Abstract
We retrospectively investigated the impact of bicuspid aortic valve on the prognosis of patients who had definite infective endocarditis of the native aortic valve.
Of 51 patients, a bicuspid aortic valve was present in 22 (43%); the other 29 had tricuspid aortic valves. On average, the patients who had bicuspid valves were younger than those who had tricuspid valves. Patients with a tricuspid valve had larger left atrial diameters and were more likely to have severe mitral regurgitation.
Periannular complications, which we detected in 19 patients (37%), were much more common in the patients who had a bicuspid valve (64% vs 17%, P = 0.001). The presence of a bicuspid valve was the only significant independent predictor of periannular complications. The in-hospital mortality rate in the bicuspid group was lower than that in the tricuspid group; however, this figure did not reach statistical significance (9% vs 24%, P = 0.15). In multivariate analysis, left atrial diameter was the only independent predictor associated with an increased risk of death (hazard ratio, 2.19; 95% confidence interval, 1.1–4.5; P = 0.031).
In our study, patients with infective endocarditis in a bicuspid aortic valve were younger and had a higher incidence of periannular complications. Although a worse prognosis has been reported previously, we found that infective endocarditis in a native bicuspid aortic valve is not likely to increase the risk of death in comparison with infective endocarditis in native tricuspid aortic valves.
Key words: Aortic valve/abnormalities/pathology/ultrasonography; aortic valve insufficiency/classification/diagnosis; echocardiography/methods; endocarditis, bacterial/complications/mortality/pathology/ultrasonography; heart defects/congenital; heart diseases/complications; heart valve diseases/classification/complications/congenital/pathology; hospital mortality; retrospective studies; risk factors
Bicuspid aortic valve is the most common congenital cardiac malformation, affecting 1% to 2% of the population.1 The incidence of infective endocarditis (IE) in the bicuspid aortic valve population ranges from 10% to 30%. Twenty-five percent of IE cases occur in a bicuspid aortic valve.2,3
Few data exist about IE in persons who have a bicuspid aortic valve, in contrast with the large amount of data involving other IE populations. Risk stratification is an important objective in the evaluation of patients who have IE, especially IE of the aortic valve. In patients with IE of the native valve, acute heart failure occurs more frequently in association with aortic valve infection (29%) than mitral valve infection (20%).4 In view of these factors, we sought to evaluate the impact of bicuspid aortic valve on complications and death related to IE that occurs in a native aortic valve.
Patients and Methods
Selection of Patients for the Study
We reviewed the medical records of 450 consecutive patients who were suspected to have active IE and who were admitted to a tertiary hospital from January 2002 through May 2006. Of those 450 patients, 51 fulfilled the modified Duke's criteria for definite IE of the native aortic valve.5 Demographic, clinical, microbiologic, pathologic, and echocardiographic data were collected from the medical records of those 51 patients. The study was approved by our local ethics committee.
Characteristics of the Patients
Fifty-one patients had definite IE of the native aortic valve. A bicuspid aortic valve was present in 22 patients (43%; the “bicuspid group”), and the aortic valves were tricuspid in the other 29 patients (57%; the “tricuspid group”). The underlying conditions related to IE were bicuspid aortic valve in 22 patients (43%), rheumatic heart disease in 12 (24%), degenerative heart disease in 6 (12%), prosthetic mitral valve in 2 (4%), and congenital heart disease in 2 (4%). Seven individuals (13%) had no cardiac abnormality, and all 7 had tricuspid aortic valves.
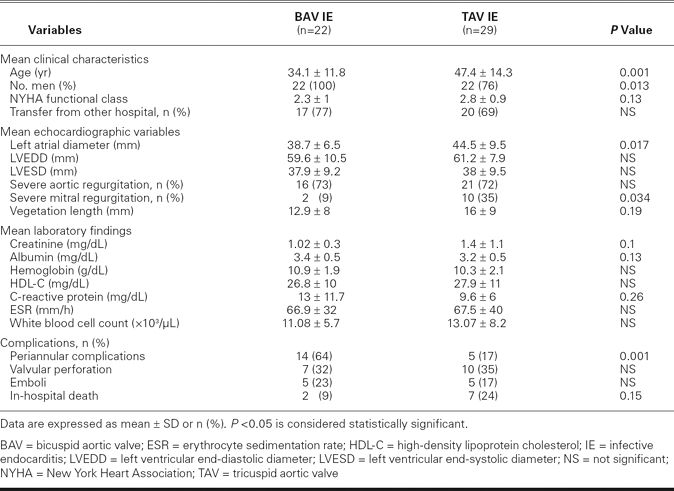
No patient had a history of intravenous drug abuse or previous endocarditis. Forty-four patients (86%) were men, and the median age of the cohort was 39 years (range, 9–75 yr). Table I shows the characteristics of the 51 patients who were included in the study.
