Abstract
Odd- and even-order distortion products (DPs), evoked by two primary tones (f1,f2,f1<f2), represent different aspects of cochlear nonlinearity. The cubic and quadratic difference tones (CDT 2f1−f2 and QDT f2−f1) are prominent representatives of the odd and even DPs. Distortion product otoacoustic emissions (DPOAEs) were measured within a primary level (L1,L2) space over a wide range of f2∕f1 ratios to compare the optimal signal conditions for these DPs. For CDT, the primary level difference decreased as L1 increased with a rate proportional to the f2∕f1 ratio. Moreover, the optimal ratio increased with L1. A set of two formulas is proposed to describe the optimal signal conditions. However, for a given level of a primary, increasing the other tone level could maximize the QDT amplitude. The frequency ratio at the maximal QDT was about 1.3 and quite constant across different primary levels. A notch was found in the QDT amplitude at the f2∕f1 ratio of about 1.22–1.25. These opposite behaviors suggest that the optimal recording conditions are different for CDT and QDT due to the different aspects in the cochlear nonlinearity. Optimizing the DPOAE recordings could improve the reliability in clinical or research practices.
INTRODUCTION
Our inner ears are nonlinear acoustic transducers where distortions are normal by-products of the conversion of sound into bioelectrical signals. The source of distortion is thought to be within the transduction process of outer hair cells (OHCs), since these cells respond to changes in membrane voltage with mechanical motion in either the cell body (Liberman et al., 2002) or the hair bundles (Kennedy et al., 2005). The OHC transduction shows a saturating nonlinearity and the resulting motile responses provide a mechanical feedback that can enhance hearing sensitivity and frequency selectivity. When stimulated by two tones (f1,f2,f2>f1), the inner ear can produce a family of distortion products (DPs) in various combinations of the primary frequencies, mf1±nf2. Depending on the sum of integers m and n, the DPs can be classified into two categories, odd- and even-order DPs. They reflect different aspects of the nonlinear transducer characteristics of OHCs in the overlapped region of traveling waves initiated by two pure tones. Odd-order DPs are relatively large at low primary levels, and representing the transducer gain of hair cells. In contrast, even-order DPs are only measurable at moderately high stimulus levels, and thus may reflect the saturation or compression of hair cell transducers. These DPs in mechanical vibrations of hair cells can propagate out of the inner ear and are measurable in the ear canal as faint sounds or distortion product otoacoustic emissions (DPOAEs). Therefore, DPOAEs provide a noninvasive tool to assess the functional status of the inner ear OHCs.
Given the possibility that DPOAEs can be used to evaluate the transducer characteristics of OHCs, the current clinical application is, however, limited to hearing screening (Lonsbury-Martin and Martin, 2003) where normal or abnormal hearing is judged by the presence or absence of emissions. Quantitative utilities, such as hearing threshold estimates, cochlear efferent function evaluation, and input∕output growth functions, are restricted to mostly research settings. One reason for the limited use of DPOAEs is the large variability in measurements. Among other factors (Garner, 2008), such as hearing sensitivity, middle ear transmission, and inner ear reflections, a source of the variability can be attributed to less optimized signal conditions of the primary tones (Johnson et al., 2006; Mills et al., 2007) that cannot evoke the largest DPOAEs. Since DP levels are correlated with the nonlinear characteristics of cochlear transduction (Lukashkin and Russell, 1999; Fahey et al., 2000; Bian et al., 2002; Bian, 2004), maximizing DPOAE magnitudes can improve the signal-to-noise ratio (SNR) so that more accurate estimates of cochlear function are possible. Therefore, there is a need for standardizing (Mills et al., 2007) or individualizing (Neely et al., 2005) the measurement procedures. Optimizing the recording conditions for DPOAEs is still an ongoing research effort.
The signal conditions for recording DPOAEs include the f2∕f1 frequency ratio and the levels of two primary tones (L1 and L2). The f2 is usually predetermined for evaluating the cochlear function at a specific frequency of interest, because the overlapped region of the two-tone excitation patterns on cochlear partition is very close to the f2 place. The frequency f1 can be determined by selecting an appropriate f2∕f1 ratio. In current clinical practice, a value of 1.22 is commonly adopted for f2∕f1 ratio regardless of frequency and level. However, the optimal ratio can range from 1.2 to 1.3 with increase in primary levels and f2 (Moulin, 2000; Johnson et al., 2006). Thus, for a certain f2, selecting the frequency ratio is tied with the choice of primary levels. Currently, the decision on signal levels is an independent process and often a simple strategy of L1 being 5–10 dB greater than L2 is used. However, studies on DPOAE amplitude within a complete L1×L2 parameter space indicate that the primary level difference (ΔL=L1−L2) is also level dependent with greater deviations towards lower levels (e.g., Whitehead et al., 1995). The optimal L1 as a linear deviation from L2 is refined with an empirical formula (Kummer et al., 2000) given the observation that ΔL diminishes at 65 dB SPL. This primary level optimization allows a more accurate measurement of DPOAE amplitudes at low stimulus levels for estimating hearing sensitivity (Kummer et al., 1998). Another relation between the optimal L1 and L2 is proposed by Johnson et al. (2006) which takes f2 into account. However, none of the approaches considers the inter-relation between the primary levels and the f2∕f1 ratio. The interaction between frequency ratio and primary levels on DPOAEs can be deduced from the fact that all these factors influence the amount of overlap between two excitation patterns in the cochlea and in turn determine the exact two-tone input to the nonlinear transduction of OHCs.
To date, all parametric studies on optimizing DPOAE recordings focus on the most prominent DP component, cubic difference tone (CDT, 2f1−f2). An obvious reason for CDT as the choice of DPOAE measure for research and application is simply its large size and SNR. Another reason is that at low signal levels CDT reflects the response growth or gain of OHC transducer and is parallel to hearing sensitivity and frequency selectivity of the inner ear (Brown et al., 1993; Gorga et al., 2003). However, CDT only represents the odd nonlinearity in cochlear transduction; thus, the estimation of hair cell function could not be complete without measuring the even-order DPs. The largest even-order DP is quadratic difference tone (QDT, f2−f1) which has not been considered clinically useful until recently. One reason for the lack of research and application is due to its low amplitude (Brown, 1993) and poor SNR. However, auditory nerve fiber responses in kittens show robust QDT that can be larger than CDT (Tubach et al., 1996). Neural QDT varies nonmonotonically with primary levels or frequencies and its behavior is different from CDT (Kim, 1980). Large QDT also presents in electrical responses in inner hair cells (Nuttall and Dolan, 1993), indicating that the source of QDT is presynaptic and possibly the OHCs. Indeed, QDT is the largest DP component found in the trans-membrane current that drives OHC somatic motility (Takahashi and Santos-Sacchi, 1999). Acoustic QDT can be associated with the saturating portions of OHC transduction, and its amplitude variation reflects the operating point (OP) shift of hair cells (Frank and Kössl, 1996). Studies in gerbils show that the QDT is enhanced or modulated by a low-frequency bias tone and the modulation patterns could be used to derive a cochlear transducer function (FTr) (Bian, 2004, 2006). This finding poses a possible clinical application in evaluating cochlear function or diagnosing inner ear disorders. Since the cochlear FTr can be quantified from low-frequency modulation of CDT in humans (Bian and Scherrer, 2007), combining this measure with QDT could provide a more comprehensive picture of cochlear function. Towards this goal, it is necessary to explore the optimal signal conditions for recording QDT, because the ideal conditions for CDT may not be applicable to QDT, especially, in humans. Therefore, the purpose of the present study is to compare the optimal conditions for CDT and QDT to provide guidelines for research and clinical practice.
