Abstract
Papillary fibroelastomas are the most common benign neoplasms of the cardiac valvular structures, and they are being recognized more frequently because of higher-resolution imaging technology. Papillary fibroelastomas are associated with substantial complications that are secondary to systemic embolism. Incidentally discovered papillary fibroelastomas are treated on the basis of their size, mobility, and associated comorbidities and symptoms. Surgical resection should be offered to all patients who have symptoms and to asymptomatic patients who have pedunculated lesions or tumors larger than 1 cm in diameter. Valve-sparing excision produces good long-term results in most instances.
Herein, we present the case of a patient who was scheduled for elective myocardial revascularization and in whom an aortic valve papillary fibroelastoma was discovered incidentally during routine intraoperative transesophageal echocardiography. The timely recognition of this asymptomatic tumor enabled a modified operative approach: the patient underwent myocardial revascularization with concomitant valve-sparing resection of the tumor. We discuss the pathophysiology of fibroelastomas of the aortic valve and operative approaches to the management of these tumors when they are discovered incidentally.
Key words: Aortic valve/pathology/surgery/ultrasonography; cardiac surgical procedures/methods; echocardiography, transesophageal; fibroma/complications/pathology/surgery/ultrasonography; heart neoplasms/diagnosis/pathology/surgery/therapy/ultrasonography
Primary cardiac tumors are rare. Papillary fibroelastomas are the 2nd most common type of cardiac tumor, surpassed only by myxomas, which arise 6 times more often.1 Papillary fibroelastomas are recognized more frequently now than in the past because of advances in imaging technology. Most of these tumors are discovered incidentally during imaging before cardiac surgery. The routine use of intraoperative transesophageal echocardiography (TEE) substantially increases the ability of the surgical team to evaluate the structural and functional anatomy of the heart before any operative procedure is undertaken.
Here, we report our treatment of an aortic valve papillary fibroelastoma that was discovered incidentally, during routine intraoperative TEE, in a patient who was scheduled for elective myocardial revascularization. We discuss the pathophysiology of fibroelastomas of the aortic valve and operative approaches for managing these tumors when they are incidentally discovered.
Case Report
In October 2007, a 66-year-old man was referred for surgical treatment of multivessel coronary artery disease. He was experiencing Canadian Cardiovascular Society class III angina pectoris, and his medical history included myocardial infarction and percutaneous coronary intervention. Among his comorbidities were chronic renal insufficiency, peripheral vascular disease, hypertension, and diabetes mellitus. A coronary angiogram revealed multivessel disease that necessitated surgical revascularization. The patient exhibited no evidence of stroke or transient ischemic attack, and physical findings upon examination were unremarkable. Preoperative transthoracic echocardiography (TTE) revealed an estimated left ventricular ejection fraction of 0.60 and no valvular abnormalities of note.
We scheduled the patient for elective coronary artery bypass grafting. After placing the patient under general anesthesia, we performed intraoperative TEE, which revealed a mobile, pedunculated, 1-cm mass on the left coronary cusp of the aortic valve (Fig. 1). As a result of this finding, we made a clinical diagnosis of fibroelastoma. We modified our operative plan to include resection of the mass on the aortic valve, along with harvesting of the autologous, reversed greater saphenous vein and the pedicled left internal mammary artery conduits. We initiated cardiopulmonary bypass with distal ascending-aortic and dual-stage venous cannulation. We reduced the patient's temperature to 32 °C (measured by use of a bladder-temperature probe) and then, after cross-clamping the aorta, we induced diastolic arrest with the use of cold-blood cardioplegic solution.
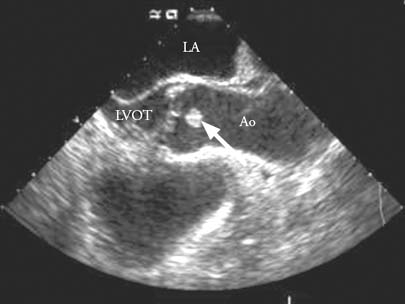
Fig. 1 Transesophageal echocardiography reveals a 1-cm mass on the aortic valve (arrow).
