Hassan K, Liu D, Tang X, El-Naggar A, et al. Loss of FHIT expression is a predictor of poor outcome in tongue cancer. Cancer Res. 2001;61:837–841. [PubMed] [Google Scholar]
- 27.Lee EJ, Lee BB, Kim JW, Shim YM, Hoseok I, Han J, et al. Aberrant methylation of Fragile Histidine Triad gene is associated with poor prognosis in early stage esophageal squamous cell carcinoma. Eur J Cancer. 2006;42:972–980. doi: 10.1016/j.ejca.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Guerin LA, Hoffman HT, Zimmerman MB, Robinson RA. Decreased fragile histidine triad gene protein expression is associated with worse prognosis in oral squamous carcinoma. Arch Pathol Lab Med. 2006;130:158–164. doi: 10.5858/2006-130-158-DFHTGP. [DOI] [PubMed] [Google Scholar]
- 29.Toledo G, Sola JJ, Lozano MD, Soria E, Pardo J. Loss of FHIT protein expression is related to high proliferation, low apoptosis and worse prognosis in non-small-cell lung cancer. Mod Pathol. 2004;17:440–448. doi: 10.1038/modpathol.3800081. [DOI] [PubMed] [Google Scholar]
- 30.Krivak TC, McBroom JW, Seidman J, Venzon D, Crothers B, MacKoul PJ, et al. Abnormal fragile histidine triad (FHIT) expression in advanced cervical carcinoma: a poor prognostic factor. Cancer Res. 2001;61:4382–4385. [PubMed] [Google Scholar]
- 31.Yoon SO. Abnormal fragile histidine triad (FHIT) expression in invasive cervical adenocarcinoma: Association with tumor aggressiveness. Hum Pathol. 2007;38:326–331. doi: 10.1016/j.humpath.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Huang LW, Chao SL, Chen TJ. Reduced FHIT expression in cervical carcinoma: Correlation with tumor progression and poor prognosis. Gynecol Oncol. 2003;90:331–337. doi: 10.1016/s0090-8258(03)00318-4. [DOI] [PubMed] [Google Scholar]
- 33.Ren CC, Miao XH, Yang B, Zhao L, Sun R, Song WQ. Methylation status of the fragile histidine triad and E-cadherin genes in plasma of cervical cancer patients. Int J Gynecol Cancer. 2006;16:1862–1867. doi: 10.1111/j.1525-1438.2006.00669.x. [DOI] [PubMed] [Google Scholar]