METHODS
Subjects
Sixteen healthy subjects with age ranging from 23 to 40 years (mean 26.3) were recruited from the students at Arizona State University (ASU). They had no history of hearing disorders. Normal outer and middle ear functions were confirmed with an otoscopic examination. A DP-gram was measured with an 8-point∕oct procedure from 1 to 8 kHz (ILO92, Otodynamics Ltd., Herts, UK). Subjects with CDT amplitudes greater than 10 dB SPL in the frequency range between 1 and 4 kHz were selected for the study. Subjects with deep notches or spectral fine structures in this region were excluded to reduce interference of DPOAEs generated from reflections at the best frequency place (Knight and Kemp, 2001; Shera, 2004). To avoid possible influence and interference, the subjects were also screened to rule out spontaneous otoacoustic emissions in the testing ear. Subjects were seated comfortably in a sound-proofed booth and instructed to be as quiet as possible during the test to reduce noises. The recruiting protocol and experimental procedures were approved by the Institutional Review Board of ASU.
Stimulus presentation
Two primary tones were generated and controlled with a personal computer using software implemented in LABVIEW (National Instruments, NI, Austin, TX). Durations of the primary tones were 1 s with a 5-ms cos2-shaped rise and fall ramp, respectively. The signals were delivered separately to two earphones (ER-2A, Etymōtic Research, Inc., Elk Grove Village, IL) through a 24-bit dynamic signal acquisition and generation card (PXI-4461, NI) and acoustically mixed in the ear canal to stimulate the inner ear. The ear-canal acoustic response was then recorded by a calibrated probe microphone (ER-10B+). The recorded signal was amplified 20 dB by the built-in preamplifier of the ER-10B+ and digitized at a rate of 131.072 kS∕s (PXI-4461). Prior to each test, the fit of ER-10B+ probe was checked and adjusted to ensure a flat ear-canal frequency response up to 5 kHz to a frequency-sweeping tone at a constant level. This can avoid the interference from standing waves in the ear canal and acoustic leaks around the probe.
Data were collected over a range of f2∕f1 ratios using a “chess-grid” protocol, namely, the L1 and L2 were swept independently from 75 to 54 dB SPL in 3-dB steps to create an 8×8 grid of data sets for each f2∕f1 ratio. Lower signal levels were not used due to a concern of poor SNR for QDT. The primary levels were automatically adjusted and calibrated at the beginning of each decrement. To ensure the completion of experiments within 90 min, the frequency of f2 was fixed at 4 kHz in the study. Based on the coverage of f2∕f1 ratio, the study was carried out in two phases. In phase 1, the f2∕f1 ratio was varied from 1.2 to 1.8 at a step size of 0.1 to explore the behaviors of CDT and QDT when f1 and f2 were separated further apart. It was initially thought that there could be a mechanical “(second) filter” just below the frequency of f2 in the cochlea (Allen and Fahey, 1993; Brown et al., 1993), so that the QDT could be “amplified” if the f2∕f1 ratio was large enough to bring the QDT frequency into the filter bandwidth. In phase 2, conventional frequency ratios from 1.15 to 1.36 with a step size of 0.035 were used to reveal the optimal signal conditions for both DPOAE components. Ratios lower than 1.1 were not used to keep the QDT above 500 Hz, since the noise floor below this frequency was higher (>−10 dB SPL). Eight subjects participated in each phase.
Data collection and analysis
At each two-tone condition, the stimuli were repeated eight times and the recorded acoustic signal was averaged in the time domain to reduce random noise. Noisy data due to body movements were discarded with an artifact rejection routine which examined the noise floor from 300 to 800 Hz. The rejection threshold was adjustable depending on the noise condition of each subject to optimize the speed of data collection. Data were analyzed offline in MATLAB (Mathworks Inc., Natick, MA) using custom-written programs. The waveform of ear-canal acoustic signal was passed through a Hanning window and converted into the frequency domain via a fast Fourier transform (FFT). Then, the spectral magnitudes at the frequencies of 2f1−f2 and f2−f1 were extracted to result in the value of a single grid in the L1×L2 level space for CDT and QDT, respectively.
To examine the combined effects of primary levels and frequency ratio, the data were merged into an 8×8 grid of CDT or QDT amplitude-ratio functions with a specific L1 and L2 combination for each grid. Thus, the DPOAE amplitude could be examined in each dimension by fixing L1, L2, or f2∕f1 ratio, respectively. For the optimal signal conditions, relations between the two primary levels under different frequency ratios or the optimal ratios as functions of the primary levels were inspected. As the influence of L1 on DPOAE amplitudes was stronger than L2, the relations among these signal variables were expressed as functions of L1. To reveal possible underlying rules, these relations were fit with appropriate linear or nonlinear functions. The curve fittings were usually performed on the averaged data and the parameters were validated by predicting the optimal conditions for each individual.
RESULTS
In this study, three signal variables, L1, L2, and f2∕f1 ratio, were varied simultaneously, creating an extra complexity in observing the behaviors of DPOAEs. It would be convenient to reduce the data into a two- or three-dimensional space by keeping one variable constant. For the optimal signal conditions, the data analysis focused on the relations between these variables. Data reported were mostly from phase 2, where common f2∕f1 ratios from 1.15 to 1.36 were used. Phase 1 data were reported in the section regarding the ratio effects.
Level effects
L2 fixed
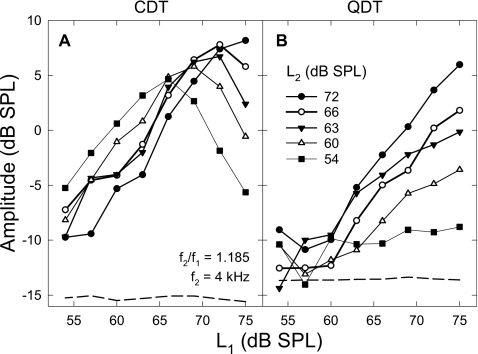
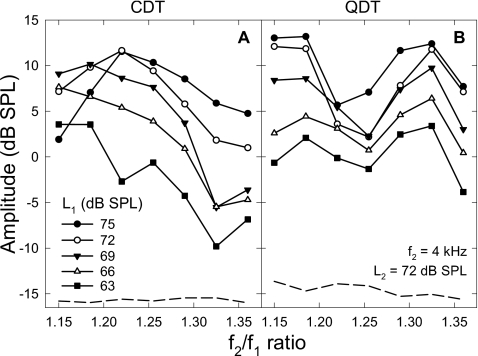
For different f2∕f1 ratios, the DPOAE magnitudes behaved similarly, but with different overall sizes. It would be reasonable to select a ratio that produced larger DPOAEs to show effects of L1 when L2 was fixed at different values. At the f2∕f1 ratio of 1.185, amplitudes of CDT and QDT averaged across eight ears showed different growth patterns (Fig. 1) with L1. The CDT amplitude showed an initial increase and a rollover as L1 was raised over L2 [panel (A)]. The level of f1 where the CDT reached the peak depended on the fixed level of f2, usually when L1 was 3–10 dB greater than L2. As the fixed L2 decreased, the peaks of CDT amplitudes showed a small shift towards lower values of L1, indicating a larger level difference. In other words, for lower levels of f2 a much larger L1 was needed to produce a peak CDT magnitude. Moreover, the peak CDT values decreased slightly (<5–10 dB) when L2 was lowered for about 20 dB. Therefore, these CDT amplitude functions were largely overlapped when L1 was below 65 dB SPL. The growth rates of CDT amplitudes were about 1 dB∕dB, suggesting that the CDT increased proportionally with L1 until the peak values were reached.
Figure 1.
DPOAE amplitudes as functions of L1 under different L2 settings. (A) CDT magnitudes rise with L1 and roll over at different L1 values. (B) QDT amplitudes increase with L1 in different growth rates depending on the L2 settings. Dashed lines indicate the averaged noise floor of DPOAE measures. Data reflect an average across eight subjects.
