Abstract
Thyroid cancer incidence has been rising in the United States, and this trend has often been attributed to heightened medical surveillance and use of improved diagnostics. Thyroid cancer incidence varies by sex and race/ethnicity, and these factors also influence access to and utilization of healthcare. We therefore examined thyroid cancer incidence rates by demographic and tumor characteristics, based on 48,403 thyroid cancer patients diagnosed during 1980–2005 from the Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute. Rates varied by histologic type, sex, and race/ethnicity. Papillary carcinoma was the only histologic type for which incidence rates rose consistently among all racial/ethnic groups. Subsequent analyses focused on the 39,706 papillary thyroid cancers diagnosed during this period. Papillary carcinoma rates increased most rapidly among females. Between 1992–1995 and 2003–2005, they rose nearly 100% among White Non-Hispanics and Black females, but only 20–50% among White Hispanics, Asian/Pacific Islanders and Black males. Increases were most rapid for localized stage and small tumors; however, rates also rose for large tumors and tumors of regional and distant stage. Since 1992–1995, half the overall increase in papillary carcinoma rates was due to rising rates of very small (≤1.0 cm) cancers, 30% to cancers 1.1–2 cm, and 20% to cancers >2 cm. Among White females, the rate of increase for cancers >5 cm almost equaled that for the smallest cancers. Medical surveillance and more sensitive diagnostic procedures cannot completely explain the observed rises in papillary thyroid cancer rates. Thus, other possible explanations should be explored.
Keywords: Incidence, Thyroid cancer, Papillary, SEER Program
Introduction
The incidence of thyroid cancer has been rising in the United States and other developed countries over the past three decades.(1–8) Recently, Davies and Welch (3) analyzed data from the Surveillance, Epidemiology, and End Results (SEER) program and estimated that thyroid incidence rates rose more than 2-fold from 1973 to 2002, while the mortality rates remained relatively constant. When the data were analyzed by histologic type and tumor size, the increase appeared predominantly among small (≤2 cm) papillary carcinomas. In a study conducted in Ontario, Canada, Kent et al.(9) reported a similar increase for differentiated cancers ≤2 cm in diameter. The authors of both studies concluded that the observed increase in disease incidence was an artifact due to the use of better diagnostic tools such as ultrasonography and fine- needle aspiration biopsy. Others have suggested that the data do not definitively support that conclusion and that there may be a true increase in thyroid cancer incidence (10) due to changes in other risk factors.(11)
The American Cancer Society estimates that 37,340 new cases of thyroid cancer will be diagnosed in the United States during 2008.(12) About 75% of these cases are expected to occur among females, making it the sixth most common cancer among women.(12) Thyroid cancer incidence rates also differ by race and ethnicity. Rates are twice as high among Whites as Blacks,(13) notably elevated among Asians, especially those from Southeast Asia,(14) and the incidence patterns by Hispanic ethnicity are unclear.(13, 15) Although sex and race/ethnicity are also associated with healthcare access and utilization these characteristics were not taken into account when evaluating trends in thyroid cancer incidence during the previous analyses.(3, 9)
Surgery alone with or without adjuvant radioiodine, is the preferred treatment for differentiated thyroid cancer regardless of tumor size,(16) and while patients experience excellent 5-year survival rates (97%), treatment-related morbidity is not insignificant. Therefore, if the reported increase in thyroid cancer is due to an upsurge in the diagnosis of subclinical tumors, maybe the clinical management of these cancers should be reconsidered. In a counter argument, Mazzaferri (17) has stressed that small, asymptomatic thyroid cancers can be metastatic and diagnostic delays can result in higher rates of distant metastasis. If incidence patterns cannot be explained completely by better detection (e.g., if not only localized but also more advanced tumors are increasing), additional descriptive and etiological studies will be necessary to determine the underlying cause(s) for the increase in disease incidence.
To address these issues, we conducted an in-depth analysis of SEER thyroid cancer incidence trends from 1980 to 2005. We focused on papillary carcinoma, the histologic type showing the largest increase in incidence over time, and then evaluated the papillary carcinoma incidence trends in terms of stage of disease and tumor size. Age-specific trends were evaluated for supporting evidence of any predominant period (improved diagnostics) or cohort (exposure) effects, and all analyses were conducted stratified by sex and race/ethnicity.
