Abstract
Many peripheral solid tumors such as sarcomas and carcinomas express tumor-specific antigens that can serve as targets for immune effector T cells. Nevertheless, overall immune surveillance against such tumors seems relatively inefficient. We studied immune surveillance against a s.c. sarcoma expressing a characterized viral tumor antigen. Surprisingly, the tumor cells were capable of inducing a protective cytotoxic T cell response if transferred as a single-cell suspension. However, if they were transplanted as small tumor pieces, tumors readily grew. Tumor growth correlated strictly with (i) failure of tumor cells to reach the draining lymph nodes and (ii) absence of primed cytotoxic T cells. Cytotoxic T cells were not tolerant or deleted because a tumor antigen-specific cytotoxic T cell response was readily induced in lymphoid tissue by immunization with virus or with tumor cells even in the presence of large tumors. Established tumors were rejected by vaccine-induced effector T cells if effector T cells were maintained by prolonged or repetitive vaccination, but not by single-dose vaccination. Thus, in addition to several other tumor-promoting parameters, some antigenic peripheral sarcomas—and probably carcinomas—may grow not because they anergize or tolerize tumor-specific T cells, but because such tumors are immunologically dealt with as if they were in a so-called immunologically privileged site and are ignored for too long.
During the past 30 years, it has been shown that many carcinomas and sarcomas express tumor-specific antigens and that specific cytotoxic T cells (CTLs) can be induced and enhanced by appropriate vaccination (1–4). Nevertheless, the fact that these antigenic tumors grow successfully in the host indicates limitations in the efficiency of immune surveillance in tumor control (5, 6). In addition, it has been known for some time that immunosuppression in humans may enhance the incidence of virally triggered tumors or tumors of lymphohematopoietic vascular origin, but sometimes has little influence on frequencies of solid peripheral tumors such as carcinomas and sarcomas (7–9).
A generally accepted model in T cell immunology has suggested that T cells require two distinct signals for activation, one from the ligation of the T cell receptor with the major histocompatibility complex (MHC)-peptide complex and a second of varying quality (10–17). It has been postulated that antigen recognition in the absence of signal two—as might occur in peripheral tissues and thus in most carcinomas or sarcomas—renders T (or B) cells nonreactive (anergic) or may delete them (10, 12, 13). In addition, other mechanisms of inhibiting, impairing, or delaying immune responses may also facilitate tumor growth; these include modulation of MHC expression, mutation of T cell epitopes, expression of FasL, and suppressive mechanisms. The often drastically increased replication rates of tumor cells may also play a major role (4).
Successful tumor growth is a consequence of the balance between the host immune response and tumor growth kinetics. Experimental tumor immunology has often selected successful tumor cells that initiate tumors after injection of few (10–104) cells. Under physiological conditions a peripheral sarcoma or carcinoma starts as one cell and grows primarily in the periphery. A tumor model situation that precludes immediate CTL priming by injected tumor cells in suspension can mimic this physiological situation and permits an analysis of the requirements for peripheral tumors to induce a protective CTL response. Therefore, tumor growth of sarcoma cells and its relationship to the CTL-immune response against a defined strong tumor-specific antigen—the glycoprotein of lymphocytic choriomeningitis virus (LCMV) transfected into the fibrosarcoma cell MC57G (MC-GP) (18)—was evaluated here in two situations: (i) When sarcoma cells were transferred as single-cell suspensions s.c., they always induced a CTL response and were rejected, indicating a strong antigenicity and immunogenicity; yet (ii) when the same sarcoma cells were transplanted as a solid tumor fragment s.c. (containing about the same number of cells), they did not induce a CTL response and generally grew. Despite the fact that s.c. localizations are not classically considered to belong to immunologically privileged sites (19), our analysis shows that peripheral solid tumors may grow because they are ignored by the immune system for too long.
MATERIALS AND METHODS
Flow Cytometric Analysis.
Surface expression was tested with anti-B7.1 fluorescein isothiocyanate, anti-B7.2 fluorescein isothiocyanate, biotinylated anti-intercellular adhesion molecule-1, anti-lymphocyte function-associated antigen-1, or anti-Db antibodies and streptavidin-phycoerythrin with a FACStar (all antibodies were from PharMingen). H-2Db+GP33–41-specific T cells were isolated from the T cell antigen receptor transgenic mouse strain 318 (20), in vitro labeled with the fluorescent dye CFSE (21), and then transferred into recipient animals (2 × 107 spleen cells). The transferred cells were followed by monitoring CFSE, Vα2 (anti-Vα2-phycoerythrin), and CD8+ (anti-CD8-tricolor).
Cell Lines, Dendritic Cells, Cr-Release Assay, and Virus.
MC57G (MC) (18), L929 EL-4, and P815 cells [from American Type Culture Collection (18)] have been used widely. Dendritic cells from bone marrow cultures of LCMV GP33–41 transgenic animals (H-8 mice) were isolated as described previously (22). The Cr-release assay and LCMV (WE strain) have been previously described (23).
PCR.