In contrast, the growths of QDT amplitudes showed no obvious signs of peaks and rollovers [Fig. 1B]. On average, the amplitudes of QDT were smaller than the CDT, especially, when L1 was less than 60 dB SPL. At these lower f1 levels, the QDT was usually within 5 dB from the noise floor (around −12–−13 dB SPL). Then, the QDT amplitude started to grow when L1 exceeded 60 dB SPL. The rate of growth and the maximal QDT level seemed to be proportional to the fixed level of f2. The slope of QDT growth function could reach about 1 dB∕dB when L2 was set at 72 dB SPL and progressively decrease with L2. QDT magnitudes usually reached their highest values when L1 was maximal (75 dB SPL). The largest QDT level could be greater than 6 dB SPL when both L1 and L2 were at the highest level. There was also a slight compression of the QDT growth functions when L1 was above 70 dB SPL.
L1 fixed
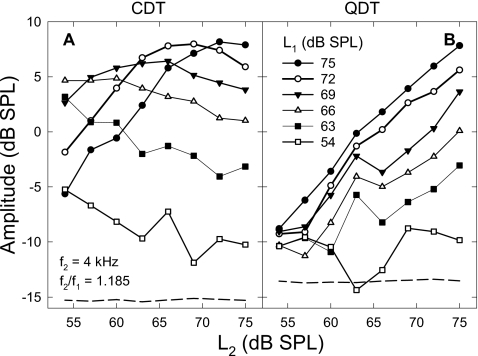
When L1 was fixed at different levels, the averaged CDT magnitude showed a range of different patterns as L2 increased [Fig. 2A]. The patterns and overall value of CDT amplitudes were predominated by the fixed levels of f1. As L1 was gradually set to lower values, the CDT amplitudes showed three types of patterns with increasing L2: (1) a compressive growth; (2) a slower initial growth with a rollover; and (3) a gradual decrease. The overall CDT magnitudes also decreased with reductions in L1. The progression of these CDT patterns reflected a dramatic shift of the CDT peak towards lower L2 along with a significant reduction in overall sizes. This indicated that CDT amplitudes could reach a higher value when L1 was set higher and the CDT growth only occurred when L2 was lower than L1. Consistent with the growth curves in Fig. 1A, the CDT peaked if L2 reached 3–10 dB below the fixed L1. When L2 was greater than these levels or L1, only a gradual decrease of CDT magnitude could be observed [Fig. 2A].
Figure 2.
DPOAE amplitudes as functions of L2 under different L1 settings. (A) CDT magnitudes show different patterns depending on the L1 settings. The CDT peak decreases with L1 and shifts to lower L2 values as L1 reduces. At lower L1 settings, only a decrease in CDT level can be observed. (B) QDT amplitudes increase with L2 in different growth rates depending on the L1 settings. Note: a notch can be observed at moderate to low levels of the f1. Dashed lines indicate the averaged noise floor of DPOAE measures. Data reflect an average across eight subjects.
In comparison, the QDT amplitude showed a less variable growth pattern with the increase of L2. When L1 was fixed above 70 dB SPL, the QDT amplitude grew quite linearly with L2. As L1 was lowered, the growth functions of QDT became nonmonotonic with the presence of a notch around the f2 levels of 63 to 67 dB SPL. There seemed to be a slight shift of the notch towards lower values of L2. The QDT magnitude grew at a 1 dB∕dB rate before the notch (lower L2), which was consistent with the linear growths at high f1 levels. If L1 was very low (<55 dB SPL), the QDT level was flattened within about 5 dB from the noise floor (−13 dB SPL). Thus, QDT magnitudes were influenced by both L1 and L2.
Both L1 and L2 varied
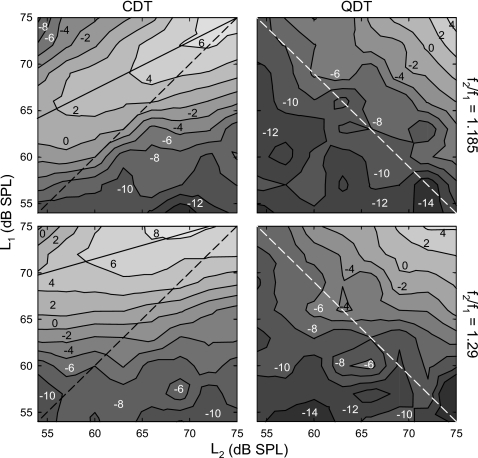
The combined effects of L1 and L2 on the averaged CDT and QDT amplitudes were evaluated with contour plots in the L1×L2 level spaces (Fig. 3). From the DPOAE contours, the optimal signal levels that produced the highest DPOAEs could be evident. The CDT amplitude contour (left panels) showed a confined ridge spanning from the upper right corner towards the moderate values of L1 and the minimal L2. The tips of contour lines on the ridge, where both L1 and L2 were minimal to evoke a certain level of CDT, reflected the optimal primary levels for CDT. The relation between the optimal L1 and L2 (solid line) indicated that L1 was preferred to be greater than L2. This ΔL became progressively larger at lower primary levels with reference to the dashed line depicting L1=L2. At the L2 of about 75 dB SPL, the optimal L1 was roughly the same as L2, i.e., no level difference. Comparing the contours from two different f2∕f1 ratios (1.185 and 1.29), the optimal ΔL became larger for the higher frequency ratio (lower left panel). In addition, the CDT magnitude was largely dominated by L1 and the influence of L2 was weak at higher ratios as indicated by the nearly horizontal contour lines.
Figure 3.
Contour plots of DPOAE amplitudes in L1×L2 spaces of two f2∕f1 ratios. Left: CDT magnitude shows a ridge spanning from the upper right corner (75,75) to moderate L1 and low L2. Solid lines indicate the optimal primary levels that yield the maximal CDT magnitude. Diagonal dashed lines indicate the condition L1=L2. Distances between the two lines represent the optimal level difference (ΔL=L1−L2). Note: ΔL is greater for higher f2∕f1 ratio (lower panel). Right: QDT magnitude contour lines are parallel to the diagonal dashed line representing L1+L2=129 dB SPL. For each fixed L1 or L2, maximizing the other primary level would optimize the QDT amplitude. Note: the slope of the contour for the larger ratio (lower panel) is shallower than the smaller ratio (top). Data reflect an average across eight subjects at an f2 of 4 kHz.
Although the maximal QDT magnitudes were also located at the upper-right corners of the QDT contour maps (Fig. 3, right panels), paths of the isoamplitude lines were quite different from the CDT. These parallel contour lines stepped down towards lower L1 and L2 with no obvious ridges formed. The direction of the contour lines followed the diagonal line from the highest L1 to the highest L2 (dashed line), meaning that a relatively large QDT could be obtained by keeping one of the primary levels high. Thus, the QDT amplitude seemed to be proportional to L1+L2, i.e., higher QDT magnitudes correlated with larger sums of the primary levels. If one of the primary levels was fixed, raising the other could increase the QDT accordingly. This diagonal direction of contour lines was unaffected by varying the f2∕f1 ratio. However, the contour gradient at the higher f2∕f1 ratio (lower right panel) was shallower than the lower ratio (top right), indicating that larger QDT magnitudes could be observed at lower primary levels.
