Abstract
Acquired left ventricular-to-right atrial communication is encountered periodically. This condition is chiefly attributable to surgical mishaps, trauma, endocarditis, or endomyocardial biopsy. In a few instances, a Gerbode-like defect develops after the repair of an atrioventricular septal defect. Our search of the worldwide medical literature revealed just 1 report of a “mirror” occurrence of a Gerbode-like defect: a shunt between the left atrium and the right ventricle.
Herein, we present the case of a 22-year-old woman who had severe mitral valve incompetence accompanying an acquired shunt between the left atrium and the right ventricle—a late sequela of the earlier repair of an atrioventricular septal defect. After surgical correction of the shunt and the associated mitral incompetence, the patient experienced a good outcome.
Echocardiographic and intraoperative findings are presented, along with a plausible explanation for the mechanism and presentation of the condition in our patient. To our knowledge, this is only the 2nd report of an acquired shunt between the left atrium and the right ventricle, and the 1st such case to be accompanied by severe mitral valve incompetence.
Key words: Cardiac surgical procedures; cardiomyopathies/etiology/ultrasonography; heart septal defects, atrial/physiopathology/surgery/ultrasonography; heart septal defects, ventricular/complications; postoperative complications/etiology; mitral valve insufficiency/etiology/surgery; reoperation; time factors; treatment outcome
Acquired left ventricular-to-right atrial communications are encountered from time to time. These are chiefly attributable to surgical mishaps, trauma, endocarditis, or even endomyocardial biopsy.1 A few cases are on record wherein endocarditis was thought to have led to a Gerbode-like defect after the repair of an atrioventricular septal defect (AVSD).2 However, we could find just 1 report of an acquired shunt between the left atrium (LA) and the right ventricle (RV).3 Here, we report a 2nd such case, that of a 22-year-old woman who also had late-onset severe mitral valve regurgitation. A point of interest is not merely the rare occurrence of this condition, but also the quest for a plausible explanation for its mechanism and presentation.
Case Report
A 22-year-old woman had undergone surgical correction of a partial defect of the atrioventricular canal when she was 5 years of age. At that time, a cleft mitral valve was repaired, and the atrial septum was reconstructed with autologous glutaraldehyde-treated pericardium. For over 15 years thereafter, she remained asymptomatic and had satisfactory echocardiographic findings upon annual follow-up examinations. However, in February 2007, she presented at our clinic with a rapidly worsening history of exertional dyspnea that had culminated in orthopnea, paroxysmal nocturnal dyspnea, and pedal edema. She was also experiencing recurrent episodes of palpitation and dizziness. There was no history of hemoptysis, chest pain, syncopal attacks, or cyanosis. The patient had experienced a 2-week episode of fever 18 months previously. Her local physicians had diagnosed and treated this ailment as enteric fever.
Upon our clinical examination, the patient had a grade 2 parasternal heave associated with a loud pulmonary 2nd sound, and a grade 3/6 pansystolic murmur over the mitral valve area with radiation to the axilla, a soft systolic ejection murmur over thepulmonary area, and substantial hepatomegaly. Chest radiography revealed cardiomegaly with a cardiothoracic ratio that exceeded 70%. An electrocardiogram showed evidence of LA and RV enlargement, normal sinus rhythm, and a left anterior hemiblock pattern. Two-dimensional and color Doppler echocardiographic evaluation revealed a severely dilated LA with preserved left ventricular volume and systolic function. The patient had a severely incompetent mitral valve with non-coapting leaflets, along with moderate tricuspid regurgitation and moderate pulmonary artery hypertension. The surprise finding was an LA-to-RV communication with bidirectional shunting. The anatomic nature of the shunt could be appropriately delineated only with the use of transesophageal echocardiography (TEE). In diastole, the flow pattern was distinctly left-to-right. A defect could be seen in the region of the repaired interatrial septum (Fig. 1).
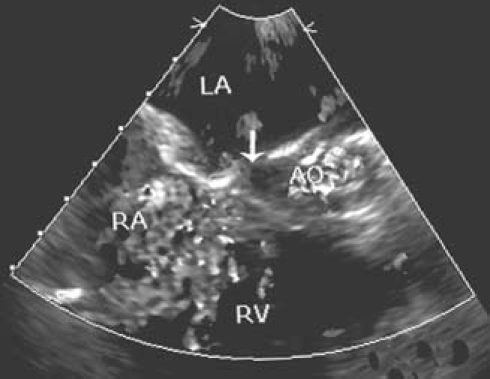
Fig. 1 Transesophageal echocardiographic appearance of the defect (arrow) between the left atrium (LA) and the right ventricle (RV).
AO = aorta; RA = right atrium
After being stabilized with diuretic agents and angiotensin-converting enzyme inhibitors, the patient underwent cardiac angiography. This study confirmed the echocardiographic findings and excluded any other intracardiac shunt. It was interesting that the regurgitant mitral valve was largely responsible for a left-to-right shunt during systole, which accounted for an oximetric step-up in the RV cavity and pulmonary artery. Because the catheterization was conducted merely for confirmatory purposes, a detailed calculation of shunt fraction was not performed.
