Abstract
Pleural effusions that are caused by congestive heart failure and refractory to medical management are rare, and the options for treating them are few and sometimes ineffective. We report here our experience, over a 2-year period, with a novel device, the Denver Biomedical PleurX® pleural catheter, in treating a series of 5 patients who had chronic, refractory, heart-failure–associated pleural effusions. The PleurX catheter is a small-bore chest tube designed to remain in place for prolonged periods, through which drainage of pleural fluid can be performed easily on a daily or less frequent outpatient basis. Placement of the catheter, in our series, was associated with no complications. In all patients, the catheter effectively drained the pleural space initially, thereby controlling the effusions and alleviating New York Heart Association functional class IV symptoms. The catheters remained in place for a period of 1 to 15 months. In 2 of the patients, the catheter was associated with no complications during the time that it remained in place. One of these patients had the catheter removed at heart transplantation, and 1 retained the catheter until death from underlying heart disease. For 1 patient, the catheter resulted in a partially loculated pleural space, and it was removed. In 2 patients, after prolonged use, it was associated with empyema, for which it was removed. We conclude that the PleurX catheter can effectively control refractory congestive-heart-failure–associated pleural effusions temporarily, but that its prolonged use can cause significant complications, most importantly empyema.
Key words: Ambulatory care; catheters, indwelling; drainage/methods; heart failure, congestive; pleural effusion
Pleural effusions are a common complication of congestive heart failure (CHF).1–4 Standard medical management of CHF, especially with diuretic agents, is generally very effective in treating these effusions.5,6 For symptomatic effusions that do not respond adequately or rapidly enough to medical management, thoracentesis is typically performed, with excellent results.5,6 Rarely, however, pleural effusions due to CHF, particularly in the settings of advanced cardiac failure or impaired renal function, prove to be recurrent or refractory to even the most aggressive medical regimens. Efforts to control such effusions have been limited to serial thoracenteses and pleurodesis.5,6 Serial thoracenteses, depending upon the frequency, are generally unsatisfactory to patients and, of course, the more frequently they are performed, the greater the risk of complication. Although pleurodesis is the preferred alternative to serial thoracenteses, it also has serious potential limitations and complications,7–11 whether performed by the traditional “closed” chest tube technique or by state-of-the-art video-assisted thoracoscopic surgery (VATS). Therefore, alternative solutions to this very difficult clinical problem are needed.12
The PleurX® Pleural Catheter (Denver Biomedical, Inc., part of Cardinal Health, Inc.; Golden, CO) was recently developed for use in patients who cannot tolerate VATS pleurodesis or who have malignant pleural effusions that traditional, closed pleurodesis often cannot control adequately.13 The PleurX catheter is essentially a small-bore chest tube that is designed to remain in place for prolonged, intermittent drainage of the pleural space through a one-way valve. The PleurX has proved to be very effective for such drainage, and complication rates have been relatively low.13–16
We hypothesized that the PleurX might be an effective tool for the medical management of refractory pleural effusions caused by CHF. We therefore placed the catheter in 5 of our CHF patients who were poor candidates for traditional pleurodesis. Herein we report our experience with the PleurX catheter, which we believe to be the 1st use of a chronically indwelling catheter system for the management of recurrent pleural effusions in CHF.
Patients and Methods
Over a period of 2 years (2006 through 2007), we considered for PleurX catheter placement those patients who had a primary diagnosis of advanced CHF and consequent refractory pleural effusion. Medically refractory effusions were those that required therapeutic thoracentesis at least every 2 weeks despite attempts by the patient's primary cardiologist to control the CHF through application of maximal medical regimens. All patients under consideration for PleurX underwent extensive pleural fluid testing, imaging studies, and liver and kidney function studies to rule out other conditions that could contribute to, or cause, pleural effusions (malignant pleural effusion, parapneumonic effusion, empyema, hepatic hydrothorax, chylothorax, or primary inflammatory pleuritis). No patient was considered for PleurX if another major condition was found, with the exception of renal dysfunction. Prospective participants were counseled extensively about the available options to treat refractory pleural effusions, and they gave written consent upon deciding to proceed with use of the PleurX catheter.
Ultimately, we placed the PleurX catheter in a total of 5 patients. Our oldest patient was a 92-year-old woman (Patient 1). Our youngest patient was a 20-year-old man (Patient 4). The mean age of our other 3 patients (2 men; 1 woman) was 79.6 ± 5 years. In Table I, we include renal comorbidity data (as indicated by the patient's baseline serum creatinine level) or by his or her need for dialysis), because it became apparent in caring for patients with refractory pleural effusions that many have some degree of renal insufficiency. The literature on CHF-induced and medically refractory pleural effusions has not heretofore identified chronic kidney disease as a specific risk factor for the development of this problem. The possible contributory effect of renal disease toward refractory pleural effusions in CHF (our series is too small to draw unequivocal conclusions) may help clinicians to anticipate management difficulties in this small but vexing patient population.
TABLE I. Congestive Heart Failure Causes and Renal Comorbidity Data
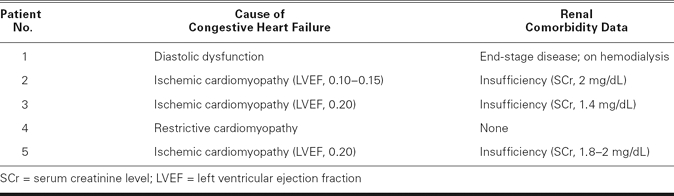
We followed the manufacturer's published procedure17 for placement of the PleurX pleural catheter (Fig. 1), which was done in the operating room under light general anesthesia. After the pleural space was entered by use of a needle, the catheter was introduced into the space over a guidewire that was placed first through the introducer needle. Using 2 small incisions (Fig. 1), we next tunneled the catheter under the skin from the pleural entry point to an exit point in the anterior low chest, about 5 cm from the entry point. The catheter was anchored in place by a subcutaneous hub (built onto the catheter), and about 5 cm of catheter remained outside of the body at the exit point. The external part of the catheter was simply covered with a gauze dressing until the catheter was ready to be used for drainage of the pleural space. Figures 2A and 2B are chest radiographs of one of our patients with a PleurX catheter in the right pleural space.
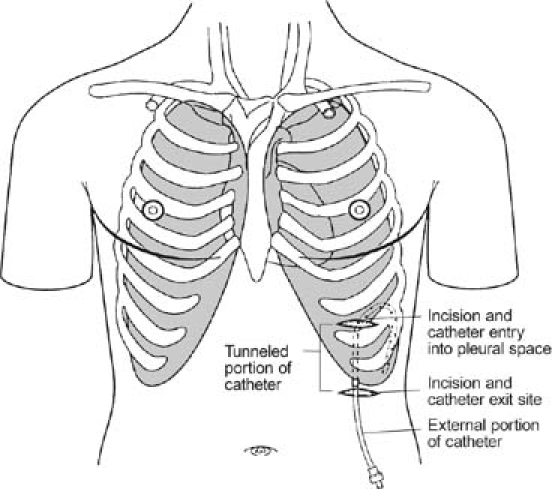
Fig. 1 Diagram shows manufacturer's recommendations for insertion of the PleurX® pleural catheter.
(Illustration courtesy of Denver Biomedical, Inc., part of Cardinal Health, Inc.; Golden, CO. All rights reserved.)
Fig. 2 A) Chest radiograph of patient with PleurX® pleural catheter inserted; B) close view of PleurX® catheter in the same patient.
We also followed the manufacturer's recommendations for drainage of the catheter, using the packaged drainage system that is illustrated in Figure 3. The system had a male adaptor (“access tip” in the illustration) that fit into the female one-way valve of the indwelling PleurX catheter. The access tip connected the PleurX to a plastic, disposable vacuum bottle that was designed to suck fluid from the pleural space. Drainage of the PleurX was done in some circumstances by home health nurses and in others by the patients themselves.
Fig. 3 Diagram shows the drainage components of the PleurX® pleural catheter.
(Illustration courtesy of Denver Biomedical, Inc., part of Cardinal Health, Inc.; Golden, CO. All rights reserved.)
The patients were closely monitored by both the cardiology and pulmonary services over the course of treatment, by means of clinical evaluation and chest radiography.
Results
There were no complications in the placement of the catheters. At the outset, all catheters effectively and completely drained the pleural space. All patients experienced significant improvement in symptoms and quality of life, initially, with catheter placement. All patients experienced an improvement of New York Heart Association functional symptoms from class IV to II. Three patients needed pleural drainage daily and 2 patients twice per week. The average amount of pleural fluid drained was 4 to 500 mL of fluid per session.
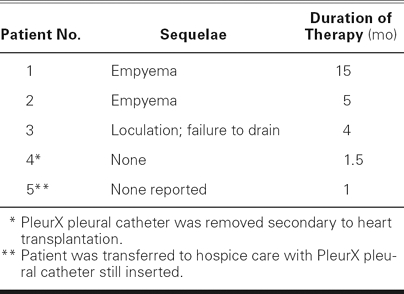
Three patients had sequelae that necessitated removal of the catheter. One patient's catheter stopped functioning (would not drain) and was associated with the development of a partially loculated pleural effusion. In the 2 months after catheter removal, that patient needed 3 subsequent thoracenteses for symptom control. However, the patient needed no additional thoracentesis after that immediate post-catheter period and has done well since. The other 2 patients developed empyema in the pleural space at the location of the PleurX catheter. For 1 of these patients, treatment consisted of catheter removal, 2 subsequent percutaneous thoracenteses, and the administration of antibiotic agents. In spite of our concerns, he did not require surgical intervention for pleural evacuation, and he recovered satisfactorily. Follow-up of this patient revealed a persistent but small and stable right pleural effusion, undoubtedly indicative of partial sclerosis of the pleural space by the empyema. However, one of the patients, a 92-year-old woman, developed full sepsis syndrome with consequent multiple-organ failure and death. For our series of patients, Table II shows the length of PleurX usage and the reasons for removal.
TABLE II. Sequelae and Duration of PleurX® Pleural Catheter Treatment after Right-Sided Insertion
One of our concerns in using the catheter for such frequent and prolonged drainage of pleural fluid lay in the question of whether patients would lose important proteins or develop electrolyte abnormalities. Although we did not specifically test for protein deficiencies, no obvious protein-deficiency problem developed in our patients. All of our patients had frequent serum electrolyte checks in order to monitor diuretic agents, and no obvious blood-chemistry problem manifested itself.
Discussion
Our small case series demonstrates that the PleurX pleural catheter can be safely placed in patients who have medically refractory pleural effusions due to CHF. The catheter appears to function well and certainly can contribute to effective control of effusions in this setting over a period of several weeks. However, prolonged use of the catheter places patients at risk of serious sequelae that include partially loculated pleural effusion, and, more importantly, empyema with consequent sepsis and death.
Pleural effusion is common in CHF. An early radiologic series1 reported that effusion was present in 58% of patients with decompensated CHF. A Mayo Clinic autopsy series2 showed that 72% of patients with CHF had an effusion of greater than 250 mL. A 2000 report3 placed the prevalence of pleural effusion in decompensated CHF as high as 87%. Depending upon the series, approximately 12% to 30% of CHF effusions are unilateral, with two thirds of those being right-sided.2–4,18 For about one quarter of patients with decompensated heart failure, the only initial manifestation is pleural effusion.3 The vast majority of such effusions can be controlled relatively easily with medications.5,6
Occasionally, effusions persist as large and symptomatic despite the usual management with diuretic agents and with even more aggressive medical maneuvers, such as preload and afterload reduction and the use of inotropic solutions. Such effusions typically prompt the performance of thoracentesis, which is easy, low risk, and very effective.5,6 For the rare circumstance in which recurrent thoracenteses are required, pleurodesis is generally recommended as the alternative next step.5,6
A review of 18 published reports on “closed” pleurodesis (the infusion of various sclerosing agents through a chest tube) shows it to provide a satisfactory outcome about 80% of the time in the treatment of persistent nonmalignant pleural effusions.7 However, in addition to the usual sequelae of pleurodesis that have been reported in other patient populations—which include pain, fever, talc embolization, empyema, adult respiratory distress syndrome, and respiratory failure8—pleurodesis for CHF can lead to the development of a contralateral effusion.19 In fact, for that reason several experts have recommended against pleurodesis in the CHF population, under any circumstances.20 There is also a theoretical concern that pulmonary edema could be worsened by pleurodesis,6 but that outcome has not yet been reported.
The standard surgical or “open” technique for pleurodesis is now VATS, and this procedure has been shown to be effective for malignant effusions, which is the most common reason for pleurodesis.8 However, the medical literature reports very little experience in the use of this technique for heart-failure–associated effusions.9–11 Although these reports are encouraging, they are too few to form a basis for recommendations. We presume that so few cases of surgical pleurodesis have been reported in the CHF population because the risk of significant chest surgery, requiring general anesthesia, is high for patients with heart failure that is sufficiently advanced to cause refractory pleural effusions.
All of the patients in our series had medically refractory effusions due primarily to CHF. Four of our 5 patients had renal insufficiency. In 3 of the patients, renal function was only mildly impaired, but 1 patient required dialysis. Chronic kidney disease has not, heretofore, been specifically identified as a comorbid condition important to the development of refractory pleural effusions in heart failure. However, it is not surprising that we found this to be so in our series, because renal impairment makes medical control of CHF much more difficult. Each of our patients had come to the point where he or she required frequent thoracentesis (at least every 2 weeks) for symptomatic control. One patient required pleural fluid drainage every 3 to 4 days. None of our patients was a good candidate for VATS pleurodesis (presumably the most reliable technique), either because surgery was too high a risk or because the potential for heart transplantation contraindicated the procedure. Closed pleurodesis in the setting of CHF was not an attractive option for us, because of our prior experience with partially loculated effusions subsequent to that approach. Therefore, we came to consider alternatives—specifically the use of the PleurX pleural catheter. Other authors also have recognized the need for innovative alternatives to this vexing clinical problem, including the chronic placement of an indwelling pleural-drainage catheter.12
The PleurX pleural catheter was recently developed for the treatment of malignant pleural effusions in patients whose disease process warrants less invasive management than pleurodesis.13 It has performed well for this indication.13–16 There are, however, no prior reports of this catheter's use in the management of transudative pleural effusions due to CHF or to any other condition. All of the catheters in our series were placed without complications. With the exception of 1 PleurX, which was removed after 4 months due to luminal obstruction by débris, the catheters functioned well while they were in place, and did not cause patients discomfort or significant inconvenience. None of the patients exhibited obvious deleterious effects associated with frequent draining. However, we did not specifically measure albumin or pre-albumin levels or other markers of protein malnutrition that could be of concern in instances of repeated pleural fluid drainage.
For 2 of our patients, the catheter performed ideally as a tool to temporarily control a refractory pleural effusion. In 1 of those patients, it provided very effective symptom control and comfort during the last several weeks of her end-stage cardiomyopathy, as part of a hospice program. For the other patient, it enabled relief of recurrent effusions and consequent dyspnea and debility over a period of 6 weeks until a heart transplantation was performed, at which time the catheter was removed.
In the other 3 patients of our series, the catheter functioned well for a 4- to 15-month period, effecting major improvement in New York Heart Association functional class and quality of life; however, it eventually caused complications. One patient, as mentioned above, had the nonfunctioning catheter removed at 4 months and was left with a partially loculated pleural effusion. The other 2 other patients developed catheter-associated empyemas. One of these patients, who had done very well with the catheter for 5 months, was treated effectively for the empyema with only catheter removal, antibiotic administration, and 2 acute thoracenteses. This patient, interestingly, required no further pleural drainage procedures after the acute treatment of his empyema, and he did very well for many months, before his sudden death. The infection, in effect, caused a pleurodesis. The other empyema case developed after 15 months of catheter use. The empyema led to septic shock, and the patient eventually died of complications due to multiple-organ-failure syndrome. The reported incidence of empyema when the PleurX is used to manage malignant effusions is 12%.14–16 We believe our incidence of empyema to be higher (40%, although our series is small), because our patients had the catheter in place for longer than is usual in cancer patients.
Conclusion
Our series demonstrates that the PleurX pleural catheter, at this time, has a limited place in the management of heart-failure–associated pleural effusions. It would seem to be an excellent option for end-stage heart-failure patients who suffer from medically refractory effusions in the setting of hospice care, to provide short-term palliation of what can, in this circumstance, be very severe symptoms. In addition, it may be considered a helpful tool within the broader therapeutic context of a short-term bridge to more decisive treatment of advanced heart failure, when that treatment has been complicated or delayed by difficult-to-control pleural effusions.
The main concern regarding use of the PleurX pleural catheter in the CHF setting is the potential for empyema development, especially when the catheter is in place for a prolonged period. Therefore, if the PleurX is used for long-term drainage of CHF-associated pleural effusions in settings other than those specified above, the attending physician must maintain high vigilance for the development of an infected pleural cavity. Indeed, attempts are warranted to detect empyema early, through the use of such methods as the serial monitoring of white blood cell count and the culturing of pleural fluid samples. Finally, the concept of a chronically accessible pleural drain for refractory effusions due to heart failure may be worth further investigation as materials, construction, and insertion techniques for chronic indwelling catheters become more effective in preventing device-associated infection.
Footnotes
Address for reprints: James P. Herlihy, MD, 6624 Fannin St., Suite 1730, Houston, TX 77030. E-mail: jph@houstonlungdocs.com
References
- 1.Logue RB, Rogers JV, Gay BB. Subtle roentgenographic signs of left heart failure. Am Heart J 1963;65:464–73.
- 2.Edwards JE, Race GA, Scheifley CH. Hydrothorax in congestive heart failure. Am J Med 1957;22(1):83–9. [DOI] [PubMed]
- 3.Kataoka H. Pericardial and pleural effusions in decompensated chronic heart failure. Am Heart J 2000;139(5):918–23. [DOI] [PubMed]
- 4.Woodring JH. Distribution of pleural effusion in congestive heart failure: what is atypical? South Med J 2005;98(5): 518–23. [DOI] [PubMed]
- 5.Light RW, Broaddus VC. Pleural effusion. In: Murray JF, Nadel JA, Mason RJ, Boushey HA, editors. Textbook of respiratory medicine. 3rd ed. Philadelphia: WB Saunders; 2000. p. 2013–41.
- 6.Kinasewitz GT. Transudative effusions. Eur Respir J 1997; 10(3):714–8. [PubMed]
- 7.Sudduth CD, Sahn SA. Pleurodesis for nonmalignant pleural effusions. Recommendations. Chest 1992;102(6):1855–60. [DOI] [PubMed]
- 8.West SD, Davies RJ, Lee YC. Pleurodesis for malignant pleural effusions: current controversies and variations in practices. Curr Opin Pulm Med 2004;10(4):305–10. [DOI] [PubMed]
- 9.Alrawi SJ, Raju R, Acinapura AJ, Cunningham JN Jr, Cane JS. Primary thoracoscopic evaluation of pleural effusion with local anesthesia: an alternative approach. JSLS 2002;6(2):143–7. [PMC free article] [PubMed]
- 10.de Campos JR, Vargas FS, de Campos Werebe E, Cardoso P, Teixeira LR, Jatene FB, Light RW. Thoracoscopy talc poudrage: a 15-year experience. Chest 2001;119(3):801–6. [DOI] [PubMed]
- 11.Panebianco V, Calanducci F, Poli A, Blandino R, Pistritto A, Ferreri ME, et al. Pleuroscopy and talc pleurodesis in recurrent pleural effusions: experience with 51 cases [in Italian]. G Chir 1995;16(10):437–41. [PubMed]
- 12.Alisky JM. Implantable central venous access ports for minimally invasive repetitive drainage of pleural effusions. Med Hypotheses 2007;68(4):910–1. [DOI] [PubMed]
- 13.Putnam JB Jr, Light RW, Rodriguez RM, Ponn R, Olak J, Pollak JS, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999;86(10):1992–9. [PubMed]
- 14.Burgers JA, Olijve A, Baas P. Chronic indwelling pleural catheter for malignant pleural effusion in 25 patients [in Dutch]. Ned Tijdschr Geneeskd 2006;150(29):1618–23. [PubMed]
- 15.van den Toorn LM, Schaap E, Surmont VF, Pouw EM, van der Rijt KC, van Klaveren RJ. Management of recurrent malignant pleural effusions with a chronic indwelling pleural catheter. Lung Cancer 2005;50(1):123–7. [DOI] [PubMed]
- 16.Putnam JB Jr, Walsh GL, Swisher SG, Roth JA, Suell DM, Vaporciyan AA, et al. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Ann Thorac Surg 2000;69(2):369–75. [DOI] [PubMed]
- 17.http://www.denverbio.com/physician_pleurx_catheter.html
- 18.Weiss JM, Spodick DH. Laterality of pleural effusions in chronic congestive heart failure. Am J Cardiol 1984;53(7): 951. [DOI] [PubMed]
- 19.Davidoff D, Naparstek Y, Eliakim M. The use of pleurodesis for intractable pleural effusion due to congestive heart failure. Postgrad Med J 1983;59(691):330–1. [DOI] [PMC free article] [PubMed]
- 20.Storm HK, Viskum K. Therapeutic pleurodesis in spontaneous pneumothorax, malignant pleural effusion, heart insufficiency and chylothorax [in Danish]. Ugeskr Laeger 1991;153 (14):989–92. [PubMed]