Effects of the f2∕f1 ratio
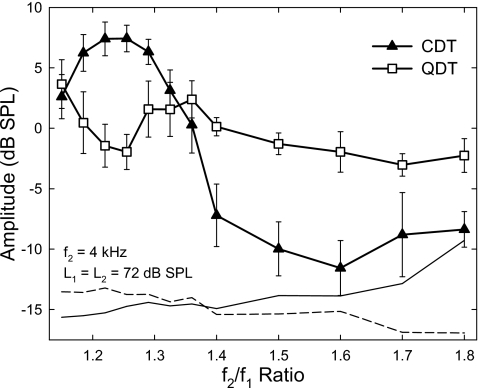
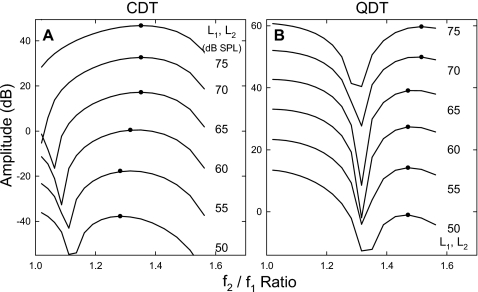
Since different ranges of f2∕f1 ratios were adopted in phase 1 and phase 2 experiments, results from both phases were pooled together to evaluate the effects of a widely changed frequency ratio. Both CDT and QDT magnitudes showed a variability which was influenced by the amplitude and frequency of the emissions. The standard error (SE) of emission amplitudes was less than 2 dB when the emission magnitude was greater than −5 dB SPL or the frequency was above 1 kHz. The SE could increase up to 5 dB if the emission frequency and amplitude were below 500 Hz and −5 dB SPL, respectively. Despite the variability, the averaged DPOAE magnitudes showed different patterns as the f2∕f1 ratio was increased from 1.15 to 1.8 (Fig. 4). As shown in this example (L1=L2=72 dB SPL), the CDT amplitude increased with the f2∕f1 ratio starting from the lowest value of 1.15 and reached a maximum of nearly 8 dB SPL at the ratios of 1.22 to 1.25. Then, the CDT magnitude decreased quickly as the ratio further increased and dropped below −5 dB SPL for the ratios above 1.4. The QDT magnitude, however, showed an opposite change when the f2∕f1 ratios were below 1.4, i.e., it produced a notch with a minimum of −2 dB SPL at the ratio of about 1.25. With further increase in the ratio, QDT levels bounced back and reached a peak at 1.36. For f2∕f1 ratios greater than 1.4, both CDT and QDT magnitudes reduced continuously. Despite a slower reduction, the QDT amplitude showed no signs of a bandpass characteristic (“the second filter”) when the f2∕f1 ratio increased to 1.8, a ratio where the QDT frequency was equivalent to the CDT generated at the ratio of 1.36. From the CDT curve, a dramatic 10-dB increase in amplitude could be observed from the ratios of 1.4 to 1.32.
Figure 4.
DPOAE amplitudes as functions of f2∕f1 ratio. Each curve is a combination of the averaged results from the two experimental phases. For frequency ratios below 1.4, the CDT amplitude reaches a peak at about 1.25, where the QDT magnitude drops to a local minimum. For f2∕f1 ratios above 1.4, both CDT and QDT amplitudes decline to minimal values at the frequency ratios of 1.6 and 1.7. Error bars represent the standard errors of the data. Solid and dashed lines indicate the averaged noise floor in CDT and QDT measures, respectively. Each data point reflects an average across eight subjects.
The opposite behaviors of CDT and QDT were also presented under the conditions of unequal primary levels. A series of averaged CDT and QDT amplitude-ratio functions was displayed to examine the influence of L1 with L2 held constant at 72 dB SPL (Fig. 5). It could be observed from panel (A) that the CDT amplitudes showed an asymmetrical bell shape as the frequency ratio varied. The peaks of CDT curves progressively shifted towards lower ratios when L1 decreased, i.e., the optimal f2∕f1 ratio for CDT was smaller (<1.2) for lower f1 levels (<66 dB SPL). For QDT magnitudes [panel (B)], a notch presented at about 1.22–1.25 for different levels of f1. However, no clear trend of shifting of the notch could be observed. On either side of the notches, the QDT level peaked around the frequency ratios of 1.18 and 1.32, respectively. For levels of f1 below 70 dB SPL, the patterns of QDT amplitude were more asymmetrical, with peaks at the ratio of 1.32 being higher.
Figure 5.
Effects of primary levels on DPOAE amplitude-ratio functions. (A) As L1 decreases, the peak of CDT amplitude shifts to lower f2∕f1 ratios. (B) QDT magnitudes show a notch around the f2∕f1 ratios of 1.22–1.25. No obvious shift of the notches can be observed with varying L1. Among the two possible optimal ratios (1.185 and 1.325), the larger one is selected as the optimal ratio for recording QDT so that the QDT frequency can be maintained above 500 Hz. Data reflect an average across eight subjects.
Optimal signal conditions
Primary level difference
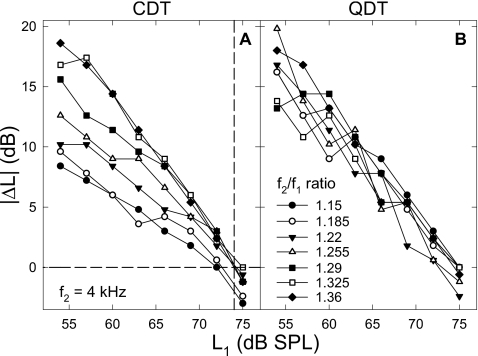
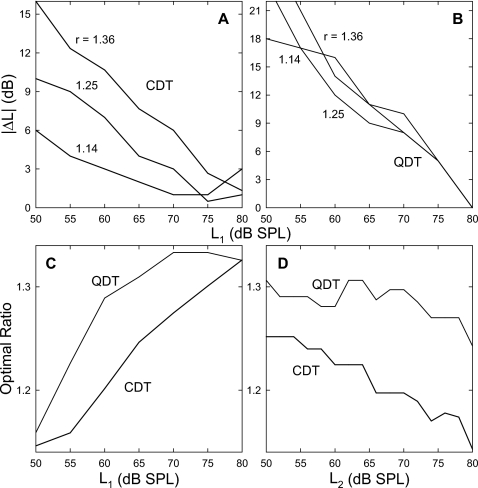
Inter-relations among the optimal signal conditions (L1, L2, and f2∕f1 ratio) were explored and quantified. As noted above, L1 should be greater than L2 to evoke a large CDT (Fig. 3 left) and the ΔL was greater at lower primary levels. The ΔL was further affected by the f2∕f1 ratio, i.e., larger for higher ratios, and the optimal ratio increased with the primary level [Fig. 5A]. These relations were depicted with a series of optimal ΔLs obtained at different frequency ratios plotted as functions of L1 (Fig. 6). For CDT [panel (A)], these ΔLs decreased linearly with the L1 and became 0 at the L1 of about 74 dB SPL. The only obvious difference among these ΔL functions was their varying slopes that decreased with the f2∕f1 ratio. Thus, the ΔL functions could be fit with a cluster of straight lines passing through a single point (74, 0),
| (1) |
where K(r) is the slope of the ΔL functions. Note that the slope K is a function of the f2∕f1 ratio (r) and the values are listed in Table 1. As can be observed, the relation between the slopes and the ratios was approximately linear with a 0.96 correlation coefficient (r2). Therefore, the slope could be obtained from K(r)=a⋅r+b, where a=−2.09, b=1.91.
Figure 6.
Effect of L1 on the optimal primary ΔL. For both CDT and QDT, the optimal ΔL declines with the increase in L1. Different lines represent different f2∕f1 ratios in each panel. (A) The slope of ΔL decrease with L1 varies depending on the f2∕f1 ratio. The maximal ΔL at the lowest L1 is proportional to the frequency ratio and ΔL approaches zero around the L1 of 74 dB SPL (dashed lines). (B) Similar trends can be observed in the ΔL for measuring QDT, but the effect of f2∕f1 ratio is minimal. Note: the absolute value of ΔL is used, i.e., ∣ΔL∣=L1−L2, because L2 could be greater than L1 for recording QDT. Data reflect an average of eight subjects.
Table 1.
Relation between the f2∕f1 ratio (r) and the slope K used for calculating the primary level difference (ΔL) in Eq. 1.
| r | 1.15 | 1.185 | 1.22 | 1.255 | 1.29 | 1.325 | 1.36 |
|---|---|---|---|---|---|---|---|
| K | −0.52 | −0.56 | −0.64 | −0.66 | −0.78 | −0.90 | −0.93 |
For QDT, ΔL was also reduced as L1 increased [Fig. 6B]. Unlike CDT, these ΔL functions for different f2∕f1 ratios were close to each other, indicating that the influence of the frequency ratio was weak. The slopes of these ΔL functions were approximately −1, meaning that the effect of primary levels was a linear decrease with increasing f2∕f1 ratio. Solving Eq. 1 with K=−1, there were two possible relations between the primary levels: one of the primaries L1 or L2=74 dB SPL, or L1=L2∕2+37, if both primaries were at moderate levels. However, the second solution was not the optimal condition, because for a given primary level the sum of L1 and L2 was not greater than when the other tone was held at the maximal value. It should be noted that L2 could be greater than L1 to evoke a large QDT.