TABLE I. Characteristics of the 51 Patients with Infective Endocarditis of the Native Aortic Valve, Grouped According to Valve Morphology
Echocardiographic Methods
Echocardiographic examinations in all 51 patients had been performed with the use of a VingMed® System FiVe cardiac ultrasonographic scanner (GE VingMed® Ultrasound; Horten, Norway). The scanner was equipped with a 2.5-MHz transthoracic probe that had 2nd-harmonic-imaging capability and with a rotary transesophageal 5-MHz multiplanar probe. All of the patients had undergone transthoracic echocardiography; 72% had also undergone transesophageal echocardiography (TEE). The TEE had been performed (optimally in fasting patients) with the use of topical pharyngeal anesthesia and conscious sedation.
Definitions of Terms
Heart failure was defined in accordance with New York Heart Association functional class. Early surgery was defined as surgical intervention before a patient's completion of a full course of antibiotic therapy. A vegetation was defined as a mobile, irregularly shaped, echogenic mass that was attached to a valve or to the myocardial surface. Abscesses were defined as abnormal echodense or echolucent areas within the valvular annulus or perivalvular tissue, confirmed by imaging in more than 1 echocardiographic plane, in the presence of valvular infection.6 When flow was detected in such a cavity, the lesion was identified as a pseudoaneurysm.7 Valvular regurgitations were evaluated semiquantitatively.8,9 On 2-dimensional images, valvular perforation was defined as an interruption of leaflet tissue continuity at a site remote from the commissures.10 On color-flow Doppler images, valvular perforation was defined as a high-velocity, eccentric, turbulent jet that traversed valve-leaflet tissue during systole (mitral valve) or diastole (aortic valve).11 A fistula was defined as abnormal communication between the aorta and the cardiac chambers with turbulent systolic and diastolic flow, as determined by continuous or color-Doppler mapping.12
Statistical Analysis
Data were analyzed with the use of SPSS for Windows, version 11.5 (SPSS Inc.; Chicago, Ill). Continuous variables were expressed as mean ± SD. Comparisons were made using the Student t test, the Mann-Whitney U test, the Fisher exact test, or the χ2 test, as appropriate. Univariate and multivariate Cox regression analyses were used to determine the independent predictors of death and periannular complications. For all analyses, P < 0.05 was considered statistically significant.
Results
Microbiologic Characteristics
Bacteria had been isolated from blood cultures in 40 of the 51 patients (78%). The causative organisms identified were Staphylococcus aureus (22%), Streptococcus viridans (20%), other streptococci (10%), enterococci (8%), other staphylococci (6%), gram-negative rods (6%), and other microorganisms (6%). Eleven patients (22%) had negative blood cultures. There were no statistically significant differences in S. aureus infection between the bicuspid group and the tricuspid group.
Clinical and Echocardiographic Findings
The patients in our bicuspid group were younger than those in our tricuspid group (mean age, 34.1 ± 11.8 vs 47.4 ± 14.3, P = 0.001). All 22 in the bicuspid group were men. Of those 22, 2 had aortic stenosis alone, 14 had aortic regurgitation alone, and 5 had a combination of aortic stenosis and regurgitation. One patient had no significant valvular stenosis or regurgitation. No significant differences were detected in severe aortic regurgitation between the bicuspid and tricuspid groups (73% vs 72%, P = 0.62). Patients in the tricuspid group had larger left atrial diameters and were more likely to have severe mitral regurgitation (Table I).