Ao = aorta; LA = left atrium; LVOT = left ventricular outflow tract
We performed 3 distal coronary anastomoses and then made an oblique aortotomy to expose the aortic valve. A soft, gelatinous, 1-cm mass was found adhering to the free edge of the left coronary cusp of the aortic valve (Fig. 2). We dissected the mass from the edge of the valve, ensuring that no tumor was left behind on the leaflet. Upon visual inspection, the resected tumor had negative margins, which pathologic analysis confirmed. Because these tumors are often friable and have a tendency to embolize, we thoroughly inspected and irrigated the ascending aorta and the left ventricle under direct vision in order to remove potential tumor fragments. Aortic valve competence was confirmed intraoperatively before the aortotomy was closed. The proximal aspects of the venous graft were anastomosed to the ascending aorta in an end-to-side configuration by use of the single-clamp technique. After appropriate de-airing maneuvers, we released the aortic cross-clamp. The patient's body was rewarmed, and spontaneous cardiac contractility resumed, which enabled successful weaning of the patient from cardiopulmonary bypass.
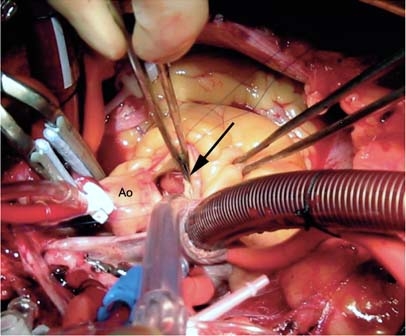
Fig. 2 Intraoperative photograph shows a mass adhering to the left coronary cusp of the aortic valve (arrow).
Ao = aorta
Postoperative TEE confirmed the functional integrity of the aortic valve. The patient required mild inotropic support during the first 8 hours after surgery. Transient atrioventricular block necessitated pacing for less than 24 hours. On postoperative day 12, nonoliguric renal failure developed secondary to acute tubular necrosis, which was treated expectantly. The patient was discharged from the hospital on postoperative day 17 in sinus rhythm and with stable creatinine levels. The final histologic findings were consistent with papillary fibroelastoma (Fig. 3). As of December 2008, the patient was doing well.
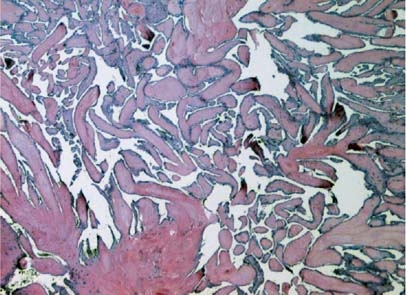
Fig. 3 Histologic photomicrograph shows a mucopolysaccharide matrix with typical filiform projections (H & E, orig. ×400).
Discussion
Papillary fibroelastomas are the most common valvular tumors of the heart, accounting for 10% of all cardiac tumors.1 More of these tumors are being reported, as a result of advances in clinical imaging. Cardiac papillary fibroelastomas have a high propensity to affect the aortic valve, the left ventricular outflow tract, and the anterior mitral leaflet. Single or multiple lesions can develop.2
Several mechanisms behind the development of papillary fibroelastoma have been proposed, but none has been proved scientifically. The most commonly accepted explanation for the origin of papillary fibroelastoma is the microthrombus theory, which posits that these lesions are acquired rather than congenital3—that they originate as small thrombi and coalesce on the coapting margins of the valves at the site of minor endothelial damage.4 The microthrombi, which can themselves dislodge and embolize, are believed to form a nidus for the subsequent progression of excrescences. These abnormal growths were first identified on the surfaces of aortic valves in 1856 by Vilem Dusan Lambl.5
Papillary fibroelastomas resemble chordae tendineae and have 2 layers—an outer, hyperplastic endothelial layer and a dense central core—that are contiguous with the underlying valve leaflet.6 The surface of a papillary fibroelastoma is covered with numerous filiform projections. An intermediate layer of loose, mucopolysaccharide-rich connective tissue is sandwiched between the outer endothelium and the central core.6 The acellular fibrous axis in the central core forms a concentric granular pattern consisting of layers of fibrin and an acid mucopolysaccharide matrix.
Even though papillary fibroelastomas are histologically benign, they can result in life-threatening complications such as stroke, acute valvular dysfunction, embolism, ventricular fibrillation, and sudden death.7 Cardiac papillary fibroelastomas appear to be firmly attached to valvular or mural endocardium, but their extreme mobility causes fragments of papillary fronds to enter the systemic circulation, which results in embolization. Most papillary fibroelastomas are located in the left heart, which increases the risk of systemic embolism.8 The vascular beds most commonly affected are the cerebral and retinal arteries; sequelae can range from transient ischemic attacks or amaurosis fugax to full stroke.8 The tumor can also occlude the coronary ostia or embolize into the coronary vessels, resulting in myocardial infarction, atypical angina, or ventricular tachycardia. In our patient, the fibroelastoma was identified only upon intraoperative TEE, after preoperative coronary artery angiography had been completed. Because these tumors can migrate and are often friable, coronary artery angiography should be performed with extreme caution, in the event that one of these tumors is present. Risks and benefits must be carefully weighed before manipulation of the coronary ostia.