Frequency ratio
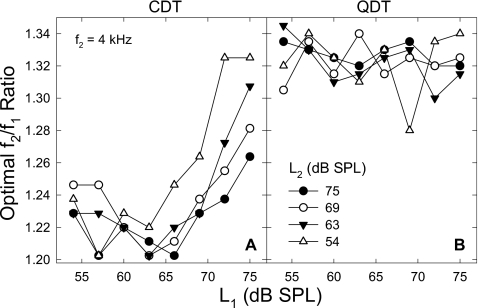
For CDT, the influence of primary levels on the optimal f2∕f1 ratio was mainly from L1, since the ratio showed only a small reduction with L2 (data not shown). At different L2 levels, the optimal ratio as functions of L1 demonstrated a series of “J”-shaped curves [Fig. 7A]. Generally, the optimal ratio declined slightly as L1 increased to 60–65 dB SPL. With further increase in L1, the ratio rose considerably. As L2 was lowered, the optimal ratio change with L1 showed three trends: (1) a steeper slope; (2) a higher maximal ratio; and (3) a shift of the minimum to lower L1. For each fixed L2, the ratio curve could be fit with a quadratic function,
| (2) |
where r0 is the minimal f2∕f1 ratio (when L1=L0) and s is the slope of ratio growth. Values of the parameters obtained from curve fitting varied with L2 (Table 2). For simplicity, linear functions were used to approximate the relations between the parameters and L2,
| (3) |
where c1=−3.65×10−4, d1=1.25, c2=−1.65×10−5, d2=1.42×10−3, c3=0.19, and d3=50.91.
Figure 7.
Influence of primary levels on optimal f2∕f1 ratio. (A) After an initial dip, the optimal frequency ratio increases nonlinearly with L1. The rate of f2∕f1 growth and the maximal value are inversely related to L2. The minimal ratios (>1.20) present at the L1 of about 55–65 dB SPL. (B) The optimal f2∕f1 ratios for QDT vary between 1.3 and 1.34 with only a slight decrease with L1. No influence from L2 can be observed. Data reflect an average of eight subjects.
Table 2.
Relation between L2 and the parameters in Eq. 2. r2: correlation coefficient of a linear regression between the parameter and the L2.
| L2 (dB SPL) | 75 | 72 | 69 | 66 | 63 | 60 | 57 | 54 | r2 |
|---|---|---|---|---|---|---|---|---|---|
| r0 | 1.223 | 1.227 | 1.230 | 1.230 | 1.224 | 1.233 | 1.236 | 1.228 | 0.62 |
| s(×10−4) | 2.31 | 1.72 | 2.65 | 1.75 | 5.52 | 4.53 | 4.33 | 5.06 | 0.74 |
| L0 (dB SPL) | 65.44 | 64.96 | 63.9 | 61.61 | 63.98 | 62.20 | 61.91 | 61.15 | 0.37 |
There could be two optimal ratios for QDT as indicated by the two peaks of the QDT amplitude-ratio patterns [Figs. 45B]. Only the larger ratio was chosen to keep the QDT frequency above 500 Hz for a better SNR. For QDT, no systematic increase of the optimal f2∕f1 ratio was observed [Fig. 7B]. The ratios fluctuated between 1.30 and 1.34 regardless of L2. Examined across different levels of L2, the frequency ratios showed a slight decrease with increasing L1. On average, the f2∕f1 ratios ranged from 1.32 to 1.33. Therefore, the influence of primary levels on the f2∕f1 ratio to record QDT was weak and a value slightly greater than 1.3 could be used as the optimal frequency ratio.
DISCUSSION
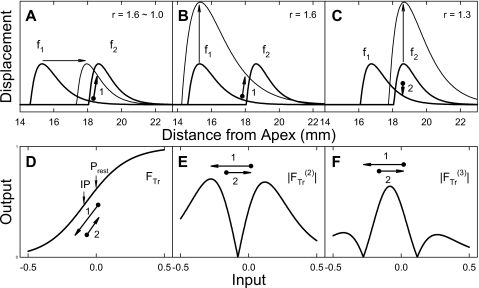
To explain the experimental results and explore the generating mechanisms of DPOAEs, a model combining the cochlear mechanics of traveling wave and the nonlinearity in hair cell transduction is used (Fig. 8). Simulated effects of manipulating the primary tones are compared with experimental data. Adding a traveling-wave component to the hair cell transducer model (Bian et al., 2002) is to determine the extent of two-tone interaction on the basilar membrane (BM) and the exact input levels to hair cell transduction.
Figure 8.
A model of DPOAE generation. Top row: two tone interaction via traveling waves. (A) Sweeping f1 to reduce the f2∕f1 ratio (r) while keeping primary levels constant (L1=L2). (B) Increasing L1 while keeping the f2∕f1 ratio constant. The overlap of traveling wave envelopes increases as f1 approaches f2 or as L1 increases. Influence of f1 on f2 or the effective L1, as indicated by the crossing point of the envelopes, rises accordingly. The direction of the crossing point change (dot arrow) marks the direction of OP shift on the FTr (path 1). (C) Raising L2 while keeping the frequency ratio constant. As L2 increases, changes in the overlapped region and the effective L1 are small. Actually, the influence of f1 on f2 is reduced. Relative to the f2 peak, the direction of the crossing point change (dot arrow) is reversed (path 2). Bottom row: the hair cell transducer nonlinearity and DP generation. The direction and path of the OP shift resulting from traveling wave interactions are indicated by the numbered dot arrows. (D) A cochlear FTr marked with the resting position (Prest) and inflection point (IP). (E) and (F) Absolute values of the second and third derivatives of the FTr, or and , respectively. Following the OP paths, the differential behaviors of QDT and CDT can be observed from panels (E) and (F).
Interaction of traveling waves
A simplified gamma-tone envelope (Carney, 1993) can be used to describe the spread of excitation or displacement along the BM in response to a pure tone,
| (4) |
where PTW is the traveling wave envelope as a function of the BM location l in millimeters from the apex and n is a constant which is unique to the traveling wave peak location (lP). Since the slope or derivative of the envelope at its peak is zero, i.e., , thus n=1∕lP. Ideally, lP is determined by the cochlear frequency map (Greenwood, 1990), where the characteristic frequency (fCF) location from the apex is lCF=(1∕α)log10(fCF∕A+k), where α=0.06 mm−1, A=165 Hz, and k=1 for humans. With increasing stimulus level, two alterations in traveling wave properties are also implemented: a basal peak shift and an envelope widening. Since the maximal peak shift is about 1∕2 octave above the fCF (Robles and Ruggero, 2001), the shift can be quantified as lP=lCF+(l1.5CF−lCF)L, where L is the signal level in pascals. Width of the traveling wave is defined as the distance between lP and the apical zero crossing (l0). Given a 35-mm-long BM, the width is a fraction (w) of the basal portion of the traveling wave (35−lP). This yields to l0=lP−w(35−lP)L1∕2, where w=1∕3. Thus, the actual traveling wave envelope is calculated as
| (5) |
where n=1∕(lP−l0) and the BM apical to l0 does not respond.