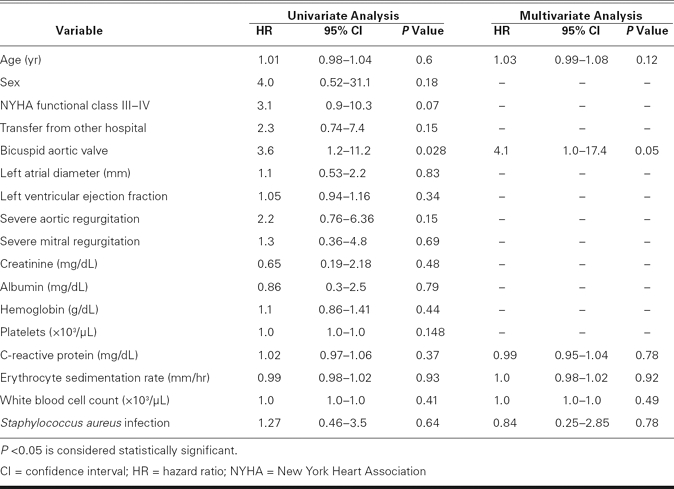
Of the 51 patients, 19 (37%) had periannular complications in the aortic ring, including 11 persons with nonruptured abscess alone (58%), 4 with pseudoaneurysm alone (21%), 2 with abscess and pseudoaneurysm (11%), 1 with abscess and aortocavitary fistula (5%), and 1 with aortocavitary fistula alone (5%). Patients in the bicuspid group were more likely to have periannular complications than were patients in the tricuspid group (64% vs 17%, P = 0.001). Multivariate Cox regression analysis identified the presence of a bicuspid aortic valve as the only significant independent predictor of periannular complications (hazard ratio, 4.1; 95% confidence interval, 1–17.4; P = 0.05) (Table II).
TABLE II. Univariate and Multivariate Relations for Prediction of Periannular Complications
Laboratory Findings
There were no statistically significant differences in laboratory values (serum creatinine, albumin, and C-reactive protein levels, white blood cell count, hemoglobin, and erythrocyte sedimentation rate) between the bicuspid and tricuspid groups.
Complications and Death
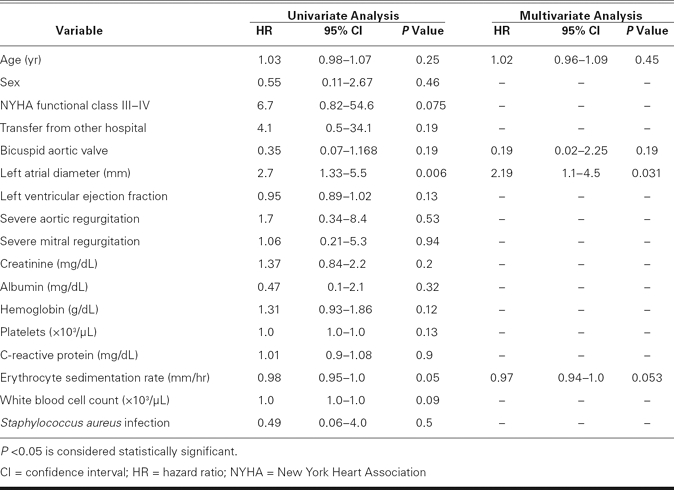
During the in-hospital period, 37 of 51 patients (73%) underwent early surgery, all within 28 days after admission. The median time was 7 days, and the mean interval between clinical presentation of IE and surgery was 10 ± 8 days. Early-surgery rates did not differ significantly between the bicuspid and tricuspid groups (77% vs 69%, P = 0.37). Nine patients died during the in-hospital period (mortality rate, 18%): 5 from severe heart failure, 2 from septic shock, 1 from cerebral hemorrhage, and 1 from acute renal failure. Although the in-hospital mortality rate in the bicuspid group was lower than that in the tricuspid group (9% vs 24%), it did not reach statistical significance (P = 0.15). In multivariate analysis, left atrial diameter was the only independent predictor that was associated with an increased risk of death (hazard ratio, 2.19; 95% confidence interval, 1.1–4.5; P = 0.031) (Table III).
TABLE III. Univariate and Multivariate Relations for Prediction of Death
Our analysis showed that patients with IE in a bicuspid aortic valve were younger, were more likely to have periannular complications, and had a lower incidence of in-hospital death than did those with IE in a tricuspid aortic valve.
Discussion
Bicuspid aortic valve is associated with significant morbidity and death, especially after the 4th decade of life. The progression of stenosis and regurgitation due to calcific changes and the sequelae of IE often requires intervention.2,13
As a substrate for IE, bicuspid aortic valve is predominantly a complication in children and young adults: in persons with a bicuspid aortic valve who are younger than 30 years of age, 55% of the deaths are attributable to IE.2 In patients who have a bicuspid aortic valve, IE causes 43% to 60% of cases of severe aortic regurgitation—from cusp perforation, in most instances.14–16
The histologic abnormality that underlies aortic-root complications in bicuspid-valve IE is cystic medial necrosis, which is characterized by a noninflammatory loss of smooth muscle cells, fragmentation of elastic fibers, and mucoid degeneration. In 1 study,17 45% of the patients with a bicuspid aortic valve had severe cystic medial necrosis, in comparison with only 9% of the patients who had a tricuspid valve.
Few clinical trial data exist regarding IE in patients who have a bicuspid aortic valve. Only 1 study, by Lamas and Eykyn,18 has concentrated chiefly on such patients, wherein the investigators compared bicuspid-valve IE with other native-valve IE (including mitral, tricuspid, and pulmonary). Their retrospective study included 50 patients who presented at a single institution from 1970 through 1998 with IE of a bicuspid aortic valve. The investigators found a very high rate of complications in those patients within 6 months of hospital admission, including heart failure (72%) and periannular abscess formation (30%), in comparison with other native-valve endocarditis (not only of the aortic valve). Our study focused distinctly on IE of the native aortic valve, in an attempt to determine the impact of a bicuspid aortic valve on complications and death. Our study supports the observation that patients with IE of a bicuspid aortic valve are more likely to experience periannular complications. Furthermore, we found the presence of a bicuspid aortic valve to be the only significant independent predictor of periannular complications.
The mortality rate in our bicuspid group was lower than that in Lamas and Eykyn's patients (9% vs 14%). The mortality rate in our bicuspid group was also lower than the given overall mortality rate of IE (9% vs 16%–25%).3,19–21 As a result of advances in diagnosis, improved antimicrobial treatment, earlier detection of complications via the frequent use of TEE, and the management of complications with early surgery, the short-term prognosis for patients with IE in a bicuspid aortic valve may be improved over that of past years.
Multivariate analysis revealed left atrial diameter as the only independent predictor that was associated with an increased risk of death in our patients. Although the causal pathway between death and increased left atrial diameter cannot be determined by our current analysis, we offer some possible explanations. Increased left atrial size is the result of elevated left atrial pressure and the cumulative effect of filling pressures over time,22 which are conditions associated with an increased risk of cardiovascular events, including death.23 During ventricular diastole, the left atrium is exposed to the pressures of the left ventricle. The elevation of filling pressure is uniformly found in the presence of symptomatic congestive heart failure. Increased left atrial diameter may indicate severe congestive heart failure in patients who have IE.
Limitations of the Study
This study was limited by its retrospective nature. All study subjects were treated in a single tertiary center, which raises the possibility of referral bias.
Conclusions
Our study shows that, despite a higher rate of periannular complications, the short-term mortality rate in patients with IE in bicuspid native aortic valves is lower than that in patients with IE in native tricuspid aortic valves. Early diagnosis of periannular complications, along with early surgery, may have produced these improved outcomes.