Materials and Methods
Data Source
Thyroid cancer incidence data were obtained from the SEER program of the National Cancer Institute which began collecting data in the early 1970s from nine population-based registries: Connecticut, Iowa, New Mexico, Utah, Hawaii, Detroit, San Francisco-Oakland, Atlanta, and Seattle-Puget Sound.(18) These SEER-9 registries include approximately 10% of the U.S. population. In 1992, four registries were added: San Jose-Monterey, Los Angeles, rural Georgia, and Alaska Natives, expanding the coverage to 14% of the U.S. population. Our analysis included data available for the racial categories of White and Black since the early years of the SEER-9 registries and for Asian/Pacific Islander (API) and Hispanic ethnicity since 1992 from the SEER-13 registries. We excluded rural Georgia and Alaska Natives from the analyses because these two registries included relatively few APIs or Hispanics.
Case Definition and Tumor Characteristics
Analyses were restricted to 48,403 patients with malignant thyroid tumors that were microscopically confirmed and not diagnosed at autopsy or identified solely through death certificates; 887 (1.8%) cases were excluded based on these criteria. Since the late 1970s, histologic type has been coded according to the first International Classification of Diseases for Oncology (ICD-O),(19) the second edition ICD-O-2 for cases diagnosed from 1992 to 2001,(20) and the third edition ICD-O-3 for cases diagnosed since 2001;(21) all cases have been recoded using the ICD-O-3. To allow adequate time for all registries to convert to ICD-O coding the first year included for analyses was 1980. Histologic categories of papillary, follicular, medullary, anaplastic, other, and unspecified were defined according to the recommendations of the International Association of Cancer Research as used in Cancer Incidence in Five Continents, Volume IX.(22) Data on stage at diagnosis (localized, regional, or distant) was determined for each case according to SEER Historic Stage A codes. Stage at diagnosis was evaluated first because this variable was available for the entire study period. Tumor size has been recorded in the SEER data for thyroid cancer cases diagnosed since 1983; however, prior to 1988 size was not stated for 23% of all thyroid tumors. Therefore size was analyzed beginning in 1988 by combining two SEER variables, “EOD 10- size” (1988–2003) and “CS tumor size” (2004+) to form a single size (cm) variable.
Data Analysis
For long-term SEER-9 trend analyses among Whites, regardless of Hispanic ethnicity, and Blacks, years of diagnosis were grouped into seven calendar-year categories: 1980–1983, 1984–1987, 1988–1991, 1992–1995, 1996–1999, 2000–2002, and 2003–2005. For Whites, stratified by Hispanic ethnicity, and APIs, shorter-term trend analyses included the last four calendar-year categories 1992–1995 to 2003–2005. All incidence rates were age-adjusted to the 2000 U.S. population and expressed per 100,000 person-years using SEER*Stat Version 6.4.4. 1 Disease incidence rates were reported if there were at least 10 cases in a given sex, race/ethnicity, and time category, and trend lines were plotted if at least two consecutive categories met this criterion. Temporal trends were plotted using semi-logarithmic scales so that slopes or rates of change could be compared; a slope of 10 degrees represents a change of 1% per year (i.e., 40 years on the horizontal axis is the same length as one logarithmic cycle on the vertical axis).(23) The percent changes between the first and last calendar-year categories were also calculated.
Results
The most common histological type among all sex and racial/ethnic groups was papillary thyroid carcinoma (range: 65%–88%) followed by follicular carcinoma (range: 9%–23%; see Tables 1 and 2). Papillary and follicular carcinoma rates were consistently 2–3 times higher among females than males. Incidence rates tended to be higher among Whites than Blacks and among White Non Hispanics than White Hispanics or APIs.
Table 1.
Incidence rates of thyroid cancer by race, sex, and histology 1980–2005, SEER-9 registries.
| Whites |
Blacks |
|||||||
|---|---|---|---|---|---|---|---|---|
| Female |
Male |
Female |
Male |
|||||
| Histology | Count | Rate | Count | Rate | Count | Rate | Count | Rate |
| Overall | 24,764 | 9.59 | 8,557 | 3.59b | 1,743 | 5.28a | 458 | 1.84a,b |
| Papillary | 20,559 | 8.00 | 6,532 | 2.70b | 1,276 | 3.80a | 299 | 1.16a,b |
| Follicular | 2,872 | 1.10 | 1,217 | 0.53b | 353 | 1.10 | 106 | 0.47b |
| Medullary | 533 | 0.20 | 349 | 0.15b | 33 | 0.10a | 24 | 0.09a |
| Anaplastic | 303 | 0.11 | 189 | 0.09 | 18 | 0.07 | 11 | 0.05 |
| Other specified | 206 | 0.08 | 141 | 0.06 | 16 | 0.06 | 9 | - |
| Unspecified | 291 | 0.11 | 129 | 0.06b | 47 | 0.15a | 9 | - |
Age-adjusted (2000 US Standard Population) rates per 100,000 person-years.
P <0.05 in comparison to same-sex Whites.
