Abstract
Photodynamic therapy was the first treatment to have been shown to significantly decrease high-grade dysplasia and cancer in patients with Barrett’s esophagus. However, its use has been limited, primarily because of the side effects, which include esophageal strictures, cutaneous photosensitivity, chest pain, and nausea and vomiting [1]. The tolerability aspects of photodynamic therapy, as well as the dosimetry, though, can be improved with existing technologies to further develop this therapy into truly a widely applicable therapy. Studies have recently been done to help identify patients more likely to suffer stricture after photodynamic therapy. In addition, there has been evidence to suggest that the efficacy of photodynamic therapy also can be limited by genetic abnormalities in the mucosa. By combining knowledge of tissue biology, optical properties of the tissue, and dosimetry issues with ablation, photodynamic therapy can still have a potentially bright future.
Potential of photodynamic therapy
Photodynamic therapy consists of three elements [2]. There is a drug, which can be administered either orally or intravenously, that is supposed to be preferentially taken up within the Barrett’s mucosa. The most commonly used oral drug, 5-aminolevulinic acid (ALA), generally is administered within 4 hours of photoradiation, while the intravenous drug sodium porfimer must be given 48 hours before photoradiation. The oral medication certainly would make therapy simpler but is not used in the United States except as a topical application. Once adequate concentrations of the drug are achieved, photoradiation can be applied. This generally uses “red” wavelengths (630-635 nm) of light, which activate the drug. The drug interacts with molecular oxygen, causing it to form a singlet oxygen state, which then can interact with the tissue, causing cell death (Fig. 1).
Fig. 1.
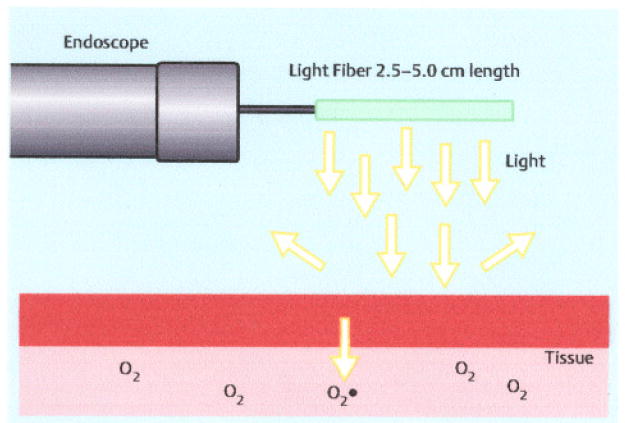
Photodynamic therapy in its current iteration. Light is used to activate a drug in the tissue. The drug absorbs the light energy and interacts with oxygen causing singlet oxygen which mediates cell death.
Although this sounds complicated, this is one of the simplest methods by which phototherapy can be delivered. Technically, it is a very easy procedure to perform which ensures that treatment can be performed without much variation in response due to variation in endoscopic skills. The administration of light can be done through very small fibers that can fit through any endoscope or even potentially come from nonendoscopic light sources. In addition, the photoradiation periods are relatively short and do not require much endoscope motion, which makes the therapy easily tolerated by most patients. The simplicity of this treatment would indicate that it has the highest chance of being effectively introduced into community practice. A great deal of the ablative therapies currently available are fairly complex and are suitable only for tertiary referral centers where the expertise exists to utilize these therapies.
This situation is analogous to esophagectomy where, if it is performed in expert centers, mortality rates are often less than 3%, even in the older population of patients who are prone to Barrett’s esophagus [3]. If one examines, though, the mortality rate in the community, they can easily approach 18% in centers where esophagectomy is not often performed. The same issues will most likely apply to complicate complex endoscopic procedures. In fact, this type of cancer therapy is probably more translatable to practice, not only in this country, but also in countries with limited healthcare resources where more complex modalities are difficult to deliver.
Current technology
A number of advances have been made, but at the current time the available technology is fairly limited. The light sources for photoradiation are limited primarily to diode lasers, which are very reliable at 630nm but are generally fairly limited in power. Older lasers such as the KTP-YAG-pumped tunable dye lasers can deliver up to 7W of power, but these are no longer manufactured. Their higher power is advantageous if longer treatment fibers are used for treatment of long segments of Barrett’s esophagus, but currently in North America only shorter fibers (< 5cm) are available, which has decreased the demand for these large-output but more complicated lasers.
Balloon diffusing fibers have also been used to deliver energy, but, though theoretically more capable for treatment, in actual practice the balloons did not seem to make a difference in increasing treatment efficacy or decreasing toxicity (Fig.2). The reason for this is unclear, although the delivery of light in a patient under conscious sedation is more difficult and esophageal motility often had a tendency to disrupt the placement of the balloon.
Fig.2.
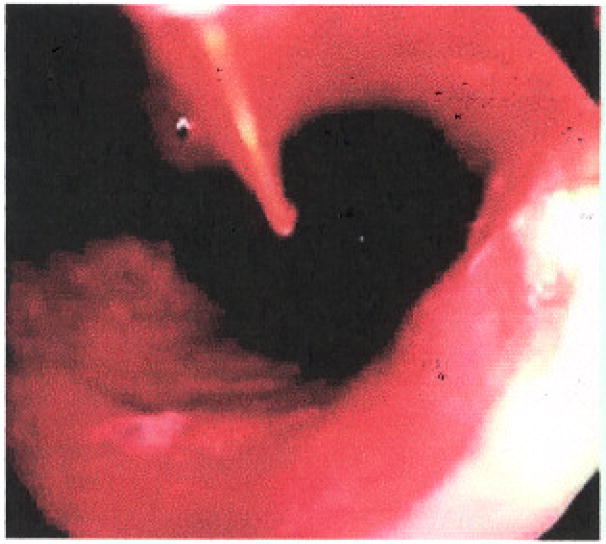
Photodynamic therapy of Barrett’s esophagus using a bare cylindrical diffusing fiber.
Drug dosimetry has been relatively static in clinical use. In North America, only sodium porfimer is approved for use in Barrett’s esophagus and is given at a dosage of 2 mg/kg, which is the same as that applied for esophageal cancer. The drug is expensive to administer since it must be reconstituted immediately before use and is dispensed in 75-mg vials such that most patients will require at least three vials for treatment. The drug must also be given intravenously 2 days before photoradiation. ALA, used most often in Europe, can be given orally and is usually given at a dosage of 60 mg/kg 4 hours before photoradiation.
Current evidence supporting photodynamic therapy in Barrett’s esophagus
Phototherapy was first approved in the gastrointestinal tract on the basis of a randomized controlled trial comparing photodynamic therapy with sodium porfimer with thermal ablative therapies using the Nd:YAG laser therapy [4]. In this study of 218 treated patients, 110 received photodynamic therapy with sodium porfimer (2 mg/kg) and 108 received treatment with Nd:YAG lasers in a total of 24 separate medical centers. The drug was administered 48 hours prior to photoradiation with a light dose of 300 J/cm fiber. Overall, the dysphagia improvement was similar between Nd:YAG and photodynamic therapy, but the latter was seen to be more efficacious for tumors in the upper and lower third of the esophagus in patients who had fairly lengthy tumors. In addition, complications were less severe in those patients who had photodynamic therapy. Perforations only occurred 1% of the patients who received photodynamic therapy versus 7% of those who received laser therapy. It is interesting that photodynamic therapy was approved because this therapy was associated with fewer complications than the standard at that time, Nd:YAG therapy. Because of these findings, photodynamic therapy was approved for the palliation of esophageal carcinoma.
Barrett’s esophagus was first treated with photodynamic therapy when it was noted that treatment of small cancers within Barrett’s esophagus led to the resolution of the segment [5]. Multiple case series were then reported that seemed to indicate that photodynamic therapy could affect the amount of metaplastic mucosa present [6,7]. This eventually led to a Barrett’s esophagus study that was the first randomized controlled treatment trial in Barrett’s esophagus with high-grade dysplasia. In this study, 485 patients were screened for entry for Barrett’s with high-grade dysplasia, and 208 were eventually accepted for the study [8]. After confirmation by a single pathologist, patients were randomized 2:1 to receive either omeprazole alone or omeprazole with photodynamic therapy with sodium porfimer. The drug dose remained at 2 mg/kg while the light dose was actually decreased to 130 J/cm because of the development of a novel treatment balloon. It was felt that the balloon should decrease the toxicities that had previously been experienced with this therapy since it would allow even light distribution, and reflective coated ends of the balloon served to reflect back into the Barrett’s segment light that would have disappeared into the stomach or into the throat. Stricture rates of 30% or more had been reported using bare fibers, although this included series where very high dosages of light were used that also produced atrial fibrillation. The selection of 130J/cm fiber for photoradiation proved to be less than ideal, with the randomized trial leading to the lowest mucosal ablation rate of any prior clinical report. Patients required multiple therapies (up to three) in order to achieve a reasonable outcome; 138 patients received active therapy versus the 70 who received omeprazole alone. At 24-month Follow-up, 77% of those patients who were treated with Barrett’s with high-grade dysplasia had remission of Barrett’s esophagus versus 39% in the control group. This high spontaneous remission rate of Barrett’s esophagus was partially due to the criterion that any patient who had decreased dysplasia on subsequent surveillance endoscopy at any point was regarded as a success. Because of this definition, 5-year results also continued to show that no high-grade dysplasia was found in 77% of patients versus only 39% of the controls. No dysplasia at all was found in 59% of treated patients versus 14% of control patients, and this was on long-term follow-up at a 5-year mark [9]. Finally, complete squamous mucosa was found in 52% of the patients who received treatment but in only 7% of those who were followed with surveillance. The appearance of a Barrett’s segment before, 48 hours after treatment, and after complete healing is shown in Fig. 3.
Fig.3.
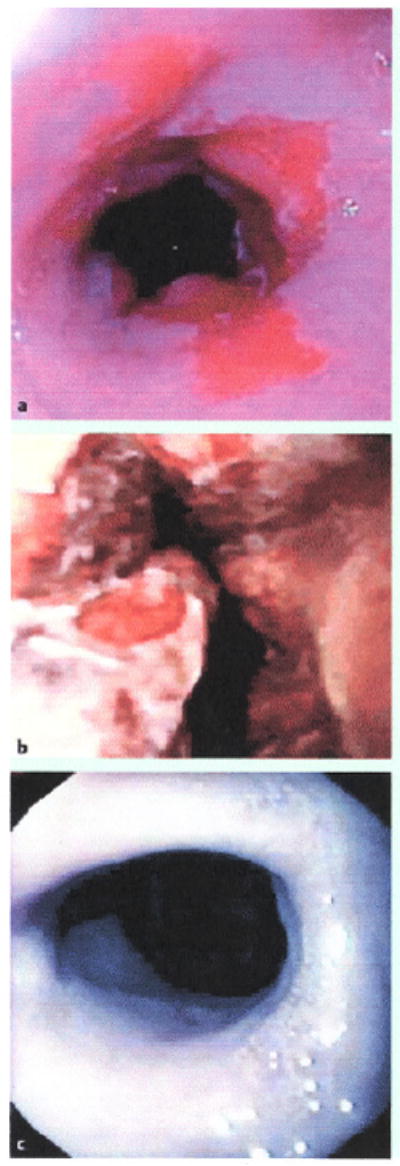
a A Barrett’s segment is treated with photodynamic therapy. b After 48 hours the mucosa sloughs. c After 3 months, the mucosa regenerates, although some fibrosis can be appreciated.
It is interesting that with biopsy alone patients could completely regress their Barrett’s esophagus segment. The rate of progression to cancer was significantly reduced in those who received photodynamic therapy, and it decreased to 15% versus 29% of those who received omeprazole. This was statistically significant, although it should be noted that the trial was not powered on cancer prevention but only on elimination of high-grade dysplasia. It also needs to be pointed out that, as to staging of the cancers that progressed, four were advanced cancers. Three were T2 and one was T3, versus only one T2 in the patients in the control group. The advanced cancers occurred primarily because of the difficulty in differentiating recurrent tumor from a photodynamic-therapy-induced stricture (Fig. 4). Unfortunately, the treatment fairly commonly produced strictures, in about 30% of patients. In addition, over 60% developed some type of cutaneous photosensitivity.
Fig.4.
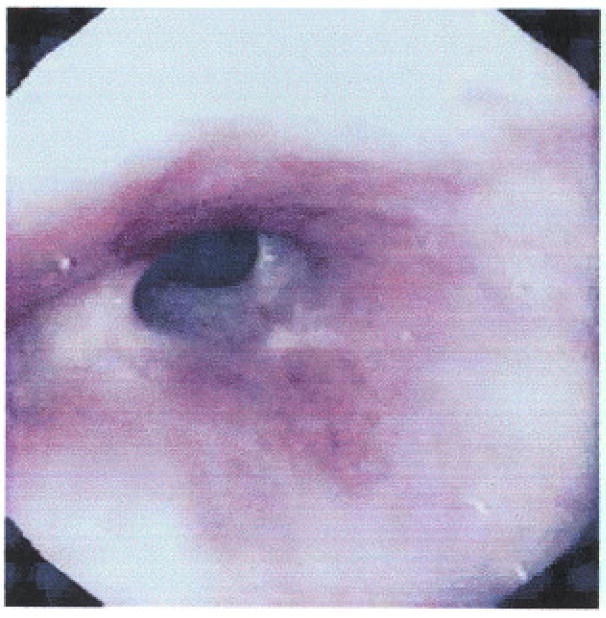
Stricture after photodynamic therapy. It is fairly stenotic with extensive fibrosis.
ALA has had a controversial role in the treatment of Barrett’s esophagus. It has been reported by certain groups to be very effective in treating Barrett’s with high-grade dysplasia and early esophageal cancer, with a 97% and 100% initial response rate, although with a relatively high recurrence rate in patients with early cancer (30%) [10]. However, other studies that investigated adding 5-ALA and varying doses of light have found less promising results, with dysplasia response rates as low as 67% and even one treatment-related death with 5-ALA due to vascular instability [11]. Another series that examined patients after mucosal resection found that photodynamic therapy with ALA did not seem to prevent recurrence of disease, especially when margins of mucosal resection were known to be positive [12]. A small series recently reported, though, that very high light dosages of up to l000J/cm fiber might be needed to eliminate dysplasia, although even at this dosage the group only found a 75% response rate [13].
Multiple outcomes studies have been published comparing photodynamic therapy to surgery or surveillance, all of which have found that photodynamic therapy for high-grade dysplasia is cost-effective in terms of quality-adjusted life years [14-16]. In addition, it was shown that photodynamic therapy was more cost-effective than no prevention of any kind. All of this evidence suggests that photodynamic therapy, despite its problems in producing side effects, is cost-efficacious compared to surveillance. The most recent cost-effectiveness study found that, although surveillance was the least costly strategy, it was also the least effective, making photodynamic therapy or esophagectomy the most cost-effective. Moreover, photodynamic therapy was less expensive than surgery and dominated the surgical strategy when willingness to pay was at a level between 100 and 3500 dollars (Canadian) per life year saved [17].
Photodynamic therapy is often combined with other treatments, such as endoscopic mucosal resection, especially in high-risk patients. Although the mucosal resections have been shown to perhaps increase the risk of stricture formation after phototherapy, using endoscopic mucosal resection allows one to properly stage any small neoplasms that may be occurring in a Barrett’s segment [18].
We examined the results of long-term follow-up of photodynamic therapy in our center [19], There were 129 patients in the photodynamic therapy group versus 70 in the surgical group. The patients who received phototherapy in this retrospective cohort study were older than those who received surgery. In addition, the Barrett’s length was significantly longer in those who had esophagectomy. However, those patients who had photodynamic therapy generally had a significantly worse performance status, which made them less likely to be surgical candidates. Seven cancers were found following photodynamic therapy in our institution for high-grade dysplasia. Four of these were intramucosal cancers, whereas three actually became submucosal. Most of these were found within 12 months, although two were detected within 18 months of therapy. Recurrent high-grade dysplasia occurred in 30% of these patients. However, it was then re-treated with ablative therapies and eradicated in 70%. Overall, at 1 year in our endoscopically treated cohort, 88% of patients had high-grade dysplasia eliminated. At 3 years, it was still absent in 86% of the patients.
Ultimately, despite the more advanced-stage recurrent cancers and advanced-age patients in the endoscopically treated group, overall mortality in the endoscopic and surgical treatment groups was similar. It should be pointed out that 80% of patients in this study had undergone mucosal resection as part of their therapy. After a median follow-up of 63 months, there was only 8.5% mortality in the surgically treated group, which had a slightly longer follow-up. In the photodynamic-therapy group, there was a 9% mortality rate at a mean 57-month mark. Most importantly for outcomes, none of the patients in either group died from esophageal-cancer-related causes.
Challenges to photodynamic therapy
At the current time, photodynamic therapy faces a number of challenges. Interestingly, efficacy is not a challenge to photodynamic therapy. As compared to other ablative therapies, photodynamic therapy’s ability to eradicate high-grade dysplasia and decrease cancer risks seems to be reasonable, although no randomized studies have been performed. None of the ablative therapies to date has been 100% successful and most are about 80% effective in eliminating high-grade dysplasia. The number of applications of photodynamic therapy is fairly limited compared to other ablative therapies, often being limited to a single application [20-22].
The primary challenge to photodynamic therapy is probably the stricture rate. In the randomized controlled trial it was about 30%. but this was the overall stricture rate of patients who were treated up to three times. Confining the figures to patients who had a single treatment, the stricture rate in our series is less than 20%. Photodynamic therapy based on ALA has been shown to decrease this rate significantly to on l%-2% [10-12]. The nature of ALA is to be concentrated in the mucosa and this may be the reason that strictures are rare. Most ablative therapies that have been more effective tend to injure transmurally. This may well be the result of the need to injure deep enough to destroy all neoplastic tissue, which often leads to transmural inflammation and strictures. Improved dosimetry could reduce this problem.
The next major challenge is that of cutaneous photosensitivity. This is quite problematic, as no other therapy has this particular issue. Patients are not very experienced in avoiding sunlight. It is fortunate that the current agents do not lead to significant injury from cutaneous photosensitivity. However, well over two-thirds of patients experience some minor degree of sunburn. Usually, this is self-limited. It can be treated with an antihistamine to relieve symptoms.
The third major challenge is the acute morbidity caused by photodynamic-therapy-induced injury. Once again, all ablative therapies are associated with some degree of chest pain and discomfort. Photodynamic therapy can cause fairly severe chest pain and nausea. Dehydration is a definite risk in the first 2 weeks after photodynamic therapy. This might be due to the increased injury and it may also be due to the nature of the temporary porphyria state induced by the drug.
Future directions of photodynamic therapy
The first step would be to improve the dosimetry with existing agents. Since phototherapy involves the application of light, being able to measure light parameters during phototherapy would improve this treatment. In particular, knowing the properties of the tissue in terms of scattering light might well lead to changes in dosimetry. All of these optical properties can be measured using fairly basic optical tools, such as reflectance spectrometry. Once these measurements of scattering and absorption in the tissue are obtained, a much more accurate light dose can be assembled. This is much like what has been done in radiation therapy, where early treatment was marked by toxicity. Currently, by taking advantage of multiple ports of overlapping radiation fields, the truly cellular-toxic doses of radiation can be concentrated specifically in tumor areas, avoiding damage to normal structures. In the case of photodynamic therapy, we can more carefully model the photon path in the tissue and ensure that only the necessary amount of light is delivered.
In addition, the tissue photosensitizer levels can usually be measured. Using similar fluorescence spectroscopy techniques, one can actually measure levels of drug in the mucosa [23], Furthermore, one can measure the treatment effect during therapy by measuring the breakdown of the drug. As the photosensitizers are activated, if additional light is received, the compounds actually will break down, a process known as “photobleaching.” Photobleaching results in the destruction of the molecule and leads to photochemical changes in the molecule, leading to decreased fluorescence, which would be a tool to measure the outcomes of therapy. Other tools to measure treatment outcome might even be to measure oxygenation status [24]. Studies have shown that decreased oxygenation during the treatment correlate with treatment efficacy. This is because one of the major targets of photodynamic therapy is the vascular supply. As treatment proceeds, the oxygenation tissue profusion at the local level is decreased.
Future directions in phototherapy also include the development of new photosensitizers. Third-generation photosensitizers have been developed that can clear the body faster and absorb light at higher wavelengths [25-31].This leads to a more limited period of photosensitivity. One of the problems with these agents is that they are so effective that, although the period of photosensitivity is much shorter, the reaction is much more intense, since the patients truly have to avoid almost all light. The development of these compounds that can target different parts of the cell, such as the lysosomes or the cell walls, might be very useful in treatment of tumors, since they could be altered or mixed to target specific cell abnormalities.
Finally, there is a need to determine the best patients for phototherapy. Biomarkers have recently been shown to predict the outcomes after ablative therapy. In particular, using fluorescence in situ hybridization, our group has recently reported that p16 copy number abnormalities in tissue lead to a decreased response to phototherapy [32].This is found in multivariate analysis to be a significant factor. In the future, it is hoped that these and other markers will help us more appropriately select the parents for the most appropriate type of treatment.
Acknowledgments
The authors would like to acknowledge the support of NIH grants R01CA111603 and R21CA122426 as well as support from the Mayo Foundation.
Footnotes
Competing Interests: K. Wang has received research funding from Axcan Pharma Inc.
References
- 1.Siersema PD. Photodynamic therapy for Barrett’s esophagus: not yet ready for the premier league of endoscopic interventions. Gastrointestinal Endoscopy. 2005;62:503–507. doi: 10.1016/j.gie.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Wilson BC, Patterson MS, Wilson BC, Patterson MS. The physics, biophysics and technology of photodynamic therapy. Phys Med Biol. 2008;53:61–R109. doi: 10.1088/0031-9155/53/9/R01. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 4.Lightdale CJ, Heier SK, Marcon NE, et al. Photodynamic therapy with porfimer sodium versus thermal ablation therapy with Nd:YAG laser for palliation of esophageal cancer: a multicenter randomized trial. Gastrointest Endosc. 1995;42:507–512. doi: 10.1016/s0016-5107(95)70002-1. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty T. Photodynamic therapy for early esophageal cancer. International Photodynamic Therapy Association Meeting. 1990 abstract. [Google Scholar]
- 6.Laukka MA, Wang KK. Initial results using low-dose photodynamic therapy in the treatment of Barrett’s esophagus. Gastrointest Endosc. 1995;42:59–63. doi: 10.1016/s0016-5107(95)70245-8. [DOI] [PubMed] [Google Scholar]
- 7.Overholt BF, Panjehpour M. Barrett’s esophagus: photodynamic therapy for ablation of dysplasia, reduction of specialized mucosa, and treatment of superficial esophageal cancer. Gastrointest Endosc. 1995;42:64–70. doi: 10.1016/s0016-5107(95)70246-6. [DOI] [PubMed] [Google Scholar]
- 8.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–498. doi: 10.1016/j.gie.2005.06.047. Erratum appears in Gastrointest Endosc 2006;63:359. [DOI] [PubMed] [Google Scholar]
- 9.Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointest Endosc. 2007;66:460–468. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Pech O, Gossner L, May A, et al. Long-term results of photodynamic therapy with 5-aminolevulinic acid for superficial Barrett’s cancer and high-grade intraepithelial neoplasia. Gastrointest Endosc. 2005;62:24–30. doi: 10.1016/s0016-5107(05)00333-0. [DOI] [PubMed] [Google Scholar]
- 11.Hage M, Siersema PD, van Dekken H, et al. 5-aminolevulinic acid photodynamic therapy versus argon plasma coagulation for ablation of Barrett’s oesophagus: a randomised trial. Gut. 2004;53:785–790. doi: 10.1136/gut.2003.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters F, Kara M, Rosmolen W, et al. Poor results of S-aminolevulinic acid-photodynamic therapy for residual high-grade dysplasia and early cancer in Barrett esophagus after endoscopic resection. Endoscopy. 2005;37:418–424. doi: 10.1055/s-2005-861198. [DOI] [PubMed] [Google Scholar]
- 13.Mackenzie GD, Jamieson NF, Novelli MR, et al. How light dosimetry influences the efficacy of photodynamic therapy with 5-aminolaevulinic acid for ablation of high-grade dysplasia in Barrett’s esophagus. Lasers in Med Sci. 2008;23:203–210. doi: 10.1007/s10103-007-0473-7. [DOI] [PubMed] [Google Scholar]
- 14.Hur C, Nishioka NS, Gazelle GS. Cost-effectiveness of photodynamic therapy for treatment of Barrett’s esophagus with high grade dysplasia. Dig Dis Sci. 2003;48:1273–1283. doi: 10.1023/a:1024146823549. [DOI] [PubMed] [Google Scholar]
- 15.Shaheen NJ, Inadomi JM, Overholt BF, Sharma P. What is the best management strategy for high grade dysplasia in Barrett’s oesophagus? A cost effectiveness analysis. Gut. 2004;53:1736–1744. doi: 10.1136/gut.2003.033837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comay D, Blackhouse G, Goeree R, et al. Photodynamic therapy for Barrett’s esophagus with high-grade dysplasia: a cost-effectiveness analysis. Can J Gastroenterol. 2007;21:217–222. doi: 10.1155/2007/791062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comay D, Blackhouse G, Goeree R, et al. Photodynamic therapy for Barrett’s esophagus with high-grade dysplasia: a cost-effectiveness analysis. Can J Gastroenterol. 2007;21:217–222. doi: 10.1155/2007/791062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad GA, Wang KK, Buttar NS, et al. Predictors of stricture formation after photodynamic therapy for high-grade dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2007;65:60–66. doi: 10.1016/j.gie.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Prasad GA, Wang KK, Buttar NS, et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2007;132:1226–1233. doi: 10.1053/j.gastro.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65:185–195. doi: 10.1016/j.gie.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Mörk H, Barth T, Kreipe HH, et al. Reconstitution of squamous epithelium in Barrett’s oesophagus with endoscopic argon plasma coagulation: a prospective study. Scand J Gastroenterol. 1998;33:1130–1134. doi: 10.1080/00365529850172458. [DOI] [PubMed] [Google Scholar]
- 22.Van Laethem JL, Cremer M, Peny MO, et al. Eradication of Barrett’s mucosa with argon plasma coagulation and acid suppression: immediate and mid term results. Gut. 1998;43:747–751. doi: 10.1136/gut.43.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung R, Solonenko M, Busch TM, et al. Correlation of in vivo photosensitizer fluorescence and photodynamic-therapy-induced depth of necrosis in a murine tumor model. J Biomed Optics. 2003;8:248–252. doi: 10.1117/1.1560011. [DOI] [PubMed] [Google Scholar]
- 24.Solonenko M. In vivo reflectance measurement of optical properties, blood oxygenation and motexafin lutetium uptake in canine large bowels, kidneys and prostates. Phys Med Biol. 2002;47:857–873. [PubMed] [Google Scholar]
- 25.Sessler JL, Miller RA. Texaphyrins: new drugs with diverse clinical applications in radiation and photodynamic therapy. Biochem Pharmacol. 2000;59:733–739. doi: 10.1016/s0006-2952(99)00314-7. [DOI] [PubMed] [Google Scholar]
- 26.Vargas A, Eid M, Fanchaouy M, et al. In vivo photodynamic activity of photosensitizer-loaded nanoparticles: formulation properties, administration parameters and biological issues involved in PDT outcome. Eur J Pharm Biopharm. 2008;69:43–53. doi: 10.1016/j.ejpb.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Saw CL, Heng PW, Olivo M, et al. Potentiation of the photodynamic action of hypericin. J Environ Pathol Toxicol Oncol. 2008;27:23–33. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i1.30. [DOI] [PubMed] [Google Scholar]
- 28.Minnes R, Weitman H, You Y, et al. Dithiaporphyrin derivatives as photosensitizers in membranes and cells. J Phys Chem B. 2008;112:3268–3276. doi: 10.1021/jp0768423. [DOI] [PubMed] [Google Scholar]
- 29.Cole CD, Liu JK, Sheng X, et al. Hypericin-mediated photodynamic therapy of pituitary tumors: preclinical study in a GH4C1 rat tumor model. J Neurooncol. 2008;87:255–261. doi: 10.1007/s11060-007-9514-0. [DOI] [PubMed] [Google Scholar]
- 30.Trachtenberg J, Bogaards A, Weersink RA, et al. Vascular targeted photodynamic therapy with palladium-bacteriopheophorbide photosensitizer for recurrent prostate cancer following definitive radiation therapy: assessment of safety and treatment response. J Urol. 2007;178:1974–1979. doi: 10.1016/j.juro.2007.07.036. discussion 1979. [DOI] [PubMed] [Google Scholar]
- 31.Taquet JP, Frochot C, Manneville V, et al. Phthalocyanines covalently bound to biomolecules for a targeted photodynamic therapy. Curr Med Chem. 2007;14:1673–1687. doi: 10.2174/092986707780830970. [DOI] [PubMed] [Google Scholar]
- 32.Prasad GA, Wang KK, Halling KC, et al. Utility of biomarkers in prediction of response to ablative therapy in Barrett’s esophagus. Gastroenterology. 2008;135:370–379. doi: 10.1053/j.gastro.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]