Although TTE can adequately screen for papillary fibroelastomas, TEE is currently the preferred method because of its high resolution and optimal imaging capabilities. Multiplanar TEE is useful for seeing and identifying the tumor's precise point of attachment, which enables the surgeon to efficiently plan aortic valve repair, if necessary.9
Papillary fibroelastomas are typically small tumors of about 9 to 12 mm in diameter. On echocardiography, they usually appear pedunculated and mobile, with a homogeneous speckled pattern and characteristic stippling along their edges.10 This stippled pattern correlates with the papillary projections on the surface of the tumor.11
The management of papillary fibroelastoma depends upon its clinical presentation. Patients who are experiencing embolic events that are cardiovascular or neurologic in origin should undergo surgical resection. In addition, papillary fibroelastomas of the aortic valve have been associated with syncope, acute myocardial infarction, and sudden death due to impingement of the tumor on the coronary ostia, which even in asymptomatic patients increases the risks associated with surgical resection.12 Cerebrovascular embolism has been associated with tumors of diameters as small as 3 mm.13 Small (diameter, < 1 cm) and sessile tumors that show no evidence of impingement on the coronary ostia may be present in asymptomatic patients.14 These patients require periodic follow-up with serial imaging studies. Surgical excision should be offered if the tumor is mobile or pedunculated, increases in size, or begins to cause symptoms. Finally, asymptomatic patients who undergo operative cardiac procedures for other reasons should be offered surgical resection if the tumor is discovered incidentally in the operating room, provided that surgery can be readily accomplished without risking additional morbidity.
Although thrombi have been reported on the surface of fibroelastomas,15 there are no guidelines for evaluating the efficacy of anticoagulation or antiplatelet therapy in affected patients. Once the diagnosis of papillary fibroelastoma is established, prophylactic anticoagulation therapy should be initiated, to guard against thrombi until surgical resection is accomplished. The optimal surgical procedure for pedunculated tumors is valve-sparing resection. More than 80% of aorticvalve papillary fibroelastomas can be treated with only a shave excision. A 3-year follow-up study of patients who underwent shave excision did not reveal any tumor recurrences.16 More extensive or sessile tumors may require a pericardial patch and reconstruction of the valve leaflet. Aortic valve resection, replacement, or reconstruction is generally not necessary for papillary fibroelastomas unless there is underlying degeneration or extensive destruction of the native valvular apparatus.17 Partial valve-sparing resection is an option for extensive lesions, especially when valve reconstruction or replacement would substantially accentuate the morbidity that could arise. Regrowth of the tumor after partial tumor resection has not been reported, and there are no long-term data concerning whether or how often such regrowth might occur. Evidence from TEE follow-up studies suggests that the true incidence of recurrence is very low.18
Although papillary fibroelastoma is benign, it is increasingly considered a matter for surgery because of such potential complications as stroke, acute myocardial infarction, ventricular arrhythmia, and sudden death.19 Surgical resection is strongly advised if any of the following conditions is present: pedunculated lesions, tumor mobility, or a history of symptoms or complications related to the tumor. Valve-sparing shave excision of the tumor can be easily accomplished in most instances, with good long-term results. Once papillary fibroelastoma is suspected, we recommend prompt TEE, anticoagulation therapy to guard against surface thrombi, and early surgical referral.
Acknowledgments
We thank Hilary D. Marks, PhD, and Stephen N. Palmer, PhD, ELS, for their editorial assistance in preparing this manuscript.