Since DPs are generated from the overlapped region of two traveling wave envelopes initiated by the primary tones [Fig. 8A], only a portion of the f1 envelope contributes to the two-tone interaction. The effective L1, indicated by the crossing point of f1 and f2 envelopes, is critical in determining the contribution of f1 and the influence of f1 on f2 (Lukashkin and Russell, 2001). Effects of f1 with respect to a fixed f2 were systematically examined by: (1) varying f1 with equal primary levels; (2) varying L1 with f1 fixed; and (3) varying L2 with a fixed f2∕f1 ratio [Figs. 8A, 8B, 8C]. As f1 approaches f2 [panel (A)], the overlapped region becomes larger and the effective L1 at the crossing point climbs up. When both f1 and f2 are fixed, increasing L1 enlarges the traveling wave overlap and the effective L1 (panel B), similar to raising f1. The manipulation of L2, however, produces a limited effect, because the basal slope of f1 envelope is shallow and expansion of apical portion of f2 pattern is small [Fig. 8C]. All three situations produce more or less similar changes of the CDT, i.e., a limited or saturated increase in CDT amplitude, since the effective L1 is limited by the f2∕f1 ratio of 1, or the fixed settings of L2 and L1. Increase in traveling wave overlap alone cannot explain the nonmonotonic changes in DPOAE magnitudes and differential behaviors of CDT and QDT (Figs. 12345). In essence, the optimal signal conditions are not solely dependent on traveling wave interactions. A second component in the model, the transducer nonlinearity, is thus necessary.
Interaction with the hair cell transducer
The nonlinearity of OHC transducer is characterized by a second-order Boltzmann function [Fig. 8D] relating cochlear responses to the BM displacement x (Bian et al., 2002),
| (6) |
where Fmax is a scaling factor determining the maximal output, (b,d) are slope constants, (c,e) are constants setting the resting position of the hair cell transducer. The main advantage of using the second-order Boltzmann function is its asymmetry. The inflection point (IP) on the FTr curve is located off zero input (resting point) and the slopes on two sides are different (Bian, 2004). These FTr asymmetries can be observed from the center notch or peak of the absolute values of its second and third derivatives (corresponding to the IP) and the sizes of sidelobes [panels (E) and (F)]. Note that there is a 180° phase difference between the sidelobes or side and center peak of the second and third derivatives, respectively. In addition to traveling wave interactions, the second and third derivatives of FTr largely dictate the behaviors of QDT and CDT.
Ratio effects
Influence of L1 on the overlapped region is not only a modification of actual input to the cochlear transducer, but also a biasing of the OP of hair cells in that region due to a tilting of hair bundles or shrinking of cell bodies caused by the f1 traveling wave. With the absence of f1 or when f1 is far away from f2, the OP at the f2 place usually is at the resting point [Fig. 8D]. When f1 approaches f2, or L1 steps up, the BM may undergo a progressive baseline shift or an OP shift [Figs. 8A, 8B]. The direction of OP shift on FTr could be from the resting position towards the IP (path 1). When the influence of f1 becomes very large, e.g., at a very small f2∕f1 ratio or an intense L1, the OP could pass the IP and eventually approach the minima on the third derivative [panel (F)]. Therefore, the CDT magnitudes, as functions of f2∕f1 ratio [Figs. 45A] or L1 [Fig. 1A], show a maximum and a rollover. Another evidence for the OP shift across the IP could be the notch presented on the QDT amplitude-ratio functions [Figs. 45B], which was also observed by Brown (1993). The simulated results of QDT and CDT (Fig. 9) well represent the scenarios of OP shifts on the second and third derivatives of FTr as the f2∕f1 ratio is reduced.
Figure 9.
Model simulation: effects of f2∕f1 ratio. (A) CDT magnitudes are maximized at the ratio between 1.2 and 1.3 and otherwise reduced. The peak CDT amplitude (dots) shifts to higher ratios with increase in primary levels (L1=L2). Note: a notch is presented at the f2∕f1 ratio of about 1.1. (B) QDT amplitudes are minimized at the frequency ratio of about 1.3 and maximized at higher (>1.4) or lower ratios. The maximal QDT magnitudes at the higher ratios are marked with dots.
A result of the simulation is that the CDT magnitudes reach a minimum and then increase when the f2∕f1 ratio is reduced below about 1.1 [Fig. 9A]. This notch could not be observed in the present study due to the limited frequency ratio selection (⩾1.15). However, the notch in CDT amplitude-ratio function has been reported in adults (Harris et al., 1989, Moulin, 2000; Londero et al., 2002) and neonates (Abdala, 1996; Lasky, 1997; Vento et al., 2004). Applying the above scenario and following path 1 of the OP shift, the CDT can reach a minimum and regain its magnitude when f1 further approaches f2 [Fig. 8F]. It has been suggested that when f2∕f1<1.1, reflection or place-fixed DPOAEs may become dominant (Stover et al., 1999; Knight and Kemp, 2001; Dhar et al., 2005) and cancel the nonlinearly generated DPs. However, the possibility that a nonlinear mechanism alone is capable of producing the notch cannot be ruled out (Lukashkin and Russell, 2001). Several investigators have measured the widths of CDT amplitude-ratio functions as the bandwidth of a “second cochlear filter” (Brown et al., 1993; Abdala, 1996; Vento et al., 2004) and found it narrower at lower primary levels. The simulated results [Fig. 9A] are consistent with these observations. Although the “second filter” is not evident by bringing the QDT frequency into the filter bandwidth (Fig. 4; Fahey et al., 2006), the shape of CDT amplitude-ratio function partly reflects the third derivative of cochlear FTr which is level dependent (Patuzzi and Moleirinho, 1998; Bian and Chertoff, 2001). Indeed, the sharpness of cochlear filter and the hair cell transducer gain may be intimately related.
Level effects
The effect of increasing L1 is similar to reducing f2∕f1 ratio where the CDT amplitude first increases and then rolls over [Fig. 1A]. The nearly linear growth of CDT with L1, which is also observed by others (Gaskill and Brown, 1990; Whitehead et al., 1995; Dreisbach and Siegel, 2005), may reflect the elevation of the effective L1 at the overlap of traveling wave envelopes [Fig. 8B]. Since the apical slope of f2 traveling wave envelope is very steep, the increase of effective L1 follows the rise of the tail of f1 envelope proportionally. When L1 exceeds L2, the traveling wave overlap reaches a maximum and the OP may shift (path 1) to the IP of FTr, thus producing a peak in CDT magnitude. Unlike the frequency ratio change, the CDT-L1 functions rarely show a notch [Fig. 1A]. However, such a notch is frequently found in gerbils (Mills, 2002) and chinchillas (Rhode, 2007), because the frequency places of the primaries are closer in small rodents and L1 is perhaps more effective in biasing OHCs at the f2 place. At the lowest f2∕f1 ratio (1.15), a notch was indeed observed on the L1 side of the L1×L2 space. It is worth noting that larger f2∕f1 ratios are used to evoke DPOAEs in rodents (Brown, 1987).
In contrast, increasing L2 may result in an opposite OP shift, because the influence of f1 on f2 diminishes as L2 rises due to unchanged effective L1 [Fig. 8C]. The direction of OP shift is from a prebiased position in the negative pressures towards the resting position of hair cell FTr (path 2). Depending on the value of L1, the initial OP shift could vary, thus resulting in variable patterns of CDT amplitude [Fig. 2A]. For high f1 levels, the initial OP shift is large and the OP can pass through the IP as L2 increases, causing CDT to rise and fall [Fig. 8E]. For lower L1, the small initial shift may not even reach the IP, and CDT can only show a decrease as the OP returns to its resting point. These different patterns are indeed observed as increasing L2 while holding L1 at various levels [Fig. 2A; Hauser and Probst, 1991]. Notches of the QDT amplitude in Fig. 2B may also indicate the OP path. However, the OP shift on QDT is less influential than the primary levels. This can be evident from the QDT contours in humans (Fig. 3, right) and rabbits (Martin et al., 2003) that show diagonal contour lines indicating increasing influences from both primary levels. In contrast, the ridge on CDT contours implies that a specific influence of L1 on L2, i.e., a unique OP shift, can maximize the amplitude. From Figs. 8D, 8E, 8F, it can be noted that CDT at lower input level is related to the center peak of the third derivative and the IP. In contrast, QDT is associated with sidelobes of the second derivative that are maximal for high input levels or large OP shifts. Moreover, sidelobes of the second and third derivatives are both related to saturation regions of the FTr. Indeed, the direction of QDT contour line is consistent with the “passive” CDT evoked at high primary levels (Mills, 1997).