P <0.01 in comparison to same-race females.
-Rates were not calculated when counts were less than 10.
Table 2.
Incidence rates of thyroid cancer by race/ethnicity, sex, and histology 1992–2005, SEER-11 registries*.
| White Non-Hispanics |
White Hispanics |
Asian/Pacific Islanders |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female |
Male |
Female |
Male |
Female |
Male |
|||||||
| Histology | Count | Rate | Count | Rate | Count | Rate | Count | Rate | Count | Rate | Count | Rate |
| Overall | 19,243 | 11.54 | 6,927 | 4.33b | 4,057 | 10.94a | 935 | 3.09a,b | 3,652 | 12.08a | 931 | 3.72a,b |
| Papillary | 16,450 | 9.94 | 5,444 | 3.38b | 3,549 | 9.34a | 772 | 2.48a,b | 3,204 | 10.53a | 775 | 3.04a,b |
| Follicular | 1,935 | 1.13 | 890 | 0.56b | 352 | 1.05 | 88 | 0.31a,b | 315 | 1.06 | 94 | 0.40a,b |
| Medullary | 358 | 0.21 | 261 | 0.16b | 67 | 0.21 | 44 | 0.16 | 40 | 0.13a | 23 | 0.09a |
| Anaplastic | 205 | 0.10 | 146 | 0.10 | 40 | 0.18a | 8 | - | 38 | 0.15a | 22 | 0.11 |
| Other | ||||||||||||
| specified | 141 | 0.08 | 104 | 0.07 | 24 | 0.09 | 16 | 0.07 | 37 | 0.13a | 6 | - |
| Unspecified | 154 | 0.08 | 82 | 0.05b | 25 | 0.09 | 7 | - | 18 | 0.07 | 11 | 0.06 |
SEER-13 registries excluding the Alaska Native and Rural Georgia registries.
Age-adjusted (2000 US Standard Population) rates per 100,000 person-years.
P ≤0.05 in comparison to same-sex White Non-Hispanics.
P ≤0.01 in comparison to same-race/ethnicity females.
-Rates were not calculated when counts were less than 10.
Histology-Specific Time Trends
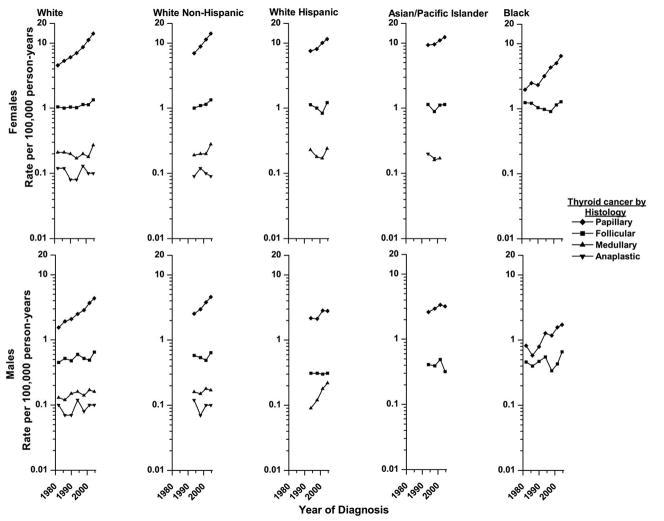
From 1980–1983 to 2003–2005, papillary carcinoma rates tripled among White and Black females and doubled among White and Black males (p-values< 0.001; see figure 1). From 1992–1995 to 2003–2005, papillary carcinoma rates rose among every racial/ethnic/sex group, ranging from 23% (p-value =0.07) among API males to 104% (p-value <0.01) among Black females.
Figure 1.
Histology-specific trends in thyroid cancer incidence in SEER* diagnosed from 1980–1983 to 2003–2005 by sex and race/ethnicity.
Rates are age-adjusted (2000 US Standard Population) and each point represents 3 or 4-years.
*Nine regions were included for the Whites and Blacks and eleven were included for the White non-Hispanics, White Hispanics and Asian/Pacific Islanders.
The trends for the other histological types were more variable. Follicular carcinoma rates rose only modestly among Whites (p-values <0.01) and Blacks (p-values >0.40). Medullary carcinoma appeared to increase dramatically among White Hispanic males (144%, p-value=0.13), but the rates were based on small numbers. Anaplastic carcinoma rates were low and did not show consistent trends. Rates for other specified and not specified histologic types were not plotted because they were low and could not provide explanation of the trends for the more common histologic types.