Footnotes
Address for reprints: Danny Chu, MD, Division of Cardiothoracic Surgery, Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, 2002 Holcombe Blvd., OCL 112, Houston, TX 77030. E-mail: dchumd@gmail.com
References
- 1.Bossert T, Gummert JF, Battellini R, Richter M, Barten M, Walther T, et al. Surgical experience with 77 primary cardiac tumors. Interact Cardiovasc Thorac Surg 2005;4(4):311–5. [DOI] [PubMed]
- 2.Davoli G, Bizzarri F, Enrico T, Carone E, Muzzi L, Frati G, Chiavarelli M. Double papillary fibroelastoma of the aortic valve. Tex Heart Inst J 2004;31(4):448–9. [PMC free article] [PubMed]
- 3.Malik MF, Sagar K, Wynsen JC, Kenny D. Evolution of a papillary fibroelastoma. J Am Soc Echocardiogr 1998;11(1):92–4. [DOI] [PubMed]
- 4.Magarey FR. On the mode of formation of Lambl's excrescences and their relation to chronic thickening of the mitral valve. J Pathol Bacteriol 1949;61(2):203–8. [DOI] [PubMed]
- 5.Lambl VD. Papillare excrescenzen an der semilunar-klappe der aorta. Wien Med Wochenschr 1856;6:244–7.
- 6.Chitwood WR Jr. Cardiac neoplasms: current diagnosis, pathology, and therapy. J Card Surg 1988;3(2):119–54. [DOI] [PubMed]
- 7.Munhoz Da Fontoura Tavares C, Araujo De Oliveira N, Miguel R, Atie J. Recurrent ventricular fibrillation secondary to aortic valve tumor. Heart Rhythm 2004;1(3):348–51. [DOI] [PubMed]
- 8.Mezilis NE, Dardas PS, Tsikaderis DD, Zaraboukas T, Hantas A, Makrygiannakis K, Anastasiadis K. Papillary fibroelastoma of the cardiac valves: a rare cause of embolic stroke. Hellenic J Cardiol 2005;46(4):310–3. [PubMed]
- 9.Shelh M. Multiplane transoesophageal echocardiography detection of papillary fibroelastomas of the aortic valve causing a stroke. Eur Heart J 1997;18(4):702–3. [DOI] [PubMed]
- 10.Klarich KW, Enriquez-Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol 1997;30(3):784–90. [DOI] [PubMed]
- 11.Watanabe T, Hosoda Y, Kikuchi N, Kawai S. Papillary fibroelastoma of the tricuspid valve in association with an atrial septal defect: report of a case. Surg Today 1996;26(10):831–3. [DOI] [PubMed]
- 12.Boulmier D, Ecke JE, Verhoye JP. Recurrent myocardial infarction due to obstruction of the RCA ostium by an aortic papillary fibroelastoma. J Invasive Cardiol 2002;14(11):686–8. [PubMed]
- 13.Grinda JM, Couetil JP, Chauvaud S, D'Attellis N, Berrebi A, Fabiani JN, et al. Cardiac valve papillary fibroelastoma: surgical excision for revealed or potential embolization. J Thorac Cardiovasc Surg 1999;117(1):106–10. [DOI] [PubMed]
- 14.Menon T, Watanabe Y, Andrews D. Concurrent primary cardiac tumors. J Thorac Cardiovasc Surg 2007;134(1):263–4. [DOI] [PubMed]
- 15.Grandmougin D, Fayad G, Moukassa D, Decoene C, Abolmaali K, Bodart JC, et al. Cardiac valve papillary fibroelastomas: clinical, histological and immunohistochemical studies and a physiopathogenic hypothesis. J Heart Valve Dis 2000;9(6):832–41. [PubMed]
- 16.Westhof FB, Chryssagis K, Liangos A, Batz G, Diegeler A. Aortic valve leaflet reconstruction after excision of a papillary fibroelastoma using autologous pericardium. Thorac Cardiovasc Surg 2007;55(3):204–7. [DOI] [PubMed]
- 17.Ngaage DL, Mullany CJ, Daly RC, Dearani JA, Edwards WD, Tazelaar HD, et al. Surgical treatment of cardiac papillary fibroelastoma: a single center experience with eighty-eight patients. Ann Thorac Surg 2005;80(5):1712–8. [DOI] [PubMed]
- 18.Sun JP, Asher CR, Yang XS, Cheng GG, Scalia GM, Massed AG, et al. Clinical and echocardiographic characteristics of papillary fibroelastomas: a retrospective and prospective study in 162 patients. Circulation 2001;103(22):2687–93. [DOI] [PubMed]
- 19.Howard RA, Aldea GS, Shapira OM, Kasznica JM, Davidoff R. Papillary fibroelastoma: increasing recognition of a surgical disease. Ann Thorac Surg 1999;68(5):1881–5. [DOI] [PubMed]