Optimal signal conditions
Descriptions of the optimal signal conditions derived from the simulation are comparable with the experimental results. For equal primary levels, the optimal frequency ratio for CDT increases with the signal level [Fig. 9A], which is consistent with the observations in the present study (Figs. 45) and works by others (e.g., Johnson et al., 2006). The level-dependent drift of the optimal ratio is probably due to narrower traveling wave envelopes at low levels so that the two tones need to be placed closer to produce an interaction. Unlike the CDT, the optimal ratio for QDT shows no obvious drift with the primary level [Figs. 5B, 9B]. One reason for this difference could be that the deep notch on the second derivative of FTr [Fig. 8E] could severely affect the QDT magnitude compared to variations in traveling wave overlap.
Another important signal parameter is ΔL, which shows two linear relations in both experimental and simulated results (Figs. 610). First, ΔL decreases linearly with L1. Such a linear decrease is predictable from the differential growths of BM vibrations at the f2 place in response to f1 and f2 (Kummer et al., 2000), i.e., linear growth of effective L1 and compression of L2. Additional sound pressure is required to compensate the insensitivity for f1 at the f2 location until about 70–75 dB SPL, where tuning at the f2 place is broadened. Second, for CDT, the slope of ΔL decrease is proportional to the f2∕f1 ratio [Figs. 6A, 10A]. Again, it becomes clear that the tuning property at the f2 place determines that a smaller ΔL is required for a more closely placed f1 to produce a certain level of interaction [Figs. 8A, 8B, 8C]. These linear trends allow the derivation of Eq. 1 to calculate the ultimate ΔL for a given frequency ratio. Other formulas (Whitehead et al., 1995; Kummer et al., 2000) are also developed in a similar way. A formula by Johnson et al. (2006), incorporates the f2 and can be applied to different frequencies. Our formula in Eq. 1, however, includes the f2∕f1 ratio and is flexible for situations where the ratio is predetermined.
Figure 10.
Model simulation: optimal signal conditions. (A), (B) Influence of L1 on primary ΔL. For both CDT and QDT, the ΔL decreases with L1. For CDT, the slope of the ΔL decrease is proportional to the f2∕f1 ratio; however, such an effect is minimal for QDT. (C), (D) Effects of the primary levels on the optimal frequency ratio. For both CDT and QDT, the ratio increases with L1 and slightly reduces with L2. These level effects are smaller for QDT. Note the optimal frequency ratio is higher for QDT than CDT.
In practice, f2 and L2 are usually preselected to assess cochlear functions at a particular frequency location. The decisions to be made are to find f1 by selecting an appropriate f2∕f1 ratio and to determine L1. From the experimental data and model simulation, it is known that the optimal f2∕f1 ratio is nonlinearly dependent on the primary level [Figs. 710C, 10D]. For CDT, the optimal ratio stays low for lower values of L1 and grows quickly at higher levels. The quadratic fit of the optimal ratio function coincides with the formula of Johnson et al. (2006), in which a squared L2 is used. The effect of L2 on the optimal frequency ratio is a slight decrease [Fig. 10D]. Moving f1 closer could produce more two tone interactions if L2 is substantial, whereas moving f1 away could avoid suppressing f2 if L2 is weak. For QDT, the simulated optimal ratios (about 1.3) are generally comparable with the data for L1>65 dB SPL [Figs. 7B, 10D], even though the measured ratios show no obvious change with the primary levels.
For CDT, the complex interactions among frequency ratio and primary levels could be determined from the abstracted relations between a collective pairs of variables. Therefore, two of the relations are formulated using ΔL and optimal ratio [Eqs. 1, 2]. Since these relations coexist, the optimal conditions should satisfy both equations. Therefore, a set of two formulas is proposed for obtaining the optimal signal conditions for CDT,
| (7) |
where r0=c1L2+d1, s=c2L2+d2, and L0=c3L2+d3. Solving both equations together, the optimal f1 and L1 could be determined. These formulas with parameters obtained from the data predict the optimal conditions for each subject in the study. Using f2∕f1 ratio as an example, the prediction accuracy is as high as 95% with an average of 73%. From Eq. 7, the calculated values of L1 and f2∕f1 are well comparable with results in humans (Kummer et al., 2000; Johnson et al., 2006) and rodents (Pibal et al., 2002; Michaelis et al., 2004). Parameters used in the formulas still need to be tested and refined by large-scaled studies with wider stimulus ranges.
SUMMARY AND CONCLUSION
Amplitudes of CDT and QDT were measured within a finite L1×L2 space across a wide range of f2∕f1 ratios. For CDT, three relations are found among the optimal signal conditions: (1) L1 should always be greater than L2, until about 74 dB SPL where the level difference diminishes; (2) the rate of primary ΔL decrease is proportional to the f2∕f1 ratio; and (3) the optimal frequency ratio increases with L1 in a nonlinear fashion. A set of two formulas is proposed to quantify the optimal signal conditions, namely, f1 and L1 for a given L2. For QDT, different relations are observed: (1) maximizing one of the primary levels can optimize the QDT amplitude; (2) the ΔL decreases with the signal level, but independent of the f2∕f1 ratio; and (3) the optimal frequency ratio was constant at just over 1.3. An important finding is the QDT notch around the optimal ratio for CDT (1.22–1.25). Therefore, the optimal signal conditions for CDT cannot be applied to QDT. If applied cautiously to avoid system distortions, maximizing the signal level could allow for recording of robust QDT and CDT that may persistent after cochlear damages (Avan et al., 2003). The present results are consistent with the notion that DPOAEs mainly originate from the OHC nonlinearity in the overlapped region of the traveling waves. Optimizing DPOAE recordings could improve the measurement reliability and repeatability, thus allowing for more accurate estimates of cochlear function and a wider range of clinical utilities.
ACKNOWLEDGMENTS
We thank the subjects for their patience and Tina Stinson for recruiting some of the participants. This work was supported by a grant (R03 DC006165) from the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health.