The patient was taken for reoperation; the goal was repair of the defect and the incompetent mitral valve. At operation, she was found to have massive biatrial enlargement. When the right atrium was opened, a single defect of approximately a centimeter in diameter was identified in the inlet portion of the RV aspect of the septum just below the septal tricuspid leaflet. By means of probing, the defect was determined to lead to the LA. Upon saline insufflation, the tricuspid valve was seen to be adequately competent. The LA was approached across the atrial septal patch. The mitral valve had a shriveled and deformed anterior leaflet, which was largely bereft of chordal harnessing. This produced free regurgitation. Some chordal remnants were visible in the left ventricular cavity. The cleft in the mitral valve, which had been sutured during the 1st operation, was intact. A triangular opening close to the fibrous trigone (Fig. 2) was communicating with the defect in the RV.The mitral valve, obviously unsuitable for repair, was replaced with a 29-mm Hall tilting-disc prosthetic valve (Medtronic, Inc.; Minneapolis, Minn), which was implanted in a supra-annular fashion with the use of interrupted, pledgeted, 2–0 everting mattress sutures (with posterior chordal preservation). The defect was closed from the RV aspect with a generously sized, expanded polytetrafluoroethylene patch, by retracting the tricuspid leaflets. The adequacy of the repair and the proper positioning of the patch were confirmed when the anesthesiologist's performance of the Valsalva maneuver enabled the LA to fill with blood. Weaning of the patient from cardiopulmonary bypass was smooth, and the adequacy of the repair was further corroborated upon TEE.
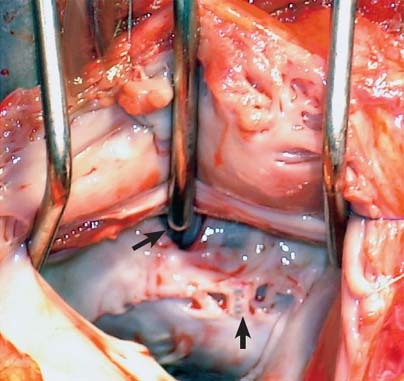
Fig. 2 Intraoperative photograph shows the left atrial aspect of the defect (top arrow), the shriveled anterior mitral leaflet, and the intact suture line where the cleavage had been repaired (bottom arrow).
The patient required sustained inotropic support until the 7th postoperative day, in order to treat her longstanding features of congestive cardiac failure. She made an otherwise uneventful recovery. After 11 postoperative days, she was discharged from the hospital on a regimen of digitalis and diuretic agents and oral anticoagulant agents. On subsequent follow-up visits, she remained free of severe symptoms and was experiencing no discomfort.
Discussion
Reoperations after repair of partial or complete AVSDs are most commonly indicated by progressively worsening late mitral regurgitation.4 In most of these patients, however, the regurgitation is either a residual or a “left-alone” incompetence that takes a turn for the worse. The postoperative reappearance of shunts may occur due to patch dehiscence quite early after the primary operation.5 Small residual shunts are known to close spontaneously.6 The appearance of a shunt between the LA and the RV, similar to a Gerbode defect, is rare after AVSD repair, with only a few reports published worldwide to date.2 In our patient, we found a “mirror” occurrence of a Gerbode-like defect. This complication was not suspected clinically, its effects having been masked in the presence of the severe mitral valve regurgitation.
As was emphasized in the only such case documented earlier,3 TEE helped to pinpoint the anatomy of the shunt, which had manifested itself late after the original corrective procedure. Inspection of the mitral valve revealed an intact suture line where the cleft had been repaired. The shriveling of the anterior mitral leaflet, the features of chordal rupture, and the punched-out, newly created defect, juxtaposed with the fibrous skeleton at the lower end of the repaired interatrial septum, can best be explained by healed, clinically unsuspected endocarditis. In our case, the patient's recent history of prolonged fever offers a plausible clue. Finally, the predominant LA-to-RV shunting during diastole occurred due to an elevated LA pressure that was caused by severe mitral regurgitation.
Footnotes
Address for reprints: Amit Banerjee, MS, MCh, Department of Cardiovascular and Thoracic Surgery, Govind Ballabh Pant Hospital, New Delhi 110002, India. E-mail: amit_banerjee@india.com
References
- 1.Katta S, Akosah K, Stambler B, Salter D, Guerraty A, Mohanty PK. Atrioventricular fistula: an unusual complication of endomyocardial biopsy in a heart transplant recipient. J Am Soc Echocardiogr 1994;7(4):405–9. [DOI] [PubMed]
- 2.Velebit V, Schoneberger A, Ciaroni S, Bloch A, Maurice J, Christenson JT, et al. “Acquired” left ventricular-to-right atrial shunt (Gerbode defect) after bacterial endocarditis. Tex Heart Inst J 1995;22(1):100–2. [PMC free article] [PubMed]
- 3.Chirillo F, Totis O, Cuzzato V. Left-atrium-to-right-ventricle shunting after surgery for atrioventricular septal defect: an unusual sequel detected by transesophageal echocardiography. Int J Cardiol 1992;37(2):253–6. [DOI] [PubMed]
- 4.Ten Harkel AD, Cromme-Dijkhuis AH, Heinerman BC, Hop WC, Bogers AJ. Development of left atrioventricular valve regurgitation after correction of atrioventricular septal defect. Ann Thorac Surg 2005;79(2):607–12. [DOI] [PubMed]
- 5.Backer CL, Mavroudis C, Alboliras ET, Zales VR. Repair of complete atrioventricular canal defects: results with the two-patch technique. Ann Thorac Surg 1995;60(3):530–7. [DOI] [PubMed]
- 6.Dodge-Khatami A, Knirsch W, Tomaske M, Pretre R, Bettex D, Rousson V, Bauersfeld U. Spontaneous closure of small residual ventricular septal defects after surgical repair. Ann Thorac Surg 2007;83(3):902–5. [DOI] [PubMed]