Stage-Specific Time Trends in Papillary Carcinomas
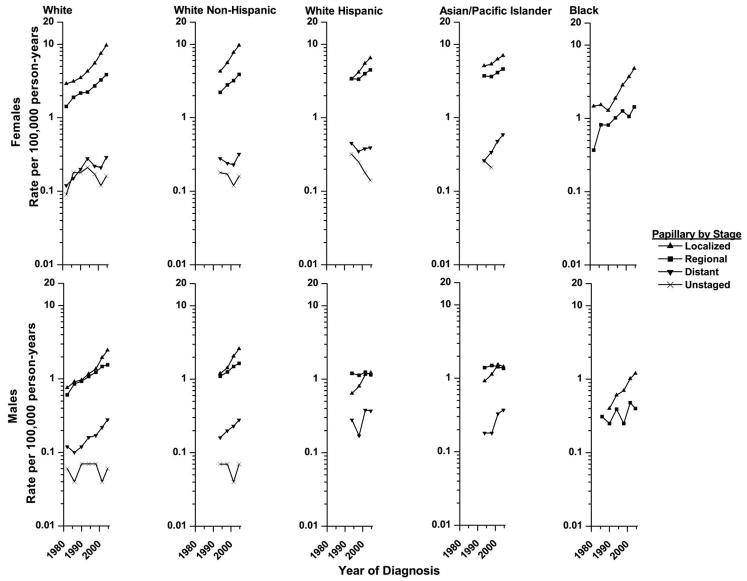
All subsequent analyses focused on papillary carcinoma because this histologic type was increasing the most rapidly and was by far the most frequent, allowing for stratification by other variables. The most common stage of disease at time of diagnosis was localized for all racial/ethnic groups except White Hispanic and API males for whom regional stage was the most frequent (Figure 2). The proportion of tumors that were localized was higher among females than males (62% vs. 50%), among Blacks than Whites (66% vs. 61%), and among White Non-Hispanics (63%) compared with White Hispanics (51%) or APIs (54%; all p-values<0.01).
Figure 2.
Stage-specific trends in papillary thyroid cancer incidence in SEER* diagnosed from 1980–1983 to 2003–2005 by sex and race/ethnicity.
Rates are age-adjusted (2000 US Standard Population) and each point represents 3 or 4-years.
*Nine regions were included for the Whites and Blacks and eleven were included for the White non-Hispanics, White Hispanics and Asian/Pacific Islanders.
Papillary thyroid cancer incidence rates rose for tumors of each stage, but the increases for localized stage tumors were the most consistent among all the racial/ethnic groups. Among Whites and Blacks, localized tumor rates increased about 200% (range: 186%–232%) since 1980–1983 and 100% (range: 97%–155%) since 1992–1995 until 2003–2005. Among APIs localized papillary cancer rates increased moderately (females: 39%, males: 57%) since 1992–1995. (All p-values <=0.01.)
Rates for regional and distant stage tumors also rose. In the more than 20-year period from 1980–1983 to 2003–2005, regional tumor rates increased 171% and 156% among White females and males, respectively, and 290% and 33% among Black females and males. Since 1992–1995, regional tumor rates increased 49%–76% among White Non-Hispanics, 32% among White Hispanic females and 25% among APIs females. Regional tumor rates were stable among White Hispanic and API males. Distant stage tumor rates more than doubled among Whites since 1980–1983 and among APIs since 1992–1995. Rates for unstaged tumors were low, and decreases could not explain the observed trends for tumors of known stage. (All p-values <0.01, except regional tumors among Black males p-value>0.40).
Tumor-Size Specific Time Trends in Papillary Carcinomas
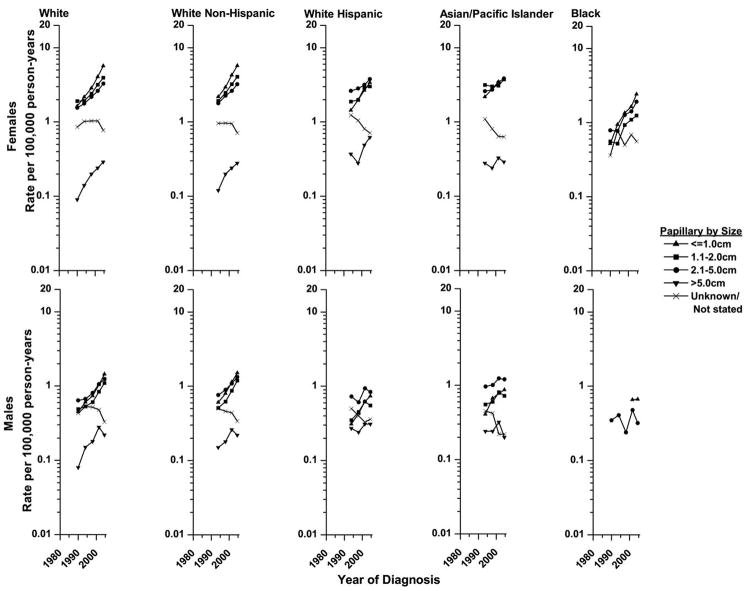
Disease incidence rates rose for tumors of all sizes, but the increases were most rapid for the smaller size tumors (Figure 3). Among White females since 1988–1991 (earliest years that tumor size were adequately recorded), rates rose 248% for tumors ≤1 cm, 106% for those 1.1–2 cm, 113% for 2.1–5 cm, and 222% for those >5 cm (p-values<0.01). Size-specific increases in disease incidence among White males and Black females were similar. During the years studied, rates for most tumor sizes also rose among White Hispanics, APIs, and Black males. About 50% of the overall increase in papillary carcinomas since 1992–95 was due to rising rates of very small cancers ≤1.0 cm, 30% to cancers 1.1–2 cm, and 20% to cancers >2 cm. Rates for tumors of unknown size decreased, most consistently among White Hispanic females, but the declines could not account for the observed trends for tumors of known size.
Figure 3.
Size-specific trends in papillary thyroid cancer incidence in SEER* diagnosed from 1988–1991 to 2003–2005 by sex and race/ethnicity.
Rates are age-adjusted (2000 US Standard Population) and each point represents 3 or 4-years.
*Nine regions were included for the Whites and Blacks and eleven were included for the White non-Hispanics, White Hispanics and Asian/Pacific Islanders.
Age-Specific Time Trends in Papillary Carcinomas
Except for the youngest (<20 years) and oldest (80+ years) age groups, which each accounted for only 2–3% of the papillary carcinomas within each racial/ethnic group, consistent increasing time trends were apparent across all age, sex, and racial/ethnic groups (Figure 4). Among females, the highest rates occurred among individuals aged 40–59, but the steepest increases were observed among those aged 60–79, whereas among males both the highest rates and largest increases over time tended to be among older individuals.
Figure 4.
Age-specific trends in papillary thyroid cancer incidence in the SEER* diagnosed from 1980–1983 to 2003–2005 by sex and race/ethnicity.
Rates are age-adjusted (2000 US Standard Population) and each point represents 3 or 4-years.
*Nine regions were included for the Whites and Blacks and eleven were included for the White non-Hispanics, White Hispanics and Asian/Pacific Islanders.
Discussion
Papillary thyroid carcinoma was the only histology for which rates rose consistently among both sexes and all racial/ethnic groups; however, significant variations in the rate of increase were observed. For papillary carcinoma, increases in disease incidence tended to be more rapid among females than males and, over the same time period, were more rapid among Whites, especially Non-Hispanic Whites, and Blacks than among APIs. Within each sex and racial/ethnic group the largest increases were for localized and smaller tumors; however, we also found that rates rose for the more advanced and larger tumors.
If all of the increase in thyroid cancer incidence was due to improved disease detection, one would expect more rapid increases in small early-stage tumors than large late-stage tumors; subsequently, rates for larger, more advanced tumors should decline. One would also expect increases across all specified histologies, except anaplastic. However, the observed trends in disease incidence do not completely support this hypothesis. Consistent increases were observed for papillary cancer only and although the greatest increases were observed among smaller early stage tumors, we did not observe declines in larger more advanced tumors. Instead, we found that tumors of all sizes increased over time. For example, rates for the smallest tumors (≤1 cm) rose 248% and those for the largest tumors (>5 cm) rose 222% among White females since 1988–1991. Furthermore, rates for all stages of disease at time of diagnosis increased. Among White males and females, localized stage disease rates rose more than 220%, regional stage rates at least 150%, and distant stage rates more than 130% since 1980–1983. In agreement with previous national and regional studies,(15, 24) the proportion of tumors that were localized was highest among Blacks, which is surprising given the racial disparities in access to and utilization of healthcare in the United States. These findings are in contrast to an ecologic analysis (25) in Wisconsin that observed the strongest correlation (r =0.41) between thyroid cancer incidence rates and percent of residents with health insurance when assessing other community-level socioeconomic status and health care access variables. Although this study gives some support to the notion that the thyroid cancer “epidemic” is related to the growth in diagnostic imaging, methodologic problems associated with ecologic studies (26) limit interpretation of these results. Analyzing data from Australia, Burgess (27) estimated that no more than 50% of the increase in thyroid cancer could be attributed to technological advances in diagnosis and case ascertainment. Since 1992–1995 we calculated that half the overall increase in papillary carcinoma rates in SEER was due to rising rates of very small (≤1.0 cm) cancers, 30% to cancers 1.1–2 cm, and 20% to cancers >2 cm. Therefore, if we assume that all the increases in the very small tumors and none of the increases in tumors >1 cm were related to improved early detection, then we also would estimate that about 50% of the observed increase in papillary carcinomas may be attributed to advances in diagnostic accuracy. If, however, changes in potential risk factors are related to the rising thyroid cancer incidence then, the estimate for the role of early detection would be lower.
The significant increase in incidence of papillary carcinomas may also have been partially affected by a change in diagnostic criteria. In 1988, a new WHO classification system (28) was introduced which recommended reclassifying tumors with follicular architecture but nuclear features characteristic of papillary carcinoma as papillary carcinomas. The change in classification may have artificially inflated papillary thyroid cancer incidence rates, but at the same time may have masked a real increase in follicular carcinoma rates. This reclassification cannot entirely account for the increase in papillary carcinomas. Although the rate for the papillary, follicular variant (morphology code 8340) rose more rapidly than for papillary carcinomas overall (235% vs. 191% from 1980–1983 to 2003–2005, data not shown) among Whites and Blacks combined, this increase accounted for only one-third of the overall rise in papillary carcinoma rates. During this time period the papillary, follicular variant comprised 20–30% of all papillary carcinomas. Another possible explanation is that information on tumor histology and stage has become more accurate and complete, resulting in reduced frequencies in the unspecified/unknown tumor categories. While some decreases in the unspecified/unknown tumor categories were observed, these changes were too small to explain the increases in the specified categories. With respect to timeliness of case ascertainment and reporting of thyroid cancers overall, delay-adjustment of the rates resulted in minimal rises in recent SEER thyroid cancer incidence rates (13) indicating that case ascertainment has been high for many years and our results, if anything, underestimate the true increases.
Radiation exposure is the major known risk factor for thyroid cancer.(14) It was used from about the 1930s to 1960s to treat several benign conditions of the head and neck, and follow-up of several of these irradiated populations demonstrated a strong radiation dose-response relationship with thyroid cancer, especially papillary carcinoma.(29) Persons treated as young children have particularly high risk, and many of them would be in the age range to develop radiation-related thyroid cancer during the study period.(30) This may at least partially explain why the steepest trends among both sexes were among individuals older than 40, which is suggestive of a cohort effect. It is difficult to sort out the relative roles of period and cohort effects, however, as there are no clear changes in the age-specific trends. Furthermore, over the last few decades there have been significant increases in the use of medical diagnostic radiation, especially CT scans which have gone from approximately 3 million performed in the United States in 1980 to 67 million in 2006.(31, 32) The young thyroid gland is very sensitive to radiation, leading some authors to speculate that the greater use of pediatric CT scanning could be accounting for a part of the thyroid cancer increase later in life.(11, 33)
As reviewed by Boas et al.(34) environmental chemicals, such as polychlorinated biphenyls (PCBs) and dioxins, have been positively correlated with thyroid stimulating hormone (TSH) levels. TSH is the principal hormone responsible for regulating the growth and function of the thyroid gland, and higher levels are associated with increased proliferation,(35) resulting potentially in an increased opportunity for mutations and the development of cancer. Results from the NHANES III study indicate that White Non-Hispanics have higher TSH levels than Black Non-Hispanics and Mexican Americans have intermediate levels,(36) which is consistent with the observed incidence rates of thyroid cancer. TSH levels also vary by sex with females tending to have higher levels,(36) especially during pregnancy.(37)
Other risk factors suspected in the rise in thyroid cancer incidence include the increase in BMI and height,(38, 39) use of fertility drugs,(40) changes in reproductive patterns,(41, 42) immigration from high incidence countries (43) and possibly insulin resistance syndrome.(44)
Conclusion
Through the examination of the SEER database and its racially/ethnic diverse national sample we were able to provide a more complete epidemiological portrait of thyroid cancer, according to common demographic and tumor-specific characteristics. Our findings indicate that implementation of more sensitive diagnostic procedures cannot completely explain the observed increases in papillary thyroid cancer incidence. Further investigation of the relationship between environmental chemicals known to interfere with thyroid function, as well as other potential risk factors and the development of thyroid cancer is, therefore, warranted.
Acknowledgments
We thank John Lahey of IMS, Inc. and David Check of the Biostatistics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute for figure development. This study was partly supported by the Intramural Research Program of the DCEG, NCI, NIH, Department of Health and Human Services and was sponsored by the United States Military Cancer Institute via the Uniformed Services University of the Health Sciences under the auspices of the Henry M. Jackson Foundation for the Advancement of Military Medicine. The information or content and conclusions do not necessarily represent the official position or policy of, nor should any official endorsement be inferred by, the Uniformed Services University of the Health Sciences, the United States Military Cancer Institute, the Department of Defense, or the U.S. Government.
Footnotes
References
- 1.Burgess JR, Tucker P. Incidence trends for papillary thyroid carcinoma and their correlation with thyroid surgery and thyroid fine-needle aspirate cytology. Thyroid. 2006;16:47–53. doi: 10.1089/thy.2006.16.47. [DOI] [PubMed] [Google Scholar]
- 2.Colonna M, Guizard AV, Schvartz C, et al. A time trend analysis of papillary and follicular cancers as a function of tumour size: a study of data from six cancer registries in France (1983–2000) Eur J Cancer. 2007;43:891–900. doi: 10.1016/j.ejca.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Leenhardt L, Grosclaude P, Cherie-Challine L. Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14:1056–60. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Semenciw R, Ugnat AM, Mao Y. Increasing thyroid cancer incidence in Canada, 1970–1996: time trends and age-period-cohort effects. Br J Cancer. 2001;85:1335–9. doi: 10.1054/bjoc.2001.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubina A, Cohen O, Barchana M, et al. Time trends of incidence rates of thyroid cancer in Israel: what might explain the sharp increase. Thyroid. 2006;16:1033–40. doi: 10.1089/thy.2006.16.1033. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds RM, Weir J, Stockton DL, Brewster DH, Sandeep TC, Strachan MW. Changing trends in incidence and mortality of thyroid cancer in Scotland. Clin Endocrinol (Oxf) 2005;62:156–62. doi: 10.1111/j.1365-2265.2004.02187.x. [DOI] [PubMed] [Google Scholar]
- 8.Smailyte G, Miseikyte-Kaubriene E, Kurtinaitis J. Increasing thyroid cancer incidence in Lithuania in 1978–2003. BMC Cancer. 2006;6:284. doi: 10.1186/1471-2407-6-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kent WD, Hall SF, Isotalo PA, Houlden RL, George RL, Groome PA. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ. 2007;177:1357–61. doi: 10.1503/cmaj.061730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Zhu Y, Risch HA. Changing incidence of thyroid cancer. JAMA. 2006;296:1350. doi: 10.1001/jama.296.11.1350-a. author reply. [DOI] [PubMed] [Google Scholar]
- 11.How J, Tabah R. Explaining the increasing incidence of differentiated thyroid cancer. CMAJ. 2007;177:1383–4. doi: 10.1503/cmaj.071464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Cancer Society. Cancer Facts and Figures 2008. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 13.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005. National Cancer Institute; Bethesda, MD: 2008. based on November 2007 SEER data submission. [Google Scholar]
- 14.Ron E, Schneider AB. Chapter 50: Thyroid Cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 3. New York, NY: Oxford University Press; 2006. pp. 975–94. [Google Scholar]
- 15.Mulla ZD, Margo CE. Primary malignancies of the thyroid: epidemiologic analysis of the Florida Cancer Data System registry. Ann Epidemiol. 2000;10:24–30. doi: 10.1016/s1047-2797(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 16.American Cancer Society. Detailed Guide: Thyroid Cancer. 2007 [cited 2008 1/22]; Available from: http://www.cancer.org/docroot/CRI/CRI_2_3x.asp?dt=43.
- 17.Mazzaferri EL. Managing small thyroid cancers. JAMA. 2006;295:2179–82. doi: 10.1001/jama.295.18.2179. [DOI] [PubMed] [Google Scholar]
- 18.Surveillance Epidemiology and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2007 Sub (1973–2005) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2005 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on the November 2007 submission.
- 19.International Classification of Diseases for Oncology. Geneva: World Health Organization; 1976. [Google Scholar]
- 20.Percy C, Van Holten V, Muir C. International Classification of Diseases for Oncology. 2. Geneva: World Health Organization; 1990. [Google Scholar]
- 21.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 22.Curado MP, Edwards B, Shin HR, et al. Cancer Incidence in Five Continents. IX. Lyon: IARC; 2007. IARC Scientific Publications No. 160 http://www-dep.iarc.fr/ed. [Google Scholar]
- 23.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–4. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 24.Morris LG, Sikora AG, Myssiorek D, DeLacure MD. The basis of racial differences in the incidence of thyroid cancer. Ann Surg Oncol. 2008;15:1169–76. doi: 10.1245/s10434-008-9812-6. [DOI] [PubMed] [Google Scholar]
- 25.Sprague BL, Warren Andersen S, Trentham-Dietz A. Thyroid cancer incidence and socioeconomic indicators of health care access. Cancer Causes Control. 2008 doi: 10.1007/s10552-008-9122-0. [DOI] [PubMed] [Google Scholar]
- 26.Greenland S, Robins J. Invited commentary: ecologic studies--biases, misconceptions, and counterexamples. Am J Epidemiol. 1994;139:747–60. doi: 10.1093/oxfordjournals.aje.a117069. [DOI] [PubMed] [Google Scholar]
- 27.Burgess JR. Temporal trends for thyroid carcinoma in Australia: an increasing incidence of papillary thyroid carcinoma (1982–1997) Thyroid. 2002;12:141–9. doi: 10.1089/105072502753522374. [DOI] [PubMed] [Google Scholar]
- 28.Hedinger C, Williams ED, Sobin LH. Histological typing of thyroid tumours, World Health Organization. 2. Berlin: Springer Verlag; 1988. [Google Scholar]
- 29.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259–77. [PubMed] [Google Scholar]
- 30.Pottern LM, Stone BJ, Day NE, Pickle LW, Fraumeni JF., Jr Thyroid cancer in Connecticut, 1935–1975: an analysis by cell type. Am J Epidemiol. 1980;112:764–74. doi: 10.1093/oxfordjournals.aje.a113049. [DOI] [PubMed] [Google Scholar]
- 31.Amis ES, Jr, Butler PF, Applegate KE, et al. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4:272–84. doi: 10.1016/j.jacr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Mettler FA, Jr, Thomadsen BR, Bhargavan M, et al. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys. 2008;95:502–7. doi: 10.1097/01.HP.0000326333.42287.a2. [DOI] [PubMed] [Google Scholar]
- 33.Baker SR, Bhatti WA. The thyroid cancer epidemic: is it the dark side of the CT revolution? Eur J Radiol. 2006;60:67–9. doi: 10.1016/j.ejrad.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Boas M, Feldt-Rasmussen U, Skakkebaek NE, Main KM. Environmental chemicals and thyroid function. Eurpoean Journal of Endocrinology. 2006;154:599–611. doi: 10.1530/eje.1.02128. [DOI] [PubMed] [Google Scholar]
- 35.Roger PP, Dumont JE. Thyrotropin is a potent growth factor for normal human thyroid cells in primary culture. Biochem Biophys Res Commun. 1987;149:707–11. doi: 10.1016/0006-291x(87)90425-6. [DOI] [PubMed] [Google Scholar]
- 36.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 37.Walker JA, Illions EH, Huddleston JF, Smallridge RC. Racial comparisons of thyroid function and autoimmunity during pregnancy and the postpartum period. Obstetrics and Gynecology. 2005;106:1365–71. doi: 10.1097/01.AOG.0000185475.61612.ea. [DOI] [PubMed] [Google Scholar]
- 38.Engeland A, Tretli S, Akslen LA, Bjorge T. Body size and thyroid cancer in two million Norwegian men and women. Br J Cancer. 2006;95:366–70. doi: 10.1038/sj.bjc.6603249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guignard R, Truong T, Rougier Y, Baron-Dubourdieu D, Guenel P. Alcohol drinking, tobacco smoking, and anthropometric characteristics as risk factors for thyroid cancer: a countrywide case-control study in New Caledonia. Am J Epidemiol. 2007;166:1140–9. doi: 10.1093/aje/kwm204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannibal CG, Jensen A, Sharif H, Kjaer SK. Risk of thyroid cancer after exposure to fertility drugs: results from a large Danish cohort study. Hum Reprod. 2008;23:451–6. doi: 10.1093/humrep/dem381. [DOI] [PubMed] [Google Scholar]
- 41.Brindel P, Doyon F, Rachedi F, et al. Menstrual and reproductive factors in the risk of differentiated thyroid carcinoma in native women in French Polynesia: a population-based case-control study. Am J Epidemiol. 2008;167:219–29. doi: 10.1093/aje/kwm288. [DOI] [PubMed] [Google Scholar]
- 42.Negri E, Dal Maso L, Ron E, et al. A pooled analysis of case-control studies of thyroid cancer. II. Menstrual and reproductive factors. Cancer Causes Control. 1999;10:143–55. doi: 10.1023/a:1008880429862. [DOI] [PubMed] [Google Scholar]
- 43.Nasseri K. Thyroid cancer in the Middle Eastern population of California. Cancer Causes Control. 2008 doi: 10.1007/s10552-008-9185-y. [DOI] [PubMed] [Google Scholar]
- 44.Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H. Introducing the thyroid gland as another victim of the insulin resistance syndrome. Thyroid. 2008;18:461–4. doi: 10.1089/thy.2007.0223. [DOI] [PubMed] [Google Scholar]