References
- Abdala, C. (1996). “Distortion product otoacoustic emission (2f1−2f2) amplitude as a function of f2∕f1 frequency ratio and primary tone level separation in human adults and neonates,” J. Acoust. Soc. Am. 10.1121/1.417234 100, 3726–3740. [DOI] [PubMed] [Google Scholar]
- Allen, J. B., and Fahey, P. F. (1993). “Asecond cochlear-frequency map that correlates distortion product and neural tuning measurements,” J. Acoust. Soc. Am. 10.1121/1.408182 94, 809–816. [DOI] [PubMed] [Google Scholar]
- Avan, P., Bonfils, P., Gilain, L., and Mom, T. (2003). “Physiopathological significance of distortion-product otoacoustic emissions at 2f1−f2 produced by high- versus low-level stimuli,” J. Acoust. Soc. Am. 10.1121/1.1525285 113, 430–441. [DOI] [PubMed] [Google Scholar]
- Bian, L. (2004). “Cochlear compression: Effects of low-frequency biasing on quadratic distortion product otoacoustic emission,” J. Acoust. Soc. Am. 10.1121/1.1819501 116, 3559–3571. [DOI] [PubMed] [Google Scholar]
- Bian, L. (2006). “Spectral fine-structures of low-frequency modulated distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 10.1121/1.2200068 119, 3872–3885. [DOI] [PubMed] [Google Scholar]
- Bian, L., and Chertoff, M. E. (2001). “Distinguishing cochlear pathophysiology in 4-aminopyridine and furosemide treated ears using a nonlinear systems identification technique,” J. Acoust. Soc. Am. 10.1121/1.1340644 109, 671–685. [DOI] [PubMed] [Google Scholar]
- Bian, L, and Scherrer, N. M. (2007). “Low-frequency modulation of distortion product otoacoustic emissions in humans,” J. Acoust. Soc. Am. 10.1121/1.2764467 122, 1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian, L., Chertoff, M. E., and Miller, E. (2002). “Deriving a cochlear transducer function from low-frequency modulation of distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 10.1121/1.1488943 112, 198–210. [DOI] [PubMed] [Google Scholar]
- Brown, A. M. (1987). “Acoustic distortion from rodent ears: A comparison of responses from rats, guinea pigs and gerbils,” Hear. Res. 10.1016/0378-5955(87)90211-5 31, 25–38. [DOI] [PubMed] [Google Scholar]
- Brown, A. M. (1993). “Distortion in the cochlea: Acoustic f2−f1 at low stimulus levels,” Hear. Res. 10.1016/0378-5955(93)90154-S 70, 160–166. [DOI] [PubMed] [Google Scholar]
- Brown, A. M., Gaskill, S. A., Carlyon, R. P., and Williams, D. M. (1993). “Acoustic distortion as a measure of frequency selectivity: Relation to psychophysical equivalent rectangular bandwidth,” J. Acoust. Soc. Am. 10.1121/1.405713 93, 3291–3297. [DOI] [PubMed] [Google Scholar]
- Carney, L. H. (1993). “A model for responses of low-frequency auditory-nerve fibers in cat,” J. Acoust. Soc. Am. 10.1121/1.405620 93, 401–417. [DOI] [PubMed] [Google Scholar]
- Dhar, S., Long, G. R., Talmadge, C. L., and Tubis, A. (2005). “The effect of stimulus-frequency ratio on distortion product otoacoustic emission components,” J. Acoust. Soc. Am. 10.1121/1.1903846 117, 3766–3776. [DOI] [PubMed] [Google Scholar]
- Dreisbach, L. E., and Siegel, J. H. (2005). “Level dependence of distortion-product otoacoustic emissions measured at high frequencies in humans,” J. Acoust. Soc. Am. 10.1121/1.1880792 117, 2980–2988. [DOI] [PubMed] [Google Scholar]
- Fahey, P. F., Stagner, B. B., and Martin, G. K. (2006). “Mechanism for bandpass frequency characteristic in distortion product otoacoustic emission generation,” J. Acoust. Soc. Am. 10.1121/1.2146088 119, 991–996. [DOI] [PubMed] [Google Scholar]
- Fahey, P. F., Stagner, B. B., Lonsbury-Martin, B. L., and Martin, G. K. (2000). “Nonlinear interactions that could explain distortion product interference response areas,” J. Acoust. Soc. Am. 10.1121/1.1308048 108, 1786–1802. [DOI] [PubMed] [Google Scholar]
- Frank, G., and Kössl, M. (1996). “The acoustic two-tone distortions 2f1−f2 and f2−f1 and their possible relation to changes in the operating point of the cochlear amplifier,” Hear. Res. 10.1016/0378-5955(96)00083-4 98, 104–115. [DOI] [PubMed] [Google Scholar]
- Garner, C. A., Neely, S. T., and Gorga, M. P. (2008). “Sources of variability in distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 10.1121/1.2939126 124, 1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill, S. A., and Brown, A. M. (1990). “The behavior of the acoustic distortion product, 2f1−f2, from the human ear and its relation to auditory sensitivity,” J. Acoust. Soc. Am. 10.1121/1.399732 88, 821–839. [DOI] [PubMed] [Google Scholar]
- Gorga, M. P., Neely, S. T., Dorn, P. A., and Hoover, B. M. (2003). “Further efforts to predict pure-tone thresholds from distortion product otoacoustic emission input∕output functions,” J. Acoust. Soc. Am. 10.1121/1.1570433 113, 3275–3284. [DOI] [PubMed] [Google Scholar]
- Greenwood, D. D. (1990). “A cochlear frequency-position function for several species-29 years later,” J. Acoust. Soc. Am. 10.1121/1.399052 87, 2592–2605. [DOI] [PubMed] [Google Scholar]
- Harris, F. P., Lonsbury-Martin, B. L., Stagner, B. B., Coats, A. C., and Martin, G. K. (1989). “Acoustic distortion products in humans: Systematic changes in amplitude as a function of f2∕f1 ratio,” J. Acoust. Soc. Am. 10.1121/1.397728 85, 220–229. [DOI] [PubMed] [Google Scholar]
- Hauser, R., and Probst, R. (1991). “The influence of systematic primary-tone level variation L2−L1 on the acoustic distortion product emissions 2f1−f2 in normal human ears,” J. Acoust. Soc. Am. 10.1121/1.400511 89, 280–286. [DOI] [PubMed] [Google Scholar]
- Johnson, T. A., Neely, S. T., Garner, C. A., and Gorga, M. P. (2006) “Influence of primary-level and primary frequency ratios on human distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 10.1121/1.2133714 119, 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, H. J., Crawford, A. C., and Fettiplace, R. (2005). “Force generation by mammalian hair bundles supports a role in cochlear amplification,” Nature (London) 10.1038/nature03367 433, 880–883. [DOI] [PubMed] [Google Scholar]
- Kim, D. O. (1980). “Cochlear mechanics: Implications of electrophysiological and acoustical observations,” Hear. Res. 10.1016/0378-5955(80)90064-7 2, 297–317. [DOI] [PubMed] [Google Scholar]
- Knight, R. D., and Kemp, D. T. (2001) “Wave and place fixed DPOAE maps of the human ear,” J. Acoust. Soc. Am. 10.1121/1.1354197 109, 1513–1525. [DOI] [PubMed] [Google Scholar]
- Kummer, P., Janssen, T., Hulin, P., and Arnold, W. (1998). “The level and growth behavior of the 2 f1−f2 distortion product otoacoustic emission and its relationship to auditory sensitivity in normal hearing and cochlear hearing loss,” J. Acoust. Soc. Am. 10.1121/1.423054 103, 3431–3444. [DOI] [PubMed] [Google Scholar]
- Kummer, P., Janssen, T., Hulin, P., and Arnold, W. (2000). “Optimal L1−L2 primary tone level separation remains independent of test frequency in humans,” Hear. Res. 10.1016/S0378-5955(00)00097-6 146, 47–56. [DOI] [PubMed] [Google Scholar]
- Lasky, R. E. (1998). “Distortion product otoacoustic emissions in human newborns and adults. I. Frequency effects,” J. Acoust. Soc. Am. 10.1121/1.421215 103, 981–991. [DOI] [PubMed] [Google Scholar]
- Liberman, M. C., Gao, J., He, D. Z. Z., Wu, X., Jia, S., and Zou, J. (2002). “Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier,” Nature (London) 10.1038/nature01059 419, 300–304. [DOI] [PubMed] [Google Scholar]
- Londero, A., Bonfils, P., and Avan, P. (2002). “Magnitudes and phases of human distortion-product otoacoustic emissions at 2f1−2f2 against f2∕f1: Effects of an audiometric notch,” Hear. Res. 10.1016/S0378-5955(02)00332-5 167, 46–56. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin, B. L., and Martin, G. K. (2003). “Otoacoustic emissions,” Curr. Opin. Otolaryngol. Head Neck Surg. 11, 361–366. [DOI] [PubMed] [Google Scholar]
- Lukashkin, A. N., and Russell, I. J. (1999). “Analysis of the f2−f1 and 2f1−2f2 distortion components generated by the hair cell mechanoelectrical transducer: Dependence on the amplitudes of the primaries and feedback gain,” J. Acoust. Soc. Am. 10.1121/1.428096 106, 2661–2668. [DOI] [Google Scholar]
- Lukashkin, A. N., and Russell, I. J. (2001). “Origin of the bell-like dependence of the DPOAE amplitude on primary frequency ratio,” J. Acoust. Soc. Am. 10.1121/1.1417525 110, 3097–3106. [DOI] [PubMed] [Google Scholar]
- Martin, G. K., Stagner, B. B., and Lonsbury-Martin, B. L. (2003). “Difference-tone response areas in rabbits,” in Biophysics of the Cochlea: from Molecules to Models, edited by Gummer A. W., Dalhoff E., Nowotny M., and Scherer M. P. (World Scientific, Singapