Abstract
Purpose
Prostate cancer cells uniformly express the immune cell inhibitory B7-H3 ligand. Enhanced B7-H3 expression correlates with increased disease progression and cancer-specific death after radical prostatectomy (RP).
Experimental Design
To further assess whether B7-H3 expression is hormone regulated and persists as a viable target during (or after) androgen-ablative therapy, we examined B7-H3 ligand expression within primary and metastatic cancer lesions in response to neoadjuvant hormone therapy (NHT) or palliative hormone deprivation. Tumor B7-H3 in RP specimens from men treated with ≥3 months of NHT was compared with B7-H3 in tumors from matched patients who received no therapy before RP. Hormone-treated and untreated metastatic lesions involving bone were also compared for levels of B7-H3 expression.
Results
Of 165 consecutive RP specimens in each cohort studied, sufficient tissues were available for 148 patients (89.7%) treated with NHT versus 127 patients (77.0%) treated with surgery alone. B7-H3 was expressed in 142 (95.9%) tumors from NHT patients compared with 122 (96.0%) tumors from patients treated with surgery alone (P = 0.91). B7-H3 expression intensity in RP specimens was not affected by NHT (P = 0.12). Bone metastases from 11 (32.4%) untreated and 23 (67.6%) androgen-ablated patients revealed that B7-H3 expression increased in response to hormone therapy (P = 0.04) relative to untreated lesions.
Conclusions
Taken together, B7-H3 expression seems to remain stable (or may even increase) in response to hormone therapy. As such, B7-H3 may represent an attractive target to improve treatment of men with high-risk hormone-treated or refractory prostate cancer.
Despite the recent implementation of highly aggressive screening programs, ∼5% of men with prostate cancer will still present with advanced forms of this malignancy (1–4). For all patients failing primary treatment or presenting with advanced prostate cancer, the mainstay of therapy continues to be androgen deprivation. However, although highly effective, androgen deprivation therapy only provides a palliative form of care to most prostate cancer patients. Taxane-based therapies have emerged as the standard of care for castration-resistant prostate cancer, but median survival is only ∼18 months (5, 6). Prompted by this, enormous attention has been placed on developing novel therapeutic treatment approaches that will either potentiate or supersede androgen-ablative treatment.
In response to the demand for improved therapies, multiple immune-based approaches to treat prostate cancer have been explored in recent years. These approaches have included, but are not limited to, vaccination with prostate-specific antigens (PSA), antigen-pulsed or genetically engineered dendritic cells, or autologous or allogeneic gene-modified prostate tumor cells (7–9). In vivo manipulations of the T-cell costimulatory pathway are also being explored as a means to evoke immune responses for treatment of prostate cancer. With regard to this latter approach, it is now well accepted that T-cell activation, antitumoral or otherwise, requires simultaneous recognition of antigen-bearing major histocompatibility complex by T-cell receptor as well as engagement of T-cell CD28 receptor by costimulatory B7.1 (CD80) and B7.2 (CD86) ligands (10). However, recent studies have also shown that activated T cells are immediately rendered susceptible to subsequent down-regulation (i.e., either impairment or death) by inhibitory signals imparted by other ligands or receptors that comprise the B7 family coregulatory pathway. Such counter-regulatory inhibitors of immune cell function include the T-cell CTLA-4 and PD-1 receptors as well as B7 family ligands, including B7.1, B7-H1 (PD-L1), B7-H3, and B7-H4 (B7x; refs. 11–13).
Related to this, aberrant tumor cell B7-H ligand expression has recently emerged as a provocative mechanism whereby human malignancies might escape host immune surveillance. Specifically, recent studies have reported expression of coregulatory B7-H ligands by multiple forms of human cancer, in which it is believed that such ligands might promote immune cell apoptosis or unresponsiveness to foster cancer progression (10, 14, 15). In support of this, we have reported that expression of B7-H1, B7-H3, and B7-H4 correlates with aggressive behavior and adverse patient outcomes for a number of genitourinary malignancies, including renal, bladder, and prostate carcinoma (16–19). Most recently, we showed that intense expression of B7-H3 by prostate tumors correlates with severe clinicopathologic features and confers a >4-fold increased risk of cancer progression in patients after radical prostatectomy (RP; ref. 19). In addition, a separate study by Zang et al. has since provided independent external validation of our observations pertaining to B7-H3 expression by prostate cancer and has further reported that adenocarcinoma of the prostate also expresses high B7-H4 (B7x) ligand levels (20).
Translational Relevance.
B7-H3 is a co-regulatory molecule known to be aberrantly expressed by prostate cancer cells. In the current study, we found that B7-H3 expression by prostate cancer is unchanged and remains predictive of prostate-specific antigen progression after radical prostatectomy in patients receiving neoadjuvant hormonal therapy. In addition, B7-H3 is strongly expressed in bone metastases and hormone refractory biopsy specimens. Given its role as a marker of adverse prognosis and the lack of change in expression with neoadjuvant therapy, B7-H3 may represent an important target for future combined therapy approaches, particularly for patients with high-risk prostate cancer.
To our knowledge, case-control studies pertaining to the impact of androgen-ablative therapy upon prostate cancer antigen expression have not been previously reported. Herein, with the use of a consecutive series of case-matched patients, we report that B7-H3 expression remains remarkably stable in primary prostate tumors treated for a minimum of 3 months with neoadjuvant hormone therapy (NHT) and may even be increased in androgen-ablated bone metastases.
Materials and Methods
Patient selection
After Institutional Review Board approval, we used the Mayo Clinic Prostatectomy Registry to identify 226 patients who received NHT in the form of a GnRH supra-agonist for a minimum of 3 mo before RP between 1990 and 1999 at the Mayo Clinic. Patients who received other hormonal therapies or radiation therapy before RP were excluded from analysis. Of these 226 cases, 61 patients who were missing clinicopathologic variables were excluded from analysis. The remaining 165 men were then matched according to age at biopsy, preoperative PSA value, Gleason score, clinical T stage, and year of biopsy against another 165 patients who contemporaneously underwent RP at our institution without prior NHT. In addition, we identified 50 prostate cancer patients with bone metastases who underwent bone biopsy for pathologic fracture between 1983 and 1998. Of these, 34 of 50 (68%) had tissue available for analysis. In this latter cohort, B7-H3 expression was compared between patients who received hormone deprivation therapy before bone biopsy (n = 23) and those who received no prior hormone deprivation before biopsy (n = 11). In addition, we analyzed biopsy specimens obtained from a group of nine patients who had developed castrate-resistant disease.
Routine pathologic analysis and B7-H3 immunohistochemical staining and scoring
RP specimens were processed, sectioned, stained with H&E, and evaluated by two urologic pathologists as previously described (21). Clinicopathologic features associated with these specimens were combined to calculate the Mayo Gleason, PSA, seminal vesicle, and margin status score, which takes into consideration preoperative serum PSA and Gleason score, seminal vesicle involvement, and positive surgical margins (22). Previous studies have confirmed the preservation of immunoreactive antigens within lesions involving bone even after extensive decalcification (23–26). As such, before B7-H3 immunohistochemical staining of prostate adenocarcinoma bone metastases, tissues were decalcified in 20% formic acid in 10% buffered formalin as previously described (27). All formalin-fixed paraffin-embedded tissues were cut into 5 μm sections, deparaffinized, and rehydrated in a graded series of ethanols. Antigen retrieval and staining for human B7-H3 was conducted with the use of affinity-purified goat anti-human B7-H3 antibody (R&D Systems; 100 μg/mL) diluted 1:80 with Da Vinci Green antibody diluent (Biocare Medical) as previously described (19). As controls for specificity of staining, B7-H3 staining was blocked with the use of 1:30 human B7-H3-Fc fusion protein (R&D Systems) but not 1:30 P-Selectin-Fc fusion protein (BD Biosciences). Percentages of tumor and adjacent nontumor cells staining positive for B7-H3 were quantified in 10% increments by a urologic pathologist (Y. Sheinin) without knowledge of patient outcome. Tumor cells were considered positive for B7-H3 if there was histologic evidence of cell surface membrane staining. Cases in which the entire tumor harbored <5% B7-H3+ tumor cells were considered negative. Intensity of B7-H3 expression was also scored as absent, weak (partial membrane staining), moderate (partial membrane and cytoplasmic staining), or marked (complete circumferential membrane and cytoplasmic staining) as previously described (19).
Statistical methods
Clinicopathologic variables were compared between NHT patients and controls with the use of rank sum, χ2, and signed rank tests as appropriate. Differences were considered significant when P values were <0.05. PSA progression (defined as a postoperative PSA ≥0.4 ng/mL) was estimated with the Kaplan-Meier method and compared through a log-rank test. Cox regression was used to assess the impact of tumor cell B7-H3 staining intensity on the time to PSA progression. All statistical analyses were carried out with the use of the Statistical Analysis System software package.
Results
Patient demographics
Of the original cohorts of 165 matched patients, 17 patients (10.4%) treated with NHT and 28 patients (17.0%) from the control group did not have tissue available for staining, leaving 148 patients who received NHT and 127 men in the control group for analysis. Table 1 provides a summary of the preoperative variables used to create the matching cohort. The delay from biopsy to RP in the patients treated with NHT (81.5 days) compared with the control group (32.0 days) was expected given the time interval during receipt of the NHT (P < 0.01).
Table 1.
Preoperative demographics
| NHT (N = 148) | No NHT (N = 127) | P | |
|---|---|---|---|
| Median age at biopsy (range) | 64.6 (42.4−76.2) | 64.7 (45.1−76.9) | 0.99 |
| Median PSA (range) | 3.1 (0.2−6.3) | 3.0 (0.3−6.5) | 0.20 |
| Biopsy Gleason score | 0.25 | ||
| ≤6 | 78 (52.7%) | 73 (57.5%) | |
| 7 | 54 (36.5%) | 45 (35.4%) | |
| 8−10 | 16 (10.8%) | 9 (7.1%) | |
| Clinical T stage | 0.04 | ||
| 1A | 0 (0%) | 1 (0.7%) | |
| 1B | 0 (0%) | 4 (2.7%) | |
| 1C | 37 (29.1%) | 42 (28.4%) | |
| 2A | 21 (16.5%) | 13 (8.8%) | |
| 2B | 29 (22.8%) | 44 (29.7%) | |
| 2C | 32 (25.2%) | 27 (18.2%) | |
| 3 | 8 (6.3%) | 17 (11.5%) | |
| Year of biopsy | 0.33 | ||
| 1990 | 1 (0.7%) | 4 (3.1%) | |
| 1991 | 1 (0.7%) | 4 (3.1%) | |
| 1992 | 3 (2%) | 4 (3.1%) | |
| 1993 | 1 (0.7%) | 1 (0.8%) | |
| 1994 | 15 (10.1%) | 8 (6.3%) | |
| 1995 | 19 (12.8%) | 19 (15%) | |
| 1996 | 28 (18.9%) | 23 (18.1%) | |
| 1997 | 25 (16.9%) | 23 (18.1%) | |
| 1998 | 36 (24.3%) | 26 (20.5%) | |
| 1999 | 19 (12.8%) | 15 (11.8%) | |
| Days: biopsy to RP | <0.01 | ||
| Median (range) | 81.5 (14.0−791.0) | 32.0 (2.0−1149.0) | |
RP pathology
The pathologic features of RP specimens for patients receiving NHT and patients treated with surgery alone are presented in Table 2. Despite matching patients for pre-operative variables, tumors from the men who received NHT were found to be significantly more likely to have a pathologic Gleason score ≥7 (82 of 148; 55.4%) than cancers from the control group (54 of 127; 42.5%; P < 0.01). Additionally, there was a significant difference in pathologic stage between the groups (P = 0.02).
Table 2.
RP pathology
| NHT (N = 148) | No NHT (N = 127) | P | |
|---|---|---|---|
| Path grade: Gleason score | <0.01 | ||
| ≤6 | 62 (43.1%) | 72 (57.2%) | |
| 7 | 62 (43.1%) | 44 (34.9%) | |
| 8−10 | 20 (13.8%) | 10 (7.9%) | |
| Pathologic stage, 1997 TNM | 0.02 | ||
| T2aN0 | 57 (38.5%) | 29 (22.8%) | |
| T2bN0 | 41 (27.7%) | 56 (44.1%) | |
| T3aN0 | 26 (17.6%) | 25 (19.7%) | |
| T3b4N0 | 15 (10.1%) | 8 (6.3%) | |
| TanyN+ | 9 (6.1%) | 9 (7.1%) | |
| Years: RP to death or last follow-up | 0.43 | ||
| Mean (SD) | 8.5 (2.21) | 8.7 (2.64) | |
| Median | 8.4 | 8.6 | |
| Q1, Q3 | 7.3, 9.9 | 7.3, 10.2 | |
| Range | (1.2−16.4) | (0.0−15.6) | |
The impact of NHT on B7-H3 expression
NHT did not significantly alter the overall frequency of B7-H3 expression in prostate tumors, as 142 of 148 (95.9%) tumors from the men who received NHT expressed B7-H3 compared with 122 of 127 (96.1%) tumors from the control group (Fig. 1 and Table 3). Similarly, NHT did not affect the percentages of tumor cells that stained positive for B7-H3 (P = 0.91) nor the intensity of B7-H3 expression by these cancers (P = 0.12; Table 3). NHT did seem to diminish the percentages of nontumor cells staining positive for B7-H3 (60.6% versus 68.3%; P < 0.01) but did not affect the staining intensities of noncancerous prostate (P = 0.52). Upon evaluating the impact of B7-H3 expression on postoperative PSA progression, we observed that increased intensity of prostate tumor B7-H3 staining correlated with an increase in the 10-year PSA progression rate for both the NHT and untreated cohorts as we have previously reported (Fig. 2).
Fig. 1.
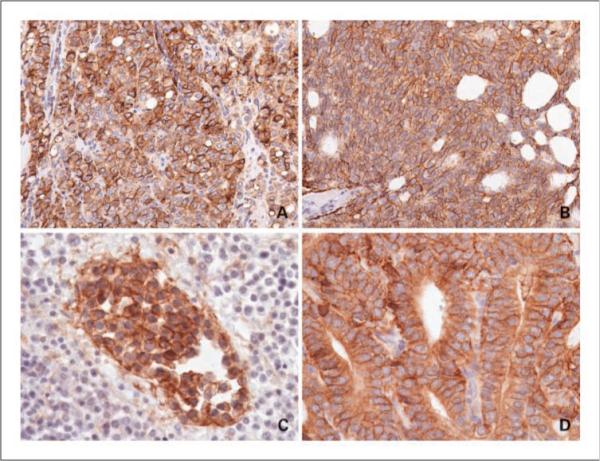
B7-H3 staining in case controls versus patients treated with NHT. A, Gleason 8 adenocarcinoma, no NHT. B, Gleason 8 adenocarcinoma treated with NHT. C, B7-H3 expression in bone metastasis. D, B7-H3 expression in castrate-resistant biopsy.
Table 3.
B7-H3 expression
| NHT (N = 148) | No NHT (N = 127) | P | |
|---|---|---|---|
| Percentage tumor cells B7-H3+ | 0.91 | ||
| Mean (minimum, maximum) | 97.5 (50, 100) | 97.1 (40, 100) | |
| Percentage nontumor cells B7-H3+ | <0.01 | ||
| Mean (minimum, maximum) | 60.6 (20, 90) | 68.3 (30, 90) | |
| Intensity of B7-H3+ tumor cell staining | 0.12 | ||
| Weak | 42 (29.6%) | 26 (21.3%) | |
| Moderate | 76 (53.5%) | 70 (57.4%) | |
| Marked | 24 (16.9%) | 26 (21.3%) | |
| Intensity of nontumor cell B7-H3+ staining | 0.52 | ||
| Weak | 129 (90.2%) | 107 (87.7%) | |
| Moderate | 14 (9.8%) | 15 (12.3%) | |
Fig. 2.
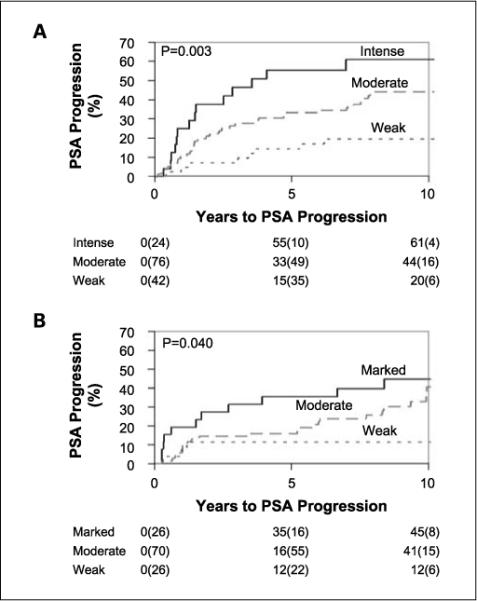
Postoperative PSA progression by staining intensity for patients who received NHT (A). Postoperative PSA progression by staining intensity for patients without NHT (B).
B7-H3 expression in bone metastases
Of 50 patients with biopsy-proven prostate cancer metastatic to bone, 34 patients (68%) had sufficient tissue available for analysis. Twenty-three patients (67.6%) received hormone deprivation therapy before bone biopsy. The majority of these patients, 17 of 23 (68%), were treated with bilateral orchiectomy alone. Weak staining intensity was seen in 3 of 11 (27.3%) patients without hormone deprivation versus 0 of 23 (0%) patients with hormone deprivation. Moderate staining was noted in 3 of 11 (27.3%) untreated patients in the control group versus 7 of 23 (30.4%) treated patients. High-intensity staining was noted in 5 of 11 (45.4%) versus 16 of 23 (69.6%) patients who were treated with hormone deprivation (P = 0.04).
B7-H3 expression in hormone-refractory biopsy specimens
Nine biopsy specimens obtained from patients with castrate-resistant disease were evaluated for B7-H3 expression. B7-H3 staining of tumor cells was present in nine (100%) of these patients (Fig. 1). Of this cohort, six patients (67%) had moderate staining whereas three patients (33%) had marked staining.
Discussion
Despite the fact that prostate cancer represents the foremost noncutaneous malignancy and the second leading cause of cancer-related death for men in the United States, only a handful of targetable cell surface prostate cancer–associated proteins have been described in the literature. In addition, even though virtually all men who die of prostate cancer will do so after receiving some interval of androgen-ablative therapy, surprisingly, little is known about the influence of hormone therapy on the expression of these proteins by primary tumors or metastases. Finally, the role of many of these prostate cancer–associated proteins is poorly understood, thus making it difficult to readily ascribe a function for these proteins in modulating prostate cancer biology and disease outcomes. Consequently, the identification of any cell surface molecule that is uniformly and stably expressed by primary and metastatic prostate tumors, especially in the context of androgen-ablative therapy, encompasses a vital finding and an opportunity to improve prostate cancer treatment. This is especially true when the function of the molecule is relatively well defined and can be readily linked to mechanisms for cancer progression.
Roth et al. (19) recently described low levels of B7-H3 expression in normal prostate epithelia as well as significantly increased B7-H3 expression in nearly all prostate cancer cells. In addition, enhanced levels of B7-H3 expression by prostate cancer cells were found to correlate with virtually every adverse clinicopathologic feature associated with this disease. Specifically, higher levels of B7-H3 expression were associated with larger tumor volume, extraprostatic extension, higher Gleason score, seminal vesicle involvement, and positive surgical margins. Moreover, multivariate adjustment for the Gleason, PSA, seminal vesicle, and margin status score showed that intense B7-H3 expression serves as a statistically significant predictor of prostate cancer progression after attempted extirpative surgery (19). Likewise, Zang et al. (20) recently reported an independent study validating the aforementioned observations and further reported that adenocarcinoma of the prostate also expresses high B7-H4 (B7x) ligand levels.
Herein, we show that B7-H3 expression by prostate cancer cells remains stable in response to hormone treatment. Moreover, we show that intense B7-H3 expression persists as a statistically significant predictor of prostate cancer progression after RP, a finding that is analogous to what we previously reported as well as to validation studies conducted by one other group (19, 20). Finally, we show that bone metastases maintained very high levels of B7-H3 expression even after prolonged androgen-ablative therapy.
Given its relatively recent discovery in 2001, the understanding of the mechanisms regulating B7-H3 expression continues to evolve. In mice, B7-H3 is constitutively expressed by resting and activated B cells, macrophages, dendritic cells, and minor subsets of CD4+ and CD8+ T cells (10). In humans, B7-H3 mRNA expression can be detected in multiple tissues and cell lines; however, protein expression on human dendritic cells and monocytes has only been shown in response to treatment with phorbol 12-myristate 13-acetate and ionomycin. In one early study, human B7-H3 was reported as a costimulator of CD4+ and CD8+ T cells, promoting T-cell proliferation and cytokine production in vitro (28). More recently, B7-H3 has been overwhelmingly implicated as a potent inhibitor of T-cell activity (29). Specifically, single or duplicate constructs of the immunoglobulin V–like and immunoglobulin C–like domains of human B7-H3 have been shown to down-regulate both T-cell proliferation and cytokine production in response to CD3/CD28-mediated costimulatory activation (29). Furthermore, it has been reported that B7-H3–deficient mice develop accelerated forms of induced airway inflammation and experimental autoimmune encephalitis, thus implicating B7-H3 as an inhibitor of TH1-mediated immunity (30). In a separate study, plate-immobilized murine B7-H3 in the presence of anti-CD3 was shown to inhibit murine CD4+ T-cell activation and interleukin 2 production, an effect completely abrogated by antibody-mediated B7-H3 blockade (28). Antibody-mediated blockade of B7-H3 also exacerbates experimental autoimmune encephalomyelitis in mice (28, 30). In human neuroblastoma studies, B7-H3 has been implicated as an inhibitor of natural killer cell–mediated lysis, and multiple receptors have now been postulated to explain the ability of B7-H3 to inhibit responses by both T cells and natural killer cells (29). To date, however, cognate receptors for B7-H3 have not been elucidated. Finally, it also seems that B7-H3 ligand expression may be regulated by host factors or tumor microenvironment as is supported by differential protein expression of B7-H3 based on tumor type or even location within a given tumor.
Of particular relevance to prostate cancer, B7-H3 is highly expressed in developing bone during embryogenesis, and its expression increases as osteoblast precursor cells differentiate into mature osteoblasts. Specifically, B7-H3 seems to be required for osteoblast differentiation (31). Interestingly, osteoblastic hyperdense bone lesions represent a signature of metastatic prostate cancer. Related to this, our evaluation of prostate cancer bone metastases obtained from 34 patients revealed a trend toward increased B7-H3 expression in patients who received prior androgen deprivation therapy. This finding may indicate that hormonal therapy up-regulates B7-H3 expression in bone metastases but not primary prostate tumors. Alternatively, androgen deprivation may facilitate the outgrowth of high-grade tumor cells in bone metastases, resulting in increased B7-H3 expression. However, larger case-matched studies will be needed to confirm these relatively preliminary studies pertaining to B7-H3 expression by prostate cancer or other osteoblastic metastases.
B7-H3 may provide some theoretical advantages over other reported markers pertaining to prostate cancer. Distinct from non–membrane-bound PSA, which is secreted by malignant prostate cancer cells and down-regulated by androgen-ablative therapy, B7-H3 is highly expressed on the outer cytoplasmic membrane surface of treated or untreated prostate cancer cells, making B7-H3 an ideal target for therapy. In this regard, B7-H3 is analogous to six-transmembrane epithelial antigen of the prostate and prostate-specific membrane antigen, which are also expressed on the surface of malignant prostate cells (32, 33). However, unlike prostate-specific membrane antigen, in which expression levels decline with metastases, reported as 44% (8 of 18) to 67% (6 of 9) of bone metastases retaining prostate-specific membrane antigen expression, we find that B7-H3 is expressed by nearly all tumor cells within 100% (34 of 34) of bone metastases that we evaluated (34, 35). Our study further suggests that B7-H3 expression within bone metastases may actually increase after androgen-ablative therapy. Collectively, these observations are consistent with a potential role for B7-H3 in mediating metastatic progression during androgen-ablative therapy, a finding that contrasts with the reported observation that prostate-specific membrane antigen functions to abrogate prostate cancer invasiveness (36).
Although the presented data provide compelling evidence that B7-H3 may serve as potential target in castrate-resistant prostate cancer, the current data are not without limitations. When generating a cohort of matched patients that were not treated with NHT, one of the parameters utilized was Gleason score. The accuracy and application of Gleason score noted in RP specimens after NHT has previously been questioned (37). However, when Gleason score after NHT is assigned by an experienced genitourinary pathologist, as was done in the current series, it remains a significant prognostic measure (38). Another potential limitation is the immunohistochemical evaluation of decalcified tissue. The decalcification process may alter the immunoreactivity; however, the staining intensity and distribution of B7-H3 in the decalcified specimens in the current study are comparable with those of the primary prostatic tumors in the current and previous reports (19).
In summary, we have found that B7-H3 expression persists after NHT and remains a predictor of PSA progression after RP. These results, together with data that show continued expression in castrate-resistant metastases, suggest that B7-H3 expression may be one mechanism by which select prostate cancer cells survive androgen deprivation therapy and may therefore represent a potential target for future therapies.
Although future studies will be required to better understand the precise events that govern B7-H3 expression and functionality, our past and present studies suggest that B7-H3 ligand may help to render prostate tumors more aggressive by imbuing such tumors with a capability to neutralize local antitumoral immune responses. As such, targeting of B7-H3 could prove useful to potentiate responses to current forms of systemic treatment for advanced prostate cancer, treatments that are primarily immunotherapeutic or antiangiogenic in nature and which, in their present form, yield relatively low rates of durable disease remission. Additionally, our data indicate that B7-H3 may prove useful as a prognostic marker to identify patients with localized tumors who are at high risk for post-surgical disease progression and who might benefit from adjuvant therapy in the clinical trial setting.
Conclusions
We found that B7-H3 expression by prostate cancer is unchanged by NHT. Moreover, B7-H3 expression remains predictive of PSA progression after RP in patients receiving NHT. In addition, B7-H3 is strongly expressed in bone metastases and hormone refractory biopsy specimens. Given its role as a marker of adverse prognosis and the lack of change in expression with androgen deprivation therapy, B7-H3 may represent an important target for future combined therapy approaches, particularly for patients with high-risk prostate cancer.
Footnotes
Disclosure of Potential Conflicts of Interest
E. Kwon, ownership interest, Medarex.
References
- 1.Khan MA, Han M, Partin AW, Epstein JI, Walsh PC. Long-term cancer control of radical prostatectomy in men younger than 50 years of age: update 2003. Urology. 2003;62:86–91. doi: 10.1016/s0090-4295(03)00404-7. [DOI] [PubMed] [Google Scholar]
- 2.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–4. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 3.Shipley WU, Thames HD, Sandler HM, et al. Radiation therapy for clinically localized prostate cancer: a multi-institutional pooled analysis. JAMA. 1999;281:1598–604. doi: 10.1001/jama.281.17.1598. [DOI] [PubMed] [Google Scholar]
- 4.Penson DF, Chan JM. Prostate cancer. J Urol. 2007;177:2020–9. doi: 10.1016/j.juro.2007.01.121. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 7.Ribas A. Genetically modified dendritic cells for cancer immunotherapy. Curr Gene Ther. 2005;5:619–28. doi: 10.2174/156652305774964758. [DOI] [PubMed] [Google Scholar]
- 8.Thomas-Kaskel AK, Waller CF, Schultze-Seemann W, Veelken H. Immunotherapy with dendritic cells for prostate cancer. Int J Cancer. 2007;121:467–73. doi: 10.1002/ijc.22859. [DOI] [PubMed] [Google Scholar]
- 9.Sonpavde G, Spencer DM, Slawin KM. Vaccine therapy for prostate cancer. Urol Oncol. 2007;25:451–9. doi: 10.1016/j.urolonc.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 11.Flies DB, Chen L. The new B7s: playing a pivotal role in tumor immunity. J Immunother. 2007;30:251–60. doi: 10.1097/CJI.0b013e31802e085a. [DOI] [PubMed] [Google Scholar]
- 12.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 13.Inman BA, Frigola X, Dong H, Kwon ED. Costimulation, coinhibition and cancer. Curr Cancer DrugTargets. 2007;7:15–30. doi: 10.2174/156800907780006878. [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7−1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 15.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100:10388–92. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7−1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 17.Krambeck AE, Thompson RH, Dong H, et al. B7−4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103:10391–6. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7−1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 19.Roth TJ, Sheinin Y, Lohse CM, et al. B7−3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 20.Zang X, Thompson RH, Al-Ahmadie HA, et al. B7−3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104:19458–63. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebo TJ, Cheville JC, Riehle DL, et al. Predicting prostate carcinoma volume and stage at radical prostatectomy by assessing needle biopsy specimens for percent surface area and cores positive for carcinoma, perineural invasion, Gleason score, DNA ploidy and proliferation, and preoperative serum prostate specific antigen: a report of 454 cases. Cancer. 2001;91:2196–204. doi: 10.1002/1097-0142(20010601)91:11<2196::aid-cncr1249>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Blute ML, Bergstralh EJ, Iocca A, Scherer B, Zincke H. Use of Gleason score, prostate specific antigen, seminal vesicle and margin status to predict biochemical failure after radical prostatectomy. J Urol. 2001;165:119–25. doi: 10.1097/00005392-200101000-00030. [DOI] [PubMed] [Google Scholar]
- 23.Shah NT, Tuttle SE, Strobel SL, Gandhi L. Prostatic carcinoma metastatic to bone: sensitivity and specificity of prostate-specific antigen and prostatic acid phosphatase in decalcified material. J Surg Oncol. 1985;29:265–8. doi: 10.1002/jso.2930290416. [DOI] [PubMed] [Google Scholar]
- 24.Athanasou NA, Quinn J, Heryet A, Woods CG, McGee JO. Effect of decalcification agents on immunoreactivity of cellular antigens. J Clin Pathol. 1987;40:874–8. doi: 10.1136/jcp.40.8.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steffens J, Friedmann W, Lobeck H. Immunohistochemical diagnosis of the metastasizing prostatic carcinoma. Eur Urol. 1985;11:91–4. doi: 10.1159/000472463. [DOI] [PubMed] [Google Scholar]
- 26.Mukai K, Yoshimura S, Anzai M. Effects of decalcification on immunoperoxidase staining. Am J Surg Pathol. 1986;10:413–9. doi: 10.1097/00000478-198606000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Cheville JC, Tindall D, Boelter C, et al. Metastatic prostate carcinoma to bone: clinical and pathologic features associated with cancer-specific survival. Cancer. 2002;95:1028–36. doi: 10.1002/cncr.10788. [DOI] [PubMed] [Google Scholar]
- 28.Prasad DV, Nguyen T, Li Z, et al. Murine B7−3 is a negative regulator of T cells. J Immunol. 2004;173:2500–6. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 29.Castriconi R, Dondero A, Augugliaro R, et al. Identification of 4Ig-B7−3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101:12640–5. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suh WK, Gajewska BU, Okada H, et al. The B7 family member B7−3 preferentially down-regulatesT helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 31.Suh WK, Wang SX, Jheon AH, et al. The immune regulatory protein B7−3 promotes osteoblast differentiation and bone mineralization. Proc Natl Acad Sci U S A. 2004;101:12969–73. doi: 10.1073/pnas.0405259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–39. doi: 10.1002/jcb.10661. [DOI] [PubMed] [Google Scholar]
- 33.Hubert RS, Vivanco I, Chen E, et al. STEAP: a prostate-specific cell-surface antigenhighly expressed in human prostate tumors. Proc Natl Acad Sci U S A. 1999;96:14523–8. doi: 10.1073/pnas.96.25.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S, Zhang HS, Reuter VE, Slovin SF, Scher HI, Livingston PO. Expression of potential target antigens for immunotherapy on primary and metastatic prostate cancers. Clin Cancer Res. 1998;4:295–302. [PubMed] [Google Scholar]
- 35.Elgamal AA, Holmes EH, Su SL, et al. Prostate-specific membrane antigen (PSMA): current benefits and future value. Semin Surg Oncol. 2000;18:10–6. doi: 10.1002/(sici)1098-2388(200001/02)18:1<10::aid-ssu3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh A, Wang X, Klein E, Heston WD. Novel role of prostate-specific membrane antigen in suppressing prostate cancer invasiveness. Cancer Res. 2005;65:727–31. [PubMed] [Google Scholar]
- 37.Bullock MJ, Srigley JR, Klotz LH, Goldenberg SL. Pathologic effect of neoadjuvant cyproterone acetate on nonneoplastic prostate, prostatic intraepithelial neoplasia, and adenocarcinoma: a detailed analysis of radical prostatectomy specimens from a randomized trial. Am J Surg Pathol. 2002;26:1400–13. doi: 10.1097/00000478-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Bently G, Dey J, Sakr WA, Wood DP, Pontes JE, Grignon DJ. Significance of the Gleason scoring system after neoadjuvant hormonal therapy. Mol Urol. 2000;4:125–31. [PubMed] [Google Scholar]


