Abstract
Systemic scleroderma is a disease that is characterized by excessive fibroblastic activity and collagen deposition in various organs, including the heart. We sought to evaluate the limits of biventricular function as derived noninvasively from pulsed-wave tissue Doppler imaging (TDI) of tricuspid and mitral annular motion in patients who had scleroderma.
We enrolled 24 patients with scleroderma (study group; mean age, 49 ± 11 yr; 20 women) and 24 healthy participants (control group; mean age, 51 ± 9 yr; 19 women). Persons with cardiovascular risk factors were excluded. We obtained images by conventional echocardiography and by pulsed-wave TDI, measuring the respective peak systolic velocities (S, Sm) and peak early (E, Em) and late (A, Am) diastolic velocities. Mean Sm, mean Em, and mean Am were averages of the 4 measured sites (anterior, inferior, lateral, and septal). We calculated noninvasive estimates of left ventricular (LV) filling pressure by dividing E velocities (from the mitral inflow) by Em velocities (E/Em ratios).
Biventricular regional Sm, regional LV myocardial Em, and ratios of myocardial Em/atrial component velocity (Em/Am) for the LV, and mean Sm, mean Em, and mean Em/mean Am ratios for the LV were significantly lower in the study group. The E/Em ratio was higher in the study group (7.3 ± 2.6 vs 5.2 ± 1.0, P = 0.01). Global LV systolic and diastolic function did not differ between the groups.
Tissue Doppler imaging complements conventional echocardiography in detecting subclinical biventricular impairment in patients with scleroderma who have normal global measurements.
Key words: Case-control studies; diastole/physiology; echocardiography/methods; heart diseases/ultrasonography; heart ventricles/ultrasonography; scleroderma, systemic/complications/diagnosis/physiopathology; sensitivity and specificity; systole/physiology; ultrasonography, Doppler, pulsed/methods; ventricular dysfunction/ultrasonography
Systemic sclerosis is a connective-tissue disease that is clinically characterized by variable involvement of the skin and the visceral organs.1 Involvement of the heart is one of the most frequent complications that has been reported in several clinical investigations and autopsy studies.1–3 When scleroderma involves the heart, fibrosis due to collagen deposition replaces damaged muscle cells with fibrous ones, which leads to atrial and ventricular arrhythmias, conduction disturbances, and right ventricular (RV) and left ventricular (LV) systolic and diastolic dysfunction.4 Cardiac involvement can exist without symptoms; once it becomes clinically apparent, the affected patient's prognosis is poor.5–7
Pulsed-wave tissue Doppler imaging (PW TDI) is a recently developed ultrasonographic technique that enables quantitative analysis of global and regional myocardial function. It has been shown that early diagnosis of ventricular dysfunction is possible by the use of newer techniques such as PW TDI.8,9 Previous investigators who used tissue Doppler echocardiography reported reduced systolic myocardial function of the LV10 and significant improvement after patients were treated with nifedipine,11 while others12 detected no alteration in the systolic function of patients who had scleroderma. Several investigators have reported LV diastolic dysfunction after using conventional echocardiography or TDI.12–15 Right ventricular diastolic impairment has also been reported.15–17 In our study, we sought to investigate RV and LV function by both conventional echocardiography and PW TDI.
Participants and Methods
For this study, we enrolled 24 patients who had scleroderma (the study group; mean age, 49 ± 11 yr; 20 women) and 24 healthy persons (the control group; mean age, 51 ± 9 yr; 19 women). All gave written informed consent to participate in the study, which was approved by our local ethics committee. Persons with any of the following were excluded from the study: prior pacemaker implantation, left or right bundle branch block, congenital or valvular heart disease, pericarditis, coronary artery disease, pulmonary emboli, or heart failure. We also excluded individuals who were receiving digitalis, β-blockers, or other antiarrhythmic drugs. Four members of the study group and 6 of the control group were smokers.
None of the 24 patients who qualified for the study group had a history of persistent or paroxysmal atrial fibrillation. All had Raynaud's phenomenon, and 10 had digital ulcers. Eighteen fulfilled the criteria for diffuse scleroderma, and 6 met the criteria for limited (cutaneous) scleroderma.17
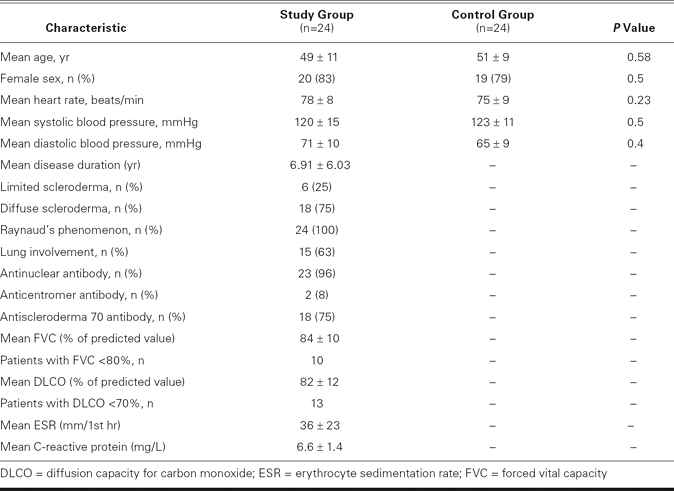
All 48 individuals underwent physical examination, laboratory testing (blood cell count, serum creatinine levels, and for anticentromere, antinuclear, and antisclerderma 70 antibodies), computed tomographic scanning, pulmonary function testing (including spirometry and the diffusion capacity for carbon monoxide), conventional echocardiography, and PW TDI. A forced vital capacity of less than 80% or a diffusion capacity for carbon monoxide of less than 70% of predicted normal values was considered to be abnormal. The baseline clinical characteristics of the patients are shown in Table I.
TABLE I. General Characteristics of the Study and Control Groups
Echocardiographic Analysis
All echocardiographic examinations were conducted with the use of a VingMed® System FiVe cardiac ultrasonographic scanner (GE VingMed® Ultrasound; Horten, Norway) and 2.5–3.5-MHz transducers. While in the left lateral decubitus position, all 48 participants were examined by precordial M-mode and 2-dimensional echocardiography, the latter in the parasternal long-axis and biplanar apical 4- and 2-chamber views. Interventricular wall thickness and LV posterior wall thickness, LV diastolic dimension, and left atrial dimension were measured from the M-mode images. The LV ejection fraction (LVEF) was derived from the biplanar apical 2- and 4-chamber views by use of a modified Simpson's method.
Conventional Doppler Echocardiographic Evaluation of Global Function
Mitral and tricuspid flow velocities were obtained with the sample volume placed at the tip of the mitral and tricuspid valve leaflets, respectively. From the mitral and tricuspid velocity profiles, the following measurements were made, in accordance with the recommendations of the American Society of Echocardiography19: 1) early (E) peak diastolic velocity; 2) late (A) peak diastolic velocity; 3) early diastolic/late diastolic (E/A) velocity ratio; and 4) E-wave deceleration time. By use of color M-mode Doppler, the propagation velocity of flow (Vp) into the LV was determined after a change to the color-coded scale so that the 1st aliasing wavefront could be clearly seen and measured. Pulmonary venous flow peak systolic (S) and diastolic (D) velocities were determined by pulsed-wave Doppler, with the sample volume placed inside the upper right pulmonary vein. The E/Vp index, which has been shown to provide good noninvasive estimates of LV filling pressure,20 was also calculated. Transtricuspid peak retrograde velocities were recorded via the continuous-wave Doppler technique, and a modified Bernoulli equation was used to estimate systolic pulmonary artery pressure.19
Tissue Doppler Imaging Evaluation of Regional Myocardial Function
Pulsed-wave TDI was performed at transducer frequencies of 3.5 MHz with as minimal an optimal gain as possible, in order to obtain the best signal-to-noise ratio. Myocardial velocities were recorded by use of a standard PW TDI technique. The sample volume was placed at the junction of the LV wall with the mitral annulus of the septal and lateral myocardial segments from the 4-chamber view, and the inferior and anterior myocardial segments from the 2-chamber view, sequentially. For the RV, the sample volume was placed at the junction of the RV and the tricuspid annulus from the 4-chamber view. Peak systolic velocity (Sm), peak early diastolic velocity (Em), and peak late diastolic velocity (Am) were measured, and an Em/Am ratio was calculated for each segment. Mean Sm, mean Em, mean Am, and mean Em/Am represented the average of the 4 measured sites. The E/Em ratio, which has been shown to provide good noninvasive estimates of LV filling pressure, was calculated by dividing the peak E velocity (obtained by pulsed Doppler from the mitral inflow) by the Em that was measured from the septal, lateral, inferior, and lateral mitral annulus by use of PW TDI.21 For the RV E/Em ratio, the peak E velocity (obtained by conventional pulsed Doppler from the tricuspid inflow) was divided by the Em that was measured from the lateral tricuspid annulus by use of PW TDI. The intraobserver and interobserver coefficients of variation (the SD of the differences between the 2 observations divided by the mean value, expressed as a percentage) varied between 3% and 4%, and 4% and 5%, respectively.