Footnotes
Address for reprints: Gokhan Kahveci, MD, Islampasa Mah., Sehitler Cad., No. 74, 53100 Rize, Turkey. E-mail: drmarist@yahoo.co.uk
References
- 1.Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002;106(8):900–4. [DOI] [PubMed]
- 2.Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000;83(1):81–5. [DOI] [PMC free article] [PubMed]
- 3.Kahveci G, Bayrak F, Mutlu B, Bitigen A, Karaahmet T, Sonmez K, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide in patients with active infective endocarditis. Am J Cardiol 2007;99(10):1429–33. [DOI] [PubMed]
- 4.Sexton DJ, Spelman D. Current best practices and guidelines. Assessment and management of complications in infective endocarditis. Cardiol Clin 2003;21(2):273–82, vii-viii. [DOI] [PubMed]
- 5.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30(4):633–8. [DOI] [PubMed]
- 6.Daniel WG, Mugge A, Martin RP, Lindert O, Hausmann D, Nonnast-Daniel B, et al. Improvement in the diagnosis of abscesses associated with endocarditis by transesophageal echocardiography. N Engl J Med 1991;324(12):795–800. [DOI] [PubMed]
- 7.Vilacosta I, Graupner C, San Roman JA, Sarria C, Ronderos R, Fernandez C, et al. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J Am Coll Cardiol 2002;39(9):1489–95. [DOI] [PubMed]
- 8.Helmcke F, Nanda NC, Hsiung MC, Soto B, Adey CK, Goyal RG, Gatewood RP Jr. Color Doppler assessment of mitral regurgitation with orthogonal planes. Circulation 1987; 75(1):175–83. [DOI] [PubMed]
- 9.Perry GJ, Helmcke F, Nanda NC, Byard C, Soto B. Evaluation of aortic insufficiency by Doppler color flow mapping. J Am Coll Cardiol 1987;9(4):952–9. [DOI] [PubMed]
- 10.Cziner DG, Rosenzweig BP, Katz ES, Keller AM, Daniel WG, Kronzon I. Transesophageal versus transthoracic echocardiography for diagnosing mitral valve perforation. Am J Cardiol 1992;69(17):1495–7. [DOI] [PubMed]
- 11.Von Reyn CF, Levy BS, Arbeit RD, Friedland G, Crumpacker CS. Infective endocarditis: an analysis based on strict case definitions. Ann Intern Med 1981;94(4 pt 1): 505–18. [DOI] [PubMed]
- 12.Anguera I, Miro JM, Vilacosta I, Almirante B, Anguita M, Munoz P, et al. Aorto-cavitary fistulous tract formation in infective endocarditis: clinical and echocardiographic features of 76 cases and risk factors for mortality. Eur Heart J 2005;26 (3):288–97. [DOI] [PubMed]
- 13.Basso C, Boschello M, Perrone C, Mecenero A, Cera A, Bicego D, et al. An echocardiographic survey of primary school children for bicuspid aortic valve. Am J Cardiol 2004;93(5): 661–3. [DOI] [PubMed]
- 14.Olson LJ, Subramanian R, Edwards WD. Surgical pathology of pure aortic insufficiency: a study of 225 cases. Mayo Clin Proc 1984;59(12):835–41. [DOI] [PubMed]
- 15.Murphy ES, Rosch J, Rahimtoola SH. Frequency and significance of coronary arterial dominance in isolated aortic stenosis. Am J Cardiol 1977;39(4):505–9. [DOI] [PubMed]
- 16.Roberts WC, Morrow AG, McIntosh CL, Jones M, Epstein SE. Congenitally bicuspid aortic valve causing severe, pure aortic regurgitation without superimposed infective endocarditis. Analysis of 13 patients requiring aortic valve replacement. Am J Cardiol 1981;47(2):206–9. [DOI] [PubMed]
- 17.de Sa M, Moshkovitz Y, Butany J, David TE. Histologic abnormalities of the ascending aorta and pulmonary trunk in patients with bicuspid aortic valve disease: clinical relevance to the Ross procedure. J Thorac Cardiovasc Surg 1999;118(4): 588–94. [DOI] [PubMed]
- 18.Lamas CC, Eykyn SJ. Bicuspid aortic valve–A silent danger: analysis of 50 cases of infective endocarditis. Clin Infect Dis 2000;30(2):336–41. [DOI] [PubMed]
- 19.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med 2001;345(18):1318–30. [DOI] [PubMed]
- 20.Hoen B, Alla F, Selton-Suty C, Beguinot I, Bouvet A, Briancon S, et al. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA 2002;288(1):75–81. [DOI] [PubMed]
- 21.Netzer RO, Zollinger E, Seiler C, Cerny A. Infective endocarditis: clinical spectrum, presentation and outcome. An analysis of 212 cases 1980–1995. Heart 2000;84(1):25–30. [DOI] [PMC free article] [PubMed]
- 22.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol 2005; 45(1):87–92. [DOI] [PubMed]
- 23.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006;47 (12):2357–63. [DOI] [PubMed]


