Abstract
Pulsatile ventricular assist devices have successfully provided circulatory support for many patients throughout the past quarter century; however, persistent complications have hindered expanded clinical application of this technology. Although the use of smaller, continuous-flow ventricular assist device pumps has reduced the frequency and severity of some adverse events, design enhancement may further improve outcomes for patients who require long-term left ventricular support. One new product, the HeartWare, Inc., miniature ventricular assist device, features a wide-bladed rotor design in an axial-flow pump with a strong, passively suspended magnetic rotor. The operating range of 16,000 to 28,000 rpm can provide up to 10 L/min of flow. The wide blades portend minimal cellular trauma.
This new device has not yet been approved for use in human beings. As a test, we implanted it in a calf, and we continuously monitored the device's performance and the hemodynamic results over 30 days. No mechanical failure occurred, and no thrombi were noted upon explantation of the device. The animal's circulation was stable during the test period, and no end-organ abnormalities were found upon autopsy. The potential benefits of this miniature ventricular assist device are its increased availability to a broader patient population, a lower risk of infection, simplified implantation procedures, and improved durability. Further in vivo testing is planned. Herein, we discuss the unique design of the HeartWare miniature ventricular assist device, our feasibility study of its performance, and the possibilities for its use in human beings.
Key words: Cardiac output/physiology, cattle, equipment design/safety, feasibility studies, heart-assist devices, heart failure/therapy, hemodynamics, prosthesis design/implantation, risk factors, treatment outcome
Implantable, pulsatile-flow ventricular assist device (VAD) systems have been used successfully for a quarter of a century as a bridge to heart transplantation, as a bridge to myocardial recovery, and as destination therapy.1–4 Early designs were influenced by the idea that mechanical blood pumps must mimic the natural heart with regard to blood flow rate and intermittent changes in pressure (pulsatility). Accordingly, VAD pumps have been designed with stroke volumes in the range of 50 to 100 mL and pumping rates of 50 to 120 beats/min. However, these devices are large and are compatible only with patients of larger body sizes. Furthermore, complications related to device operation or implantation have hampered the effectiveness of these VADs. Design technology has evolved so that newer, smaller, and more reliable VADs can now create continuous blood flow, unlike that produced by natural-heart or pulsatile VADs.
The miniature VAD (MVAD™, HeartWare®, Inc.; Miramar, Fla) is a small pump with a wide-bladed rotor design and magnetically suspended impeller that generates axial blood flow. This design portends improved durability and performance. The MVAD is not yet approved for use in human beings. In this report, we present the unique design features of this new VAD, our feasibility study of its performance in a calf, and the device's possibilities for use in human patients who require long-term left ventricular support.
Materials and Methods
Device Description
The HeartWare MVAD is a small axial-flow pump that can generate blood flow up to 10 L/min (Fig. 1). The displacement volume is 15 cc. The cylindrical titanium housing contains an electromagnetic motor stator with an external power supply (Fig. 2). Within the housing is a wide-bladed rotor that creates axial flow through the device during operation, when it is positioned within the cardiovascular system. The rotor is constructed of magnetic and biocompatible material, which is hard enough to render a coating unnecessary. The hydrodynamic thrust bearings are larger and wider than the area between the blades where blood flows (Fig. 3); this affords sufficient area for passive radial rotor suspension, and for the accommodation of magnets that create a strong electromagnetic coupling. The configuration confers good systemic power efficiency and passive motor constraint. The passive rotor-suspension system obviates mechanical bearings and other rotor-support structures. The outflow is angled at 90° from the pump housing and is attached to a 10-mm double-woven outflow graft for anastomosis to the aorta. Small wires from the pump motor are tunneled subcutaneously and exit through the skin, where they are connected to external power and control units. The pump speed is adjustable from 16,000 to 28,000 rpm, and the current can be monitored continuously to ensure proper functioning of the pump. Pump flow is monitored by means of an ultrasonic flow probe on the outflow graft.

Fig. 1 The HeartWare MVAD™ pump, held in an adult human hand.
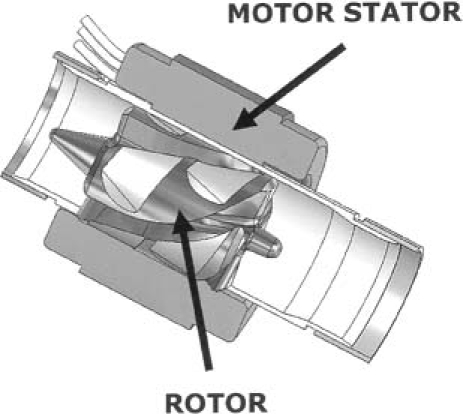
Fig. 2 The internal components of the HeartWare MVAD™ pump, and the locations of the motor stator and the rotor (arrows).
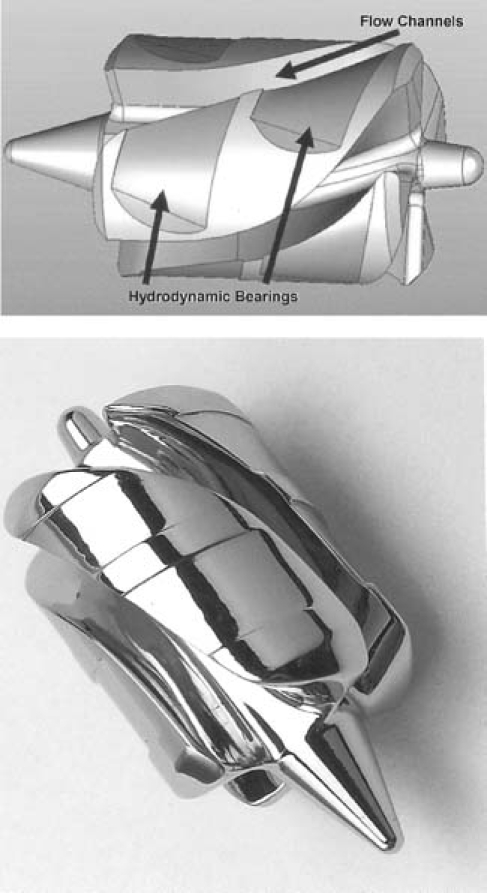
Fig. 3 A) Schematic drawing of the wide-bladed rotor of the HeartWare MVAD™, with the blood-flow channels and hydrodynamic bearings identified. B) Photograph of the rotor.
Feasibility Study in a Calf
We implanted the HeartWare MVAD in a Jersey calf for an intended 30-day pilot test. The animal was prepared for surgery in standard fashion, and all care was provided in accordance with the “Guide for Care and Use of Laboratory Animals” (NIH publication 85-23, revised 1996). Baseline laboratory values, including blood cell counts, coagulation test results, and blood chemistries, were obtained before the induction of anesthesia. Anesthesia was induced by use of intravenous thiopental (10 mg/kg) and was maintained by means of inhaled isoflurane (1%–2%). Electrocardiographic (ECG) readings, arterial line pressure, and pulse oximetry were monitored continuously during implantation, and also postoperatively. A left thoracotomy between the 5th and 6th ribs exposed the left ventricular apex and the descending thoracic aorta. A pulmonary artery catheter was inserted via the right atrial appendage, and hemodynamic data were recorded, including central venous pressure, pulmonary artery pressure, and systolic, diastolic, and mean arterial blood pressures. Heparin (300 U/kg) was administered for anticoagulation before anastomosis of the outflow graft to the descending aorta. Cardiopulmonary bypass was not necessary during this operation. After the attachment of a sewing ring to the left ventricular apex, a ventriculotomy was performed with a circular knife. The MVAD pump was inserted into the left ventricle and was secured to the sewing ring by the tightening of a locking screw. After complete de-airing of the system, the clamp on the outflow graft was removed, and normal operation of the MVAD was initiated. A 10-mm Transonic flow probe (Transonic Systems Inc.; Ithaca, NY) was attached to the outflow graft and was monitored by use of an HT-205 flow meter (Transonic). Once the MVAD was operating satisfactorily, hemodynamic and laboratory data were recorded. The heparin was reversed with protamine sulfate (500 mg over 30 min). The MVAD driveline and flow-probe cable were positioned so that they would exit the thoracotomy toward the back of the animal. Chest tubes were placed, and the thoracotomy was closed. Anesthesia was discontinued; the animal was placed in a stanchion and was then transferred to a recovery area. Postoperatively, the calf's vital signs and the pump-operating values were recorded daily, at scheduled intervals.
Results of the Feasibility Study
No mechanical or medical complications were observed during the postimplantation period, and the animal's hemodynamic levels were stable throughout the study (Fig. 4). Weekly laboratory values were within normal ranges (or they reflected expected changes), with the exception of the hemoglobin and hematocrit values, which decreased during week 2 (Table I). These values returned to normal by week 4. The calf received no transfusions during the implantation procedure or the study period. The pump speed of the MVAD ranged from 19,500 to 21,300 rpm, and mean daily left VAD flow rates of 4.25 ± 0.75 L/min (range, 3.5–5.1 L/min) were maintained (Fig. 5). Thermodilutional cardiac output measurements during the initial 8 postoperative days indicated that the VAD flow rate was, on average, 79.4% of the total cardiac output. Arterial blood pressure was maintained within a normal range; the average daily systolic, diastolic, and mean arterial pressures were 120 ± 15.2, 73.4 ± 11.8, and 89.0 ± 12.1 mmHg, respectively (Fig. 6). The average pulse pressure was 46.8 ± 9.2 mmHg, indicating that some pulsatility was maintained and that there was less than maximal unloading of the left ventricle. The plasma free hemoglobin peaked at 100 mg/dL on the 1st postoperative day. This value stabilized at 10 mg/dL during weeks 3 and 4. On day 31, the calf was euthanized in accordance with protocol, and a same-day autopsy was performed. The explanted pump was free of thrombi, and no thermal damage to it was identified. No renal, hepatic, splenic, or brain abnormalities were evident in the calf.
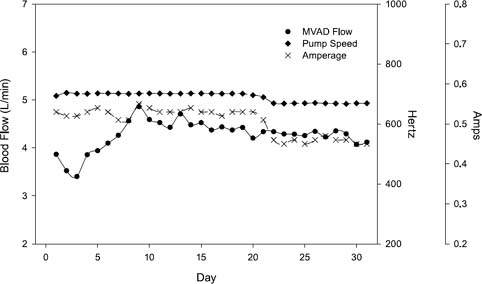
Fig. 4 Plots of the average daily values of flow, pump-speed setting, and amperage, after the implantation of the HeartWare MVAD™ in a calf.
MVAD = miniature ventricular assist device
TABLE I. Laboratory Values at Baseline and during the Study Period
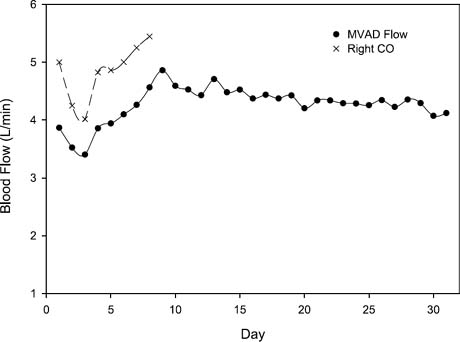
Fig. 5 Daily flow rates were 4.25 ± 0.75 L/min (range, 3.5–5.1 L/min). Thermodilutional cardiac output measurements during the initial 8 postoperative days indicated that the MVAD™ flow rate was, on average, 79.4% of the total cardiac output.
MVAD = miniature ventricular assist device
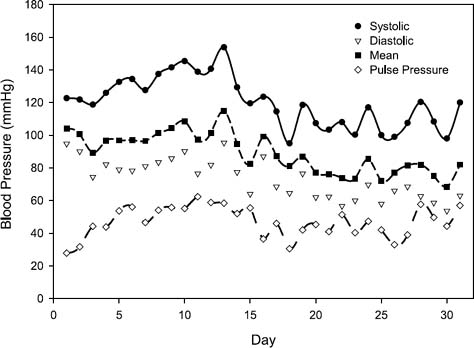
Fig. 6 Plots of the average daily values of the arterial systolic, diastolic, mean, and pulse pressures, over the duration of the study.
Discussion
Continuous-flow VADs afford several potential advantages over pulsatile-flow VADs. The smaller size of continuous-flow VADs enables their application in patients of a broader range of body sizes. Smaller pumps can be implanted with less-invasive surgery. Unlike the pulsatile-flow VADs, they generally do not need to be placed in an abdominal pocket: a difference that reduces patients' susceptibility to infection. These continuous-flow devices are simpler than the pulsatile devices, with a single moving part—the rotor—which portends greater durability. Most axial-flow VADs contain a rotor held in position by 2 bearings that are lubricated by circulating blood.5–7 In contrast, most blood pumps that have magnetically suspended impellers generate flow by centrifugal force and do not use bearings, which reduces component wear. A theoretical advantage of magnetically suspended pumps with non-contact bearings is that there is no friction or heat generation8; the absence of frictional heat should result in less thrombogenesis and thus less need for anticoagulation therapy in patients.
Complications in human patients who have received VAD support have been reduced by improved supportive care gained from experience, and by the refinement of blood-pump technology.3,9 Smaller axial-flow VADs have been shown in clinical studies to be more durable and lead to fewer complications than the older, pulsatile VADs.10–13 Axial-flow devices can be implanted without the use of cardiopulmonary bypass.14 Smaller left VADs require less invasive surgery for implantation, which minimizes postoperative bleeding complications. Less surgical and coagulopathic bleeding reduces infection rates, which is noteworthy because postoperative bleeding and infection continue to be the chief causes of morbidity and death after VAD implantation.15–17
During this pilot study of the HeartWare MVAD, the ideal pump speed for optimal clinical support was not fully studied. The maximum speed setting is 28,000 rpm; in this study, the speed was set at 21,300 rpm at highest. The thermodilutional cardiac output values and the calculated pulse pressure indicate that there was continuous but submaximal unloading of the left ventricle. While this may be the optimal support, further characterization of this system is necessary.
The MVAD features additional refinements that could continue the trend toward better long-term outcomes in VAD usage. The small, efficient rotor allows the housing to be miniaturized and to be manufactured in a variety of configurations. The MVAD can be placed within the left ventricle, eliminating the need for an inflow conduit and placement in an extracardiac pocket. Smaller pumps have less surface area for bacterial colonization, and intraventricular placement appears to minimize left VAD infections because of the continued exposure to blood flow.18
The wide hydrodynamic thrust bearings in the MVAD pump should minimize shear stress on cellular components of the blood. Furthermore, this pump did not generate heat from friction and should thus reduce or eliminate heat-induced platelet activation. The small surface area of this VAD and its low shear rate should also result in lower rates of coagulopathy and postoperative bleeding. This in turn reduces complications associated with blood transfusion, such as pulmonary hypertension, right heart dysfunction, and hepatic dysfunction. It is possible that this VAD will necessitate only minimal anticoagulation therapy in patients, because of its potential for low blood trauma and low thrombogenicity. More study on this effect is needed.
The outcome of our pilot test of the new HeartWare MVAD has confirmed its in vivo functional feasibility. Additional animal studies are planned in order to evaluate this device further and to verify our initial findings.
Footnotes
Address for reprints: Mark S. Slaughter, MD, 201 Abraham Flexner Way, Suite 1200, Louisville, KY 40202-3841. E-mail: mscabg@aol.com
*Certified Cardiovascular Perfusionist
References
- 1.Frazier OH, Rose EA, Oz MC, Dembitsky W, McCarthy P, Radovancevic B, et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg 2001;122(6):1186–95. [DOI] [PubMed]
- 2.Portner PM, Jansen PG, Oyer PE, Wheeldon DR, Ramasamy N. Improved outcomes with an implantable left ventricular assist system: a multicenter study. Ann Thorac Surg 2001;71 (1):205–9. [DOI] [PubMed]
- 3.Long JW, Kfoury AG, Slaughter MS, Silver M, Milano C, Rogers J, et al. Long-term destination therapy with the HeartMate XVE left ventricular assist device: improved outcomes since the REMATCH study. Congest Heart Fail 2005;11(3): 133–8. [DOI] [PubMed]
- 4.Frazier OH, Myers TJ. Left ventricular assist system as a bridge to myocardial recovery. Ann Thorac Surg 1999;68(2): 734–41. [DOI] [PubMed]
- 5.Burke DJ, Burke E, Parsaie F, Poirier V, Butler K, Thomas D, et al. The Heartmate II: design and development of a fully sealed axial flow left ventricular assist system. Artif Organs 2001;25(5):380–5. [DOI] [PubMed]
- 6.Myers TJ, Gregoric ID, Tamez D, Jarvik RK, Frazier OH, Inman RW, et al. Development of the Jarvik 2000 intraventricular axial-flow left ventricular assist system. J Congest Heart Fail Circ Support 2000;1:133–40.
- 7.Tayama E, Olsen DB, Ohashi Y, Benkowski R, Morley D, Noon GP, et al. The DeBakey ventricular assist device: current status in 1997. Artif Organs 1999;23(12):1113–6. [DOI] [PubMed]
- 8.Hoshi H, Shinshi T, Takatani S. Third-generation blood pumps with mechanical noncontact magnetic bearings. Artif Organs 2006;30(5):324–38. [DOI] [PubMed]
- 9.Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation 2007;116 (5):497–505. [DOI] [PubMed]
- 10.Selzman CH, Sheridan BC. Off-pump insertion of continuous flow left ventricular assist devices. J Card Surg 2007;22(4):320–2. [DOI] [PubMed]
- 11.Wilhelm MJ, Hammel D, Schmid C, Rhode A, Kaan T, Rothenburger M, et al. Long-term support of 9 patients with the DeBakey VAD for more than 200 days. J Thorac Cardiovasc Surg 2005;130(4):1122–9. [DOI] [PubMed]
- 12.Smart FW, Palanichamy N. Left ventricular assist device therapy for end-stage congestive heart failure: from REMATCH to the future. Congest Heart Fail 2005;11(4):188–93. [DOI] [PubMed]
- 13.Frazier OH, Shah NA, Myers TJ, Robertson KD, Gregoric ID, Delgado R. Use of the Flowmaker (Jarvik 2000) left ventricular assist device for destination therapy and bridging to transplantation. Cardiology 2004;101(1–3):111–6. [DOI] [PubMed]
- 14.Frazier OH. Implantation of the Jarvik 2000 left ventricular assist device without the use of cardiopulmonary bypass. Ann Thorac Surg 2003;75(3):1028–30. [DOI] [PubMed]
- 15.Zierer A, Melby SJ, Voeller RK, Guthrie TJ, Ewald GA, Shelton K, et al. Late-onset driveline infections: the Achilles' heel of prolonged left ventricular assist device support. Ann Thorac Surg 2007;84(2):515–20. [DOI] [PubMed]
- 16.Hunt SA. Mechanical circulatory support: new data, old problems. Circulation 2007;116(5):461–2. [DOI] [PubMed]
- 17.Holman WL, Pamboukian SV, Blood M, Tallaj JA, McGiffin DC, Kirklin JK. Managing device infections: are we progressing or is infection an insurmountable obstacle? ASAIO J 2005;51(4):452–5. [DOI] [PubMed]
- 18.Siegenthaler MP, Martin J, Pernice K, Doenst T, Sorg S, Trummer G, et al. The Jarvik 2000 is associated with less infections than the HeartMate left ventricular assist device. Eur JCardiothorac Surg 2003;23(5):748–55. [DOI] [PubMed]