Statistical Analysis
Continuous data were expressed as mean ± SD and the categorical data as percentages. The continuous data were compared by means of Student's t test, and the categorical data by the χ2 test. The relationship between parameters was determined by using either the Spearman or the Pearson correlation test. The SPSS statistical software package version 9.05 (SPSS Inc.; Chicago, Ill) was used to perform all statistical calculations. A P value <0.05 was considered statistically significant.
Results
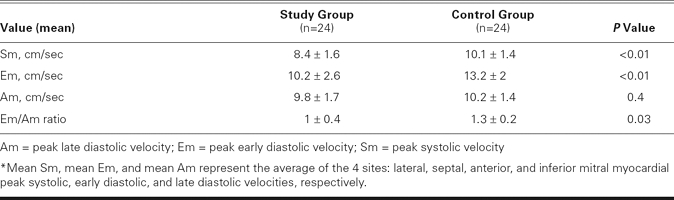
All 24 scleroderma patients were in sinus rhythm during the study's duration. There were no significant differences between the study group and the control participants with respect to age, sex, resting heart rate, systolic and diastolic blood pressure, and global measurements of LV diastolic function (Tables I and II). The LV end-diastolic and end-systolic diameters, LVEFs, and left atrial diameters were similar between the groups (Table II). Tricuspid Am was significantly higher, and the tricuspid inflow E/A ratio was significantly lower in the study group than in the control group, which indicated a relaxation abnormality of the RV in the study group (P = 0.03; Table II). The study group had higher pulmonary artery pressure than the control group (37 ± 8 vs 23 ± 4 mmHg, P = 0.004). Pulsed-wave TDI showed reduced regional Sm values for both the LV and the RV in the study group, compared with the control group (Table III). The Em values, as measured by PW TDI at the lateral, septal, anterior, and inferior mitral annulus, were significantly lower in the study group than in the control group. The Em/Am ratio at the lateral, septal, and anterior regions of the LV was significantly lower in the study group, showing reduced regional distensibility. The E/Em ratio obtained at the lateral, septal, anterior, and inferior segments of the LV was significantly higher in the study group. Mean Sm and Em values and the mean Em/Am ratio of the LV were significantly lower in the study group (Table IV).
TABLE II. Conventional Echocardiographic Data*
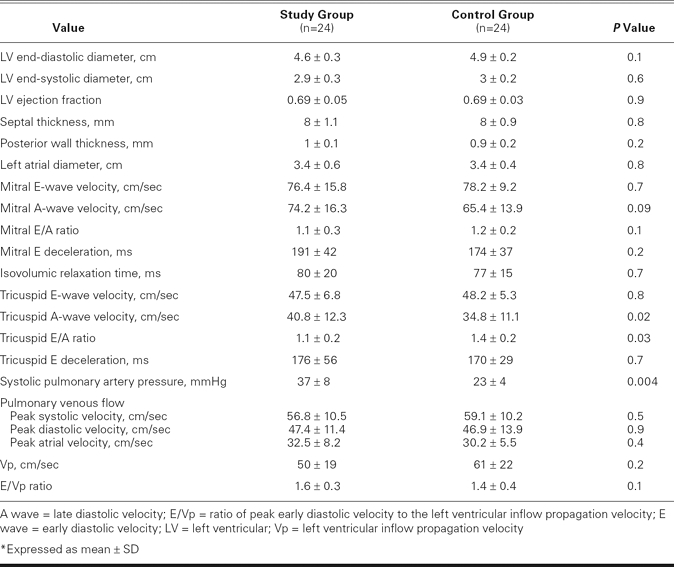
TABLE III. Pulsed-Wave Tissue Doppler Variables*
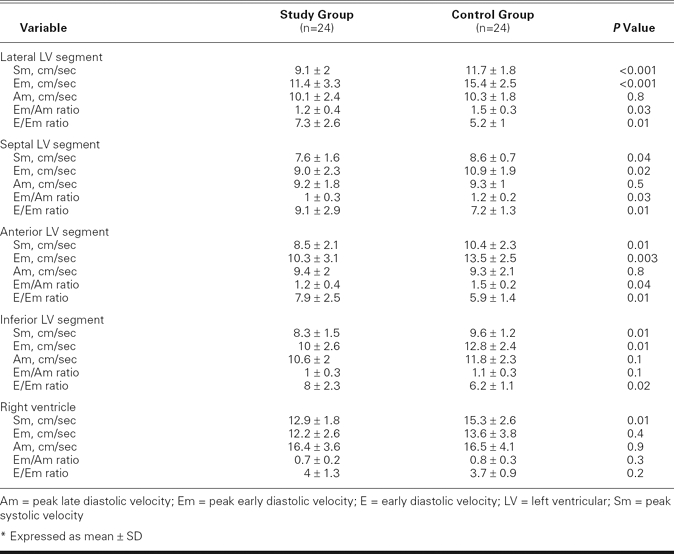
TABLE IV. Mean Values* of Tissue Doppler Myocardial Velocities
Our chief findings indicated that LV regional systolic and diastolic myocardial function was affected in the study group, as was evidenced by PW TDI, and that RV regional systolic and global diastolic function was altered, as was shown by PW TDI and conventional echocardiography, respectively.
Discussion
Scleroderma is a connective-tissue disease that is characterized by widespread vascular lesions and fibrosis of the skin and internal organs. Primary myocardial involvement (common in scleroderma) is recognized as a poor prognostic factor.5,22–24 “Patchy” fibrosis, thus termed due to its characteristic distribution in the myocardium, is considered to be the hallmark of sclerodermic heart involvement.22 In a study of 16 patients who had scleroderma with normal LV systolic function and a normal electrocardiogram, Fernandes and colleagues25 used endomyocardial biopsy to identify abnormal myocardial collagen deposition in 15 of those patients. Some histologic examinations have shown diffuse patchy fibrosis with contraction-band necrosis unrelated to epicardial coronary artery stenosis26; others have shown concentric intimal hypertrophy associated with fibrinoid necrosis of intramural coronary arteries.25 Some studies have also shown a high prevalence of myocardial involvement, by use of single-photon-emission computed tomography (SPECT).27,28 Vignaux and associates11 discovered segmental myocardial perfusion defects by using magnetic resonance imaging in patients who had scleroderma. These defects did not correspond to any epicardial coronary artery distribution, which suggested microvascular alteration.
During the last 30 years, there has been a considerable change in the causes of death in patients who have scleroderma. Due to the widespread use of angiotensin-converting enzyme inhibitors, death from renal crisis has decreased from 31% to 3% of all deaths. Cardiac involvement accounts for 5% of scleroderma-related deaths; in contrast, lung disease (pulmonary hypertension and pulmonary fibrosis) accounts for 60%, making it the primary cause of such deaths today.23
When cardiomyopathy is suspected, conventional echocardiography is often the 1st method used in order to evaluate LV and RV function. The routinely used values, such as end-diastolic diameter, fractional shortening, and LVEF, are load-dependent and do not reflect the contractile state of the myocardium. Tissue Doppler echocardiography is a new, less load-dependent method that enables the direct, reliable measurement of regional myocardial systolic and diastolic abnormalities.29 In our study, patients with scleroderma did not show systolic dysfunction when conventional measurements of LVEF were used. Global LV diastolic function was also within normal range, compared with the control group; on the other hand, there was relaxation abnormality in the RV, as was evidenced by the lower tricuspid inflow E/A ratio. Our PW TDI analysis showed reduced regional Sm values for the LV and the RV. The PW TDI also showed a reduced Em and Em/Am ratio in the LV, which indicated diastolic dysfunction, and a higher E/Em ratio, which has been shown previously to be a noninvasive estimate of filling pressure.
Previous TDI studies of scleroderma patients led to different results with regard to systolic and diastolic function. Plazak and co-authors12 used TDI to evaluate 19 patients who had scleroderma and normal LVEFs and found no significant difference in systolic myocardial velocities; however, early diastolic velocities were significantly lower than in their control group. Their patients had reduced transmitral E/A flow. In contrast, we noted in our study that transmitral E/A flow was normal. This discrepancy can be explained by the absence in our patients of LV hypertrophy, systemic hypertension, and renal insufficiency, which are conditions likely to affect global LV diastolic function in patients who have scleroderma.30 Plazak's control group also tended to be younger (43.6 yr, vs 51.7 yr in the scleroderma group), which may account for part of the difference. Hsiao and colleagues16 evaluated 40 patients who had scleroderma and normal LVEFs but found no difference in TDI-derived systolic and diastolic velocities from the LV lateral wall. In our study, we evaluated 4 regions of the LV, and all displayed altered systolic and diastolic function on TDI. The Hsiao investigation also determined that the RV had lower systolic velocity, as obtained by TDI, and that the RV ejection fractions were significantly lower than in their control group. We also detected that the RV has a lower Sm value, similar to the results of Hsiao and colleagues.16 Meune and coworkers10 examined 17 patients who had scleroderma. All 17 exhibited myocardial perfusion abnormalities on SPECT, and normal LVEFs on radionuclide ventriculography; TDI analysis showed reduced regional myocardial contractility and diastolic dysfunction of the posterior wall. Our study, which showed reduced LV Sm values despite normal LVEFs, and abnormal diastolic function as evidenced on TDI, is in accord with that study.10 Lindqvist and co-investigators17 studied 26 patients who had scleroderma and normal LVEFs, and—as did we—found no alteration in LV conventional diastolic measurements (such as Em, Am, and E/A ratio). However, the RV Am value was significantly higher and the tricuspid inflow E/A ratio was lower in their patients than in their control group, a finding that indicated reduced wall distensibility in their scleroderma group. Those investigators found no RV regional diastolic dysfunction when they used TDI analysis in their scleroderma patients. Our results were in accord with their results. The Lindqvist study17 did not evaluate LV regional function by use of TDI.
Our study showed impaired regional systolic and diastolic function of the LV, and impaired regional systolic and global diastolic function of the RV. There was no significant impairment of global LV systolic and diastolic function. Conventional echocardiographic evaluation revealed global RV diastolic dysfunction but could not detect LV diastolic dysfunction. This is probably due to ischemia or fibrosis in scleroderma, either of which could have affected the subendocardial region of the myocardium more than the subepicardial part. Intermittent coronary vasospasm (Raynaud's phenomenon) has been shown to be prevalent in patients who have scleroderma.31 We used PW TDI to evaluate long-axis contraction and relaxation, which is mainly a function of subendocardial fibers that are particularly sensitive to ischemia.32
Limitations
In this study, we have shown that RV Sm is reduced, but we did not evaluate RV ejection fraction. Our patients with scleroderma had higher systolic pulmonary artery pressures than did the control participants, which might have affected our results, yet none of the patients exhibited signs or symptoms of RV failure. The low number of patients in our limited-scleroderma subgroup precluded an effective comparison of echocardiographic values between that patient subgroup and the subgroup of patients who had diffuse scleroderma.
Conclusion
On PW TDI, patients with scleroderma exhibit abnormal LV systolic and diastolic function despite normal global LVEF. Right ventricular regional systolic myocardial function and global diastolic function are also altered, as we determined from PW TDI and conventional echocardiographic measurements, respectively. The early echocardiographic detection of cardiac involvement in scleroderma patients is desirable for optimal treatment and for implementation of preventive measures in the early stages of the disease. A PW TDI analysis complements conventional echocardiography in the detection of subclinical biventricular impairment in patients who have scleroderma.
Footnotes
Address for reprints: Ilknur Can, MD, Alparslan Turkes Sok. Gokyel Siteleri, A blok 2/9 Selcuklu, 42080 Konya, Turkey. E-mail: ilknur1973@gmail.com
References
- 1.Medsger TA Jr. Systemic sclerosis (scleroderma): clinical aspects. In: Koopman WJ, editor. Arthritis and allied conditions: a textbook of rheumatology. 14th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 1590–624.
- 2.Follansbee WP, Miller TR, Curtiss EI, Orie JE, Bernstein RL, Kiernan JM, Medsger TA Jr. A controlled clinicopathologic study of myocardial fibrosis in systemic sclerosis (scleroderma). J Rheumatol 1990;17(5):656–62. [PubMed]
- 3.Ferri C, Valentini G, Cozzi F, Sebastiani M, Michelassi C, La Montagna G, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002;81 (2):139–53. [DOI] [PubMed]
- 4.Ferri C, Giuggioli D, Sebastiani M, Colaci M, Emdin M. Heart involvement and systemic sclerosis. Lupus 2005;14(9):702–7. [DOI] [PubMed]
- 5.Follansbee WP, Curtiss EI, Medsger TA Jr, Steen VD, Uretsky BF, Owens GR, Rodnan GP. Physiologic abnormalities of cardiac function in progressive systemic sclerosis with diffuse scleroderma. N Engl J Med 1984;310(3):142–8. [DOI] [PubMed]
- 6.Butrous GS, Dowd PM, Milne J, Dymond DS, Caplin J, Camm AJ. Non-invasive assessment of early cardiac involvement in systemic sclerosis. Postgrad Med J 1985;61(718): 679–84. [DOI] [PMC free article] [PubMed]
- 7.Tassan-Mangina S, Brasselet C, Nazeyrollas P, Collot-Bigot M, Costa B, Blaise AM, et al. Value of pulsed Doppler tissue imaging for early detection of myocardial dysfunction with anthracyclines [in French]. Arch Mal Coeur Vaiss 2002;95 (4):263–8. [PubMed]
- 8.Dandel M, Wellnhofer E, Hummel M, Meyer R, Lehmkuhl H, Hetzer R. Early detection of left ventricular dysfunction related to transplant coronary artery disease. J Heart Lung Transplant 2003;22(12):1353–64. [DOI] [PubMed]
- 9.Seferovic PM, Ristic AD, Maksimovic R, Simeunovic DS, Ristic GG, Radovanovic G, et al. Cardiac arrhythmias and conduction disturbances in autoimmune rheumatic diseases. Rheumatology (Oxford) 2006;45 Suppl 4:iv39–42. [DOI] [PubMed]
- 10.Meune C, Allanore Y, Pascal O, Devaux JY, Dessault O, Duboc D, et al. Myocardial contractility is early affected in systemic sclerosis: a tissue Doppler echocardiography study. Eur J Echocardiogr 2005;6(5):351–7. [DOI] [PubMed]
- 11.Vignaux O, Allanore Y, Meune C, Pascal O, Duboc D, Weber S, et al. Evaluation of the effect of nifedipine upon myocardial perfusion and contractility using cardiac magnetic resonance imaging and tissue Doppler echocardiography in systemic sclerosis. Ann Rheum Dis 2005;64(9):1268–73. [DOI] [PMC free article] [PubMed]
- 12.Plazak W, Zabinska-Plazak E, Wojas-Pelc A, Podolec P, Olszowska M, Tracz W, Bogdaszewska-Czabanowska J. Heart structure and function in systemic sclerosis. Eur J Dermatol 2002;12(3):257–62. [PubMed]
- 13.Mizuno R, Fujimoto S, Nakano H, Nakajima T, Kimura A, Nakagawa Y, Dohi K. Atrial conduction abnormalities in patients with systemic progressive sclerosis. Eur Heart J 1997;18 (12):1995–2001. [DOI] [PubMed]
- 14.Kazzam E, Waldenstrom A, Landelius J, Hallgren R, Arvidsson A, Caidahl K. Non-invasive assessment of left ventricular diastolic function in patients with systemic sclerosis. J Intern Med 1990;228(2):183–92. [DOI] [PubMed]
- 15.Gullulu S, Kaderli AA, Ekbul A, Ozdemir B, Baran I, Gullulu M, et al. Tissue Doppler echocardiography and myocardial performance index in patients with scleroderma. J Int Med Res 2005;33(4):417–24. [DOI] [PubMed]
- 16.Hsiao SH, Lee CY, Chang SM, Lin SK, Liu CP. Right heart function in scleroderma: insights from myocardial Doppler tissue imaging. J Am Soc Echocardiogr 2006;19(5):507–14. [DOI] [PubMed]
- 17.Lindqvist P, Caidahl K, Neuman-Andersen G, Ozolins C, Rantapaa-Dahlqvist S, Waldenstrom A, Kazzam E. Disturbed right ventricular diastolic function in patients with systemic sclerosis: a Doppler tissue imaging study. Chest 2005;128(2): 755–63. [DOI] [PubMed]
- 18.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15(2): 202–5. [PubMed]
- 19.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 2002;15(2):167–84. [DOI] [PubMed]
- 20.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation 2000;102(15): 1788–94. [DOI] [PubMed]
- 21.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997;30(6):1527–33. [DOI] [PubMed]
- 22.D'Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969;46(3):428–40. [DOI] [PubMed]
- 23.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007;66(7): 940–4. [DOI] [PMC free article] [PubMed]
- 24.Medsger TA Jr, Masi AT, Rodnan GP, Benedek TG, Robinson H. Survival with systemic sclerosis (scleroderma). A life-table analysis of clinical and demographic factors in 309 patients. Ann Intern Med 1971;75(3):369–76. [DOI] [PubMed]
- 25.Fernandes F, Ramires FJ, Arteaga E, Ianni BM, Bonfa ES, Mady C. Cardiac remodeling in patients with systemic sclerosis with no signs or symptoms of heart failure: an endomyocardial biopsy study. J Card Fail 2003;9(4):311–7. [DOI] [PubMed]
- 26.James TN. De subitaneis mortibus. VIII. Coronary arteries and conduction system in scleroderma heart disease. Circulation 1974;50(4):844–56. [DOI] [PubMed]
- 27.Kahan A, Devaux JY, Amor B, Menkes CJ, Weber S, Nitenberg A, et al. Nifedipine and thallium-201 myocardial perfusion in progressive systemic sclerosis. N Engl J Med 1986; 314(22):1397–402. [DOI] [PubMed]
- 28.Kahan A, Devaux JY, Amor B, Menkes CJ, Weber S, Venot A, et al. Nicardipine improves myocardial perfusion in systemic sclerosis. J Rheumatol 1988;15(9):1395–400. [PubMed]
- 29.Nagueh SF, Bachinski LL, Meyer D, Hill R, Zoghbi WA, Tam JW, et al. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation 2001; 104(2):128–30. [DOI] [PMC free article] [PubMed]
- 30.Aguglia G, Sgreccia A, Bernardo ML, Carmenini E, Giusti De Marle M, Reali A, Morelli S. Left ventricular diastolic function in systemic sclerosis. J Rheumatol 2001;28(7):1563–7. [PubMed]
- 31.Gustafsson R, Mannting F, Kazzam E, Waldenstrom A, Hallgren R. Cold-induced reversible myocardial ischaemia in systemic sclerosis. Lancet 1989;2(8661):475–9. [DOI] [PubMed]
- 32.Pellerin D, Sharma R, Elliott P, Veyrat C. Tissue Doppler, strain, and strain rate echocardiography for the assessment of left and right systolic ventricular function. Heart 2003;89 Suppl 3:iii9–17. [DOI] [PMC free article] [PubMed]