DNA was extracted from lymph nodes by using the Qiamp Tissue Kit (Qiagen, Chatsworth, CA). LCMV-GP-specific nested PCR was performed as described previously (24) but primer pairs were adapted. The primers were RC1 and DM1 for the primary reaction and RCM and 333 for the secondary. For the secondary reaction, cycling conditions were 94°C for 70 sec, 50°C for 1 min, and 72°C for 30 sec. Positive control DNA was obtained from RNase-treated DNA extracted from MC57 cells infected 48 hr previously with LCMV (WE strain) at a multiplicity of infection of 0.04 (24). One water control was performed per primary and secondary reaction and was uniformly negative. PCR specific for perforin exon 3 was done without modifications as described (24). Sequences of primers are: 333 –CTGACGATGCCCAATGC; RCM –GGTACTGATAGCTTGTTTGGCTGCACC; RC1 –GAGCTCTGCAGCAAGGATCATCC; and DM1 –GAATTCTATCCAGTAAAAGGATGG.
RESULTS
Characterization of the Tumor Model.
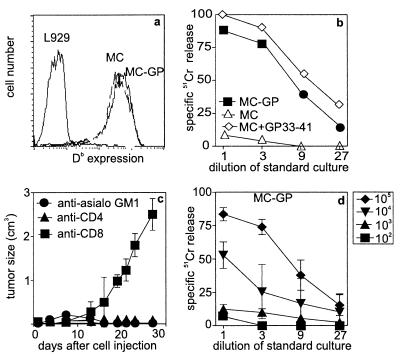
Tumor cells were grown as monolayers in tissue culture flasks and used as a single-cell suspension after trypsinization. To obtain specifically sized tumor pieces of 2 × 2 × 2 mm in the absence of immune T cells, MC-GP cells were injected s.c. into the flank of T cell immunodeficient mice (H-2b RAG-1−/− or C57BL/6 nu/nu). A dissected piece of solid tumor of 2 × 2 × 2 mm was found to contain about 2–5 × 106 tumor cells; therefore the same number of single cells was used for injection s.c. into the flank of C57BL/6 mice for comparative experiments. The MC-GP cells expressed H-2Db equal to MC (Fig. 1a), but did not express B7.1, B7.2, MHC class II, intercellular adhesion molecule-1, or lymphocyte function-associated antigen-1 above background levels (18) (not shown). Tumor cells expressing GP were lysed to an extent comparable to peptide (GP33–41)-pulsed MC57 cells by anti-LCMV-specific T cells from C57BL/6 (H-2b) mice (Fig. 1b). Depletion of CD8+ T cells enhanced tumor growth when MC-GP cell suspensions were injected s.c., whereas depletion of CD4+ T cells or natural killer (NK) cells (anti-asialo GM1 treatment) had no effect on tumor growth in vivo (Fig. 1c). Single-cell suspensions of live MC-GP injected at various doses s.c. primed CTL responses with >104 cells (Fig. 1d). Injection of irradiated cells s.c. failed to induce a CTL response at doses of up to 107 cells (not shown). In contrast, injection of irradiated (8,000 rad, and therefore non proliferating) MC-GP cells directly into the spleen primed CTL responses with 104 cells (data not shown, but comparable to those shown in Fig. 1d). This result indicates a >1,000-fold greater efficiency of CTL induction by tumor cells in secondary lymphoid organs compared with tumor cells located s.c. in the periphery.
Figure 1.
Characterization of the MC-GP tumor cells, tumor growth, and immune response. (a) MHC class I (Db) expression of MC-GP cells vs. L929 (H-2k) cells; MC-GP cells were negative for intercellular adhesion molecule-1, B7.1, B7.2, and lymphocyte function-associated antigen-1 (not shown). (b) MC-GP and MC57 cells with or without peptide labeling (GP33–41) were used as target cells in vitro in a 51Cr-release assay. (c) MC-GP cells (2 × 106) were injected s.c. in both flanks of C57BL/6 mice that were depleted of NK-cells [anti-asialo GM1 (Wako Biochemicals, Osaka)], 30 μl diluted in 200 μl balanced salt solution i.v. on day −1), of CD4+ T cells [200 μl i.p. of anti-CD4 antibodies (YTS191.1) on days −3 and −1], or of CD8+ T-cells [200 μl i.p of anti-CD8 antibodies (YTS169.4.2) on day −3 or −1]. Tumor growth was followed during 30 days. Tumor volume was calculated by the formula/V = πxabc/6, where a, b, and c are the orthogonal diameters. Titrated doses of (d) live MC-GP were injected s.c. into the flank of C57BL/6 animals. Eight days later, splenocytes were restimulated in vitro on irradiated GP33–41-pulsed splenocytes for 5 days and the CTL activity was determined then in a Cr51-release assay.
Correlation Between Peripheral Tumor Growth with Absence of Tumor Cells in Secondary Lymphoid Organs.
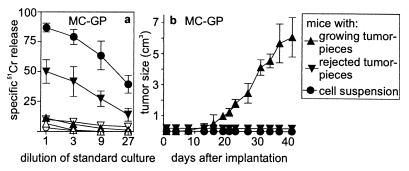
C57BL/6 mice treated s.c. with 2–5 × 106 MC-GP cells in suspension generated Db-restricted CTL responses specific for LCMV-GP (Fig. 2a). Although all tumors initially grew up to a diameter of 2–3 mm, they were rejected 10 to 12 days after initiation of the experiment. After the implantation of small MC-GP tumor pieces, 74.3% of C57BL/6 mice developed a tumor (i.e., 119 growing tumors of a total of 160 transplanted, Table 1). In some mice, tumors were rejected and a few transplanted small tumor pieces did not grow presumably for technical reasons because either the fragment was necrotic or contained too few viable tumor cells. Mice exhibiting growing tumors and those without tumors (Fig. 2b) were tested for tumor specific antigen-specific CTL priming (Fig. 2a) and for indications that tumor cells had reached the local lymph nodes or spleen (Fig. 3B). There was a strict correlation between absence of priming of CTL against LCMV-GP on one hand and absence of tumor-derived DNA in lymph nodes or spleen on the other hand (Fig. 3B) in mice with growing tumors (Fig. 2a, Fig. 3 B and D). In contrast, all mice given 2 × 106 single tumor cells were positive both for LCMV-GP-specific PCR and primed CTL activity. All mice tested that had rejected either cell suspensions forming transiently small tumors or initially growing small tumor pieces were subsequently negative by PCR on day 10, 15, or 21, but all were positive by secondary CTL at the same time points (not shown); these results indicated that rejection of tumor cells was complete.
Figure 2.
Comparison of tumor take vs. induction of CTL responses against MC-GP tumor cells or tumor pieces. MC-GP cells (2 × 106) were injected as single cells s.c. in both flanks (●), or small tumor pieces containing comparable numbers of tumor cells were implanted at similar locations (▴, ▾). According to the results, secondary CTL activity (a) and tumor growth (b) were divided and presented as two groups: growing tumors (▴) and rejected tumors (▾). The induction of a CTL response was assessed by a 51Cr-release assay after 5 days of restimulation in vitro of local lymph node or spleen cells taken 8 days after injection of the tumor-cell suspension (●, ○). Open symbols represent killing against unpulsed EL-4 target cells, closed symbols against GP33–41-pulsed EL-4 target cells (a). CTL activity was similarly assessed 8 days (not shown) and 2 weeks (not shown) after implantation of tumor pieces and at the end of the experiment (after about 40 days). CTL activity is given for each group as mean ± SD. One of three comparable experiments is shown (error bars indicate SD). After each in vivo experiment, tumor cells were cultured and all tested positive for Db-GP33–41 expression (not shown).
Table 1.
Tumor growth and CTL induction after transfer of MC-GP cell suspension or tumor pieces in C57BL/6 or ALY × ALY mice
| Transferred cells, dose | Recipient | Tumor growth*
|
CTL priming†
|
||
|---|---|---|---|---|---|
| Growing | Tumor free | Growing tumors, CTL+/total tested | No tumors, CTL+/total tested | ||
| MC-GP (2 × 106) Cell suspension | C57BL/6 | 0 | 30 | Not testable | 15/15 |
| MC-GP (2–5 × 106) Tumor piece | C57BL/6 | 119 | 41 | 0/22 | 10/15‡ |
| MC-GP (107) Cell suspension | ALY × ALY | 6 | 0 | 0/6 | Not testable |
| MC-GP (2–5 × 106) Tumor piece | ALY × ALY | 6 | 0 | 0/6 | Not testable |
Tumor size was followed for 4 weeks or longer. Tumors reaching a volume of 1 cm3 or more were counted as growing tumors.
CTL activity was determined at day 8 after injection of cell suspensions or 4 weeks after transplantation of solid tumor pieces after in vitro stimulation with GP33-41-pulsed splenocytes during 5 days; the numbers shown represent number of mice responding with secondary CTLs (CTL+) over total number of mice tested in each category.
Five transplanted tumor pieces did not grow because of transplantation failure, depending on the quality of the transplanted tumor pieces.
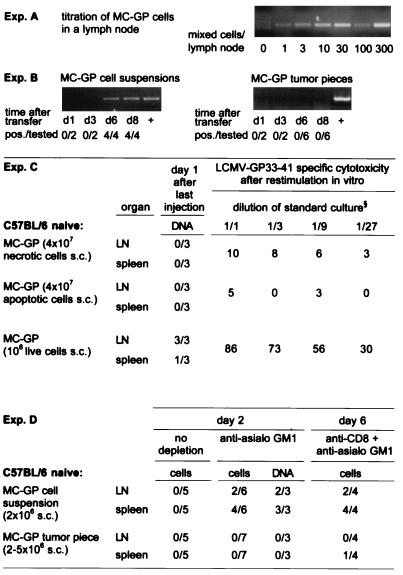
Figure 3.
Homing of MC-GP tumor cells into local lymph nodes and spleen. DNA was extracted from draining inguinal lymph nodes or spleen at the indicated time points after injection of a tumor cell suspension or after the implantation of small tumor pieces in both flanks of C57BL/6 mice. A LCMV GP-specific nested PCR was performed with the primers described in Materials and Methods. (A) The sensitivity of the assay was determined in vitro by mixing tumor cells with a constant number of 106 lymph node cells from untreated C57BL/6 mice. (B) The number of mice with positive lymph nodes over the total number of mice tested is given for each time point in each group. Lymph nodes from control C57BL/6 mice, which had not received tumor cells, and water were tested in parallel and were negative in all experiments shown. The integrity of the DNA extracted from lymph nodes was successfully tested by a perforin exon 3-specific PCR (not shown). (C) Dead tumor cells s.c. did not lead to a PCR signal or to a primed CTL response in contrast to live cells (106 s.c.). MC-GP cells (107) were either treated by freeze-thawing (necrotic, trypan blue positive, not shown) or kept on 42°C for 24 hr (apoptotic, trypan blue negative hypodiploid DNA peak in propidium iodide staining and flow cytometry, details not shown) and then injected repetitively (4 times) on alternate days in both flanks of C57BL/6 mice. One day after the last injection, DNA was prepared from spleen and from draining lymph nodes and tested by nested PCR for LCMV GP-specific DNA. Values indicate number of PCR-positive samples per total number of samples tested. At the same time point, splenocytes were restimulated in vitro for 5 days and then tested in a 51Cr-release assay. Values indicate percent specific 51Cr release as mean of three animals at the dilution of standard culture indicated. One of two comparable experiments is shown. (D) No live tumor cells could be isolated from the spleen or lymph nodes of untreated C57BL/6 mice on day 2, 4 (not shown), or 6 (not shown). Live tumor cells and GP-specific DNA could be detected in vitro after depletion of NK cells [30 μl of anti-asialo GM1 (Wako Biochemicals, Osaka) diluted in 200 μl balanced salt solution i.v. on day −1] on day 2 and after CD8 [200 μl i.p. anti-CD8 (YTS169.4.2) on days −3 and −1] plus NK depletion on day 6 after injection of MC-GP tumor cell suspensions, but not after transplantation of solid MC-GP tumor pieces. To detect live cells, lymph nodes (LN) or spleens were passed through a fine-mesh stainless steel grid, and the resulting single-cell suspension was cultured on selection medium/[0.8 mg/ml G-418 (GIBCO/BRL)]. Values indicate positive samples over total number of mice tested.
Correlation of the PCR Signal with Presence of Viable but Not Processed Tumor Cells in Secondary Lymphoid Organs.
The PCR signal was not detected in draining lymph nodes or spleen on day 1 after four s.c. injections of 107 MC-GP that had been killed by freeze-thawing (necrotic) or by incubation for 24 hr at 42°C (apoptotic cells) (Fig. 3C). Similarly, tumor cells injected i.p. or s.c. into LCMV-immune memory mice did not lead to a PCR signal in mesenteric lymph nodes or spleen 2 or 4 days later (data not shown). These results showed that the LCMV-GP PCR signal detected in lymph nodes derived from live tumor cells or cells dying locally very recently and could not stem from an ongoing immune response destroying tumor cells. In support of this, in vitro expanding tumor cells were found on days 2, 4, and 6 after s.c. injection in mice in draining lymph nodes and spleen. Reisolation of live tumor cells was possible only when mice had been depleted of NK cells by anti-asialo GM1 treatment (Fig. 3D). Because NK cells had no effect on overall growth in vivo (as shown in Fig. 1c), NK cells apparently reduced the numbers of viable tumor cells detectable after in vitro culture of spleen and lymph node single-cell preparations. On day 6 after tumor cell injection s.c. when a CTL response was already induced, no tumor cells could be grown from spleen or lymph nodes from anti-asialo GM1-treated recipient mice unless CD8+ T cells were also depleted (Fig. 3 D). With the same protocol, a PCR signal was found in draining lymph nodes on day 4 (not shown).
A crucial role of draining lymph nodes in induction of an immune response against peripheral tumors was illustrated in a second series of experiments by using mice with a spleen, but that for genetic reasons lacked all other secondary lymphoid organs [ALY × ALY mice on the C57BL/6 background (23)]. 107 MC-GP single cells injected s.c. always caused tumors in ALY × ALY mice, but never in C57BL/6 control mice (Table 1), 105 cells caused tumors in two of five ALY × ALY mice (not shown) and in none of the control mice, indicating a difference in tumor resistance of at least 10- to 100-fold. Again, as before, all ALY × ALY mice with growing tumors were not primed for GP-specific CTL responses. However, when 2 × 106 MC-GP tumor cells were injected directly into the spleen, they readily induced a CTL response in ALY × ALY mice (not shown). Thus, ALY × ALY mice could induce a CTL response against MC-GP tumor cells if the cells reached the spleen; however, if after s.c. injection they could not reach draining lymph nodes—because they are absent in ALY × ALY mice—no CTL response was induced.
So far the studies established that MC-GP tumor cells formed successfully growing tumors if they failed to reach secondary lymphoid organs. The observed differences in tumor take and CTL priming between transplanted tumor pieces and injected cell suspensions could be correlated strictly with the ability of tumor cells to reach local lymph nodes and were not caused by differences in tumor matrix or vascularization of the tumors for the following reasons: First, after injection of 2–5 × 106 single tumor cells a small (2–3 mm diameter) established and vascularized tumor could be observed by day 8, which was then readily rejected (Fig. 2b) [see also Kohler et al. (25)]. Secondly, established tumor pieces are accessible to CTL and can be rejected after efficient priming (shown in the last section of this study where immunitation protocols against established tumors were studied).
MC-GP Tumor Cells Induce a CTL Response Directly.
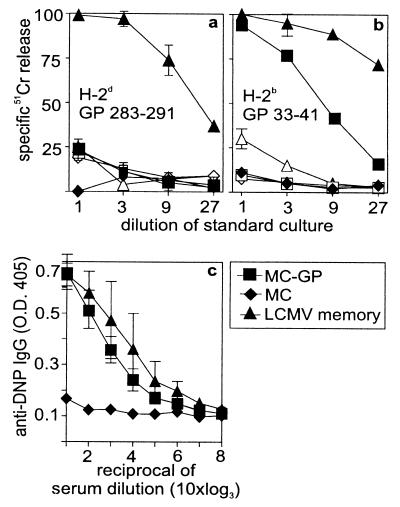
To evaluate the potential role of APCs, MC-GP cells were injected repetitively s.c. either with 107 apoptotic or necrotic cells; these immunizations failed to prime GP-specific secondary CTL responses (Fig. 3C). Mice with large tumors (>2–5 cm3) and with necrotic centers should be expected to offer plenty of material for APC to process and present to T cells. However, as shown in Fig. 2a, mice with large tumors were not primed. In addition, injection of large numbers of live or freeze-thawed (not shown) MC-GP (H-2b) into (C57BL/6 H-2b × B10.D2 H-2d)F1 mice failed to reveal a H-2d-restricted GP-specific CTL response restricted to H-2d plus the corresponding GP283–291 epitope (26) (Fig. 4a); but live cells induced a strong CTL response to the GP33–41-Db epitope (Fig. 4b). GP-specific H-2d-restricted CTLs were clearly induced in LCMV immune F1 mice (Fig. 4a). To demonstrate that processing of tumor antigens did occur and that such processing resulted in MHC class II-restricted T cell responses, the following experiment was performed. MHC class II negative fibrosarcoma MC-GP cells were injected into C57BL/6 (H-2b) mice. These mice were then checked for primed T-helper cell activity specific for the tumor antigen LCMV-GP by challenging them with the defined H-2b T helper epitope P13 (GP60–80) to which the classical hapten determinant 2,4-dinitrophenol (DNP) had been coupled (27). Whereas naive control mice or mice primed with control tumor cells failed to show a DNP-specific IgG response, those mice primed with either LCMV or with single-cell suspensions of MC-GP exhibited an enhanced IgG anti-DNP response, indicating that primed T help (27) was present (Fig. 4c). Therefore, although in several other tumor models crosspriming by means of APC has been shown to contribute to tumor immunity (28), in our model priming of tumor-specific CTL seems to be directly by tumor cells in organized lymphoid tissue and apparently not by means of crossprocessing.
Figure 4.
Examination of crosspriming vs. direct induction of a CTL response by MC-GP tumor cells. Tumor cells (5 × 106 MC57 or MC-GP) were injected four times on alternate days i.p into (C57BL/6 H-2b × B10.D2 H2d)F1 animals. On day eight after the first injection, the splenocytes were restimulated on GP283–291 (H-2d)- or GP33–41 (H-2b)-labeled F1 splenocytes for 5 days. CTL activity was then tested on (a) GP283–291-labeled P815 (H-2d) or (b) GP33–41-labeled EL4 (H-2b) cells. Closed symbols represent peptide-labeled targets, open symbols represent unlabeled targets. (c) C57BL/6 (H-2b) mice treated with the same repetitive injection protocol were challenged with 20 μg of DNP coupled to P13 [helper epitope GP60–80 in H-2b (27)] 2 days after the last injection of cells. Seven days later, anti-DNP IgG titers were measured by ELISA. LCMV-primed (−60 days, 102 pfu LCMV i.v.) mice served as positive controls. The mean and the SD of three animals per group are shown. One of two comparable experiments is shown.
Absence of CTL Anergy or Deletion.
The finding that mice with growing MC-GP tumors of up to about 5 cm3 did not possess primed tumor-specific CTL could reflect anergy or deletion of these T cells. However, when mice with large GP-expressing tumors were infected with LCMV they all promptly generated GP33–41-specific CTL responses (Fig. 5h). This result demonstrated that strictly peripheral tumors had not anergized, exhausted, or deleted GP-specific CTL. Absence of T cell deletion was confirmed by adoptive transfer of CD8+ T cells expressing a transgenic T cell receptor 318 specific for LCMV-GP33–41-Db (20), which had been marked with a stable fluorescent dye (21). The kinetics of survival of these cells during 10 days in recipients bearing >5 cm3 tumors were comparable to those in control C57BL/6 recipients. Numbers of CD8+ Vα2+ and CFSE-labeled T cells detected in the blood on day 1 after transfer were similar in tumor-free mice (3.8/3.1% of total CD8+cells) and in mice with tumors (2.8/2.6/3.2%) and did not change for 10 days (tumor-free mice: 3.2/3.3%; tumor-positive mice: 2.6/3.1/2.8%). Neither were the 318 T cells activated to a detectable extent nor were T cell antigen receptor levels down modulated (fluorescence-activated cell sorter data not shown). Thus, the polyclonal endogenous CTL response or the indicative transgenic T cell antigen receptor response yielded evidence neither for T cell anergy or deletion nor for activation, compatible with results from different models (29, 30).
Figure 5.
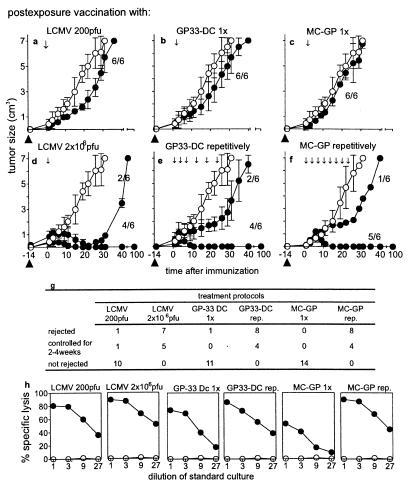
Immunotherapy of peripheral MC-GP tumors. Mice with growing MC-GP tumors of ≥5 mm on day 14 after transplantation were selected for immunotherapy experiments; tumor growth after the different priming strategies was followed up to 100 days. Closed circles indicate animals from the different experimental groups and open circles represent untreated control animals (a–f). The numbers shown indicate the proportion of tumor growth (or/rejection) per tumors tested. Each experiment was repeated twice with similar results. A low dose of LCMV (2 × 102 pfu) (a) or a high dose (2 × 106 pfu) (d) was injected intravenously 14 days after transfer/transplantation of tumor cells; this time point is now taken as day 0 of therapy. The day of transplantation is indicated as ▴. Dendritic cells were isolated from GP33–41 transgenic mice (H-8) (22) (GP33-DC) and injected either once on day 0 (b) or repetitively during 3 weeks as indicated (e) (105 cells per injection). MC-GP cells were also injected once (c) or repetitively every other day during 3 weeks (2 × 106 cells per injection) (f). The outcome of all performed experiments is summarized in g. All the treated animals mounted a CTL response when tested in a 51Cr release assay after in vitro restimulation, whereas the untreated controls did not (h). (●), CTL response of animals 40 days after the beginning of the immunotherapy; (○), animals without immunotherapy.
Tumor growth also was not caused by immune escape or MHC class I modulation, as shown by analyzing at least 10–15 growing tumors from each of the various protocols (Table 1) individually at the end of each experiment. Direct tests were technically not feasible because of poor viability and high spontaneous 51Cr release of cells isolated directly from tumors by trypsinization. After subculture for 3 days, the isolated tumor cells were all found to be susceptible to LCMV-GP-specific effector CTL, and Db expression was at control levels (data not shown; results are comparable to those shown in Fig. 1 a and b). In addition, as shown in the next section growing tumors were susceptible to rejection if a strong and long-lasting CTL response was induced and maintained in vivo.
Immunization Against Established Tumors.
The possibility was examined that tumors may lose susceptibility to rejection by immune effector cells or tumors may grow because too few effector T cells may be induced to reject efficiently growing tumors. In most available studies on protective immunity against tumors, vaccination or adoptive transfer of immune effector cells had been initiated before, on the day of, or in rare cases only a few days after tumor transfer; here we waited 14 days after tumor transplantation before a T cell response was induced by a “postexposure” vaccination. Mice given a small tumor piece of MC-GP s.c. were monitored for tumor take 14 days after transplantation, when its take could be judged reliably. Mice were then immunized once with 200 plaque-forming units (pfu) of LCMV-WE i.v. (Fig. 5a) or 105 LCMV-GP33–41-expressing dendritic cells isolated from the GP33–41 transgenic mouse H-8 (22) (GP33-DC) (Fig. 5b) or with 2 × 106 single MC-GP tumor cells injected s.c. that reach draining lymph nodes and spleen (Fig. 5c). By these immunizations of short duration (7–12 days) tumor growth was retarded by 1–2 days after immunization with MC-GP, by 2 days with GP33-DC, and by about 10 days in 200 pfu LCMV-WE-immunized mice. However, by day 30 after immunization the differences in tumor size between those treated by postexposure vaccination and untreated recipients had become negligible, despite the fact that all vaccinated mice now possessed primed CTLs (Fig. 5h). A comparable minimal effect of a single vaccination had been shown earlier in the case of a GP33–41-positive insulinoma (31). If, however, 2 weeks after tumor transplantation mice were immunized either with a high dose of 2 × 106 pfu LCMV-WE (Fig. 5d) causing a prolonged and widely spreading infection with long-term CTL activation, or alternatively by repeated injections of GP33-DC (Fig. 5e) or MC-GP (Fig. 5f) in 2- to 5-day intervals for 3 weeks, most tumor pieces failed to grow further and eventually disappeared completely during an observation period of longer than 100 days (Fig. 5g). Thus, an antigen-driven prolonged CTL response maintained for around 3–4 weeks was apparently needed to reject a peripheral tumor of 5 × 106–2 × 107 cells corresponding to a tumor diameter of about 5 mm. The surprising fact that tumor cells, themselves injected repeatedly, were capable of inducing an efficient CTL response rejecting small tumors is of greatest interest in the context of this study. This finding illustrates that besides localization, the time at which tumor cells reach secondary lymphoid organs and the duration of the antigenic stimulus are key for overall efficiency of immune surveillance. These factors are similar to the requirements of sustained antigen-driven activation of effector T cells to control Mycobacterium tuberculosis in granulomas or some persistent virus infections by “infection or concomitant immunity” (32).
These latter results are compatible with earlier experiments showing that in transgenic mice expressing LCMV-GP in β-islet cells, diabetes was induced by infection with LCMV but not by infection with a recombinant vaccinia virus expressing LCMV-GP; absence of diabetes correlated with the 100-fold weaker CTL response after the latter infection (33). Obviously, although this apparent rather high threshold against destruction of peripheral cells that are “self” by activated T cells protects against easy induction of autoimmune disease, it is a disadvantage for effective immune surveillance against strictly peripheral tumors.
DISCUSSION
Our experiments illustrate in a model situation the following simple concept: antigens that do not enter organized lymphoid tissues at sufficient levels do not induce an efficient CTL response; thus many strictly peripherally expressed self antigens (33) and also highly antigenic and successfully growing peripheral tumors are ignored by the immune system. If the same so far ignored antigen enters lymphoid organs, a T cell response is induced. A late, weak, or time-limited immune response does not suffice to cause tumor rejection, whereas vigorous and sustained T cell responses can achieve complete rejection. The overall rejection success therefore depends on the relative kinetics of tumor cell numbers (or tumor size, influenced by growth rate and several other tumor parameters) vs. kinetics and relative numbers of effector T cells over time. The importance of this balance is demonstrated here by the finding that postexposure vaccination was inefficient after a single vaccination but was able to control the small tumors completely with multiple vaccinations (Fig. 5 d–f).
Thus, three situations may be distinguished that may have clinical parallels: (i) A low dose of single tumor cells or small s.c. solid tumors may grow locally as a consequence of none or too few tumor cells reaching draining lymph nodes during early tumor development; these tumors are therefore ignored immunologically and neither induce nor tolerize T cells. The surprisingly successful formation of tumor by small but not by great numbers of experimental tumor cells injected s.c. had been reported earlier and has been described as “sneaking through” (1). (ii) Sufficient numbers of cells from many initially growing tumors may eventually reach draining lymph nodes, induce an efficient CTL response, and therefore be rejected; such tumors will usually not become clinically apparent unless this immune response comes too late (see ref. 3), MHC class I or tumor antigens are modulated, fast replication rates develop so that tumors outrun CTL responses, or other escape mechanisms evolve. (iii) Great numbers of experimental tumor cells will grow, as in clinically manifest tumors that have already reached a large size, despite induction of effector cells because the relative low numbers of antitumor-specific T cells are too inefficient to control large numbers of peripheral tumor cells (29, 31). This latter situation may also be found in human sarcomas and carcinomas that metastasize “relatively” early and where specific tumor-infiltrating lymphocytes (34) or even lymphatic metastasis may be found; effector T cells are apparently induced, but too late — or the T cell response is too weak — to control/reject the established tumor masses. Our example studied in Fig. 5 d–f impressively illustrates the demanding requirements: The few slightly (<5-fold) larger tumors tended to resist complete rejection even by repetitive vaccinations. Drastic reduction of tumor cell load, e.g. by surgery, and enhancement of T cell responses by expansion in vitro, or continued boosting in vivo by appropriate postexposure vaccination as shown here, may reverse an unfavorable balance. Overall, the present experiments illustrate the possibility that strictly peripheral sarcomas (or probably also carcinomas) grow because such tumors stay outside the immune system and are therefore immunologically ignored for too long.
Acknowledgments
We thank A. Oxenius for providing P13-coupled DNP, A. Macpherson, K. Maloy, and S. Oehen for critical reading, and A. Althage for excellent technical assistance. This work was supported by Swiss National Science Foundation Grant 31-50900.97 to R.M.Z and by the Kanton Zurich.
ABBREVIATIONS
- CTL
cytotoxic T cell
- LCMV
lymphocytic choriomeningitis virus
- MHC
major histocompatibility complex
- DNP
2,4-dinitrophenyl
- pfu
plaque-forming unit
- NK cell
natural killer cell
References
- 1.Old L J, Boyse E A, Clarke D A. Ann N Y Acad Sci. 1962;101:80–106. [Google Scholar]
- 2.Pardoll D M. Nat Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 3.Boon T, Cerottini J C, van-den-Eynde B, van-der-Bruggen P, Van-Pel A. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 4.Möller G. Immunol Rev. 1995;145:1–250. [Google Scholar]
- 5.Andrews E J. J Natl Cancer Inst. 1974;52:729–732. doi: 10.1093/jnci/52.3.729. [DOI] [PubMed] [Google Scholar]
- 6.Maraveyas A, Hrouda D, Dalgleish A G. In: Molecular Aspects of Cancer and Its Therapy. Mackiewicz A, Sehgal P B, editors. Basel: Birkhauser; 1998. pp. 73–86. [Google Scholar]
- 7.Burnet F M. Transplant Rev. 1971;7:3–25. doi: 10.1111/j.1600-065x.1971.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 8.Möller G, Möller E. J Natl Cancer Inst. 1975;55:755–759. doi: 10.1093/jnci/55.4.755. [DOI] [PubMed] [Google Scholar]
- 9.Kelly D M, Emre S, Guy S R, Miller C M, Schwartz M E, Sheiner P A. Cancer. 1998;83:1237–1243. [PubMed] [Google Scholar]
- 10.Bretscher P, Cohn M. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 11.Lafferty K J, Cunningham A J. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz R H. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 13.Matzinger P. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Ashe S, Brady W A, Hellstrom I, Hellstrom K E, Ledbetter J A, McGowan P, Linsley P S. Cell. 1992;71:1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz R H. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 16.Townsend SE, Allison J P. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 17.Allison J P, Hurwitz A A, Leach D R. Curr Opin Immunol. 1995;7:682–686. doi: 10.1016/0952-7915(95)80077-8. [DOI] [PubMed] [Google Scholar]
- 18.Kündig T M, Bachmann M F, DiPaolo C, Simard J J L, Battegay M, Lother H, Gessner A, Kühlcke K, Ohashi P S, Hengartner H, et al. Science. 1995;268:1343–1347. doi: 10.1126/science.7761853. [DOI] [PubMed] [Google Scholar]
- 19.Medawar P B. Proc R Soc London Ser B. 1958;148:145–153. doi: 10.1098/rspb.1958.0058. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann C, Brduscha-Riem K, Blaser C, Zinkernagel R M, Pircher H. J Exp Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oehen S, Brduscha R K, Oxenius A, Odermatt B. J Immunol Methods. 1997;207:33–42. doi: 10.1016/s0022-1759(97)00089-6. [DOI] [PubMed] [Google Scholar]
- 22.Ludewig B, Ehl S, Karrer U, Odermatt B, Hengartner H, Zinkernagel R M. J Virol. 1998;72:3812–3818. doi: 10.1128/jvi.72.5.3812-3818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karrer U, Althage A, Odermatt B, Roberts C W M, Korsmeyer S, Miyawaki S, Hengartner H, Zinkernagel R M. J Exp Med. 1997;185:2157–2170. doi: 10.1084/jem.185.12.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klenerman P, Hengartner H, Zinkernagel R M. Nature (London) 1997;390:298–301. doi: 10.1038/36876. [DOI] [PubMed] [Google Scholar]
- 25.Kohler M, Rüttner B, Cooper S, Hengartner H, Zinkernagel R M. Cancer Immunol Immunother. 1990;32:117–124. doi: 10.1007/BF01754208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Most R G, Sette A, Oseroff C, Alexander J, Murali-Krishna K, Lau L L, Southwood S, Sidney J, Chesnut R W, Matloubian M, et al. J Immunol. 1996;157:5543–5554. [PubMed] [Google Scholar]
- 27.Oxenius A, Bachmann M F, Zinkernagel R M, Hengartner H. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 28.Carbone F R, Kurts C, Bennet S R M, Miller J F A P, Heath W R. Immunol Today. 1998;19:368–373. doi: 10.1016/s0167-5699(98)01301-2. [DOI] [PubMed] [Google Scholar]
- 29.Wick M, Dubey P, Koeppen H, Siegel C T, Fields P E, Chen L, Bluestone J A, Schreiber H. J Exp Med. 1997;186:229–238. doi: 10.1084/jem.186.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prévost-Blondel A, Zimmermann C, Stemmer C, Klumburg P, Rosenthal F M, Pircher H. J Immunol. 1998;161:2187–2194. [PubMed] [Google Scholar]
- 31.Speiser D E, Miranda R, Zakarian A, Bachmann M F, McKall F K, Odermatt B, Hanahan D, Zinkernagel R M, Ohashi P S. J Exp Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackaness G B. Infect Immun. 1964;9:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohashi P S, Oehen S, Aichele P, Pircher H, Odermatt B, Herrera P, Higuchi Y, Buerki K, Hengartner H, Zinkernagel R M. J Immunol. 1993;150:5185–5194. [PubMed] [Google Scholar]
- 34.Rosenberg S A, Spiess P, Lafreniere R. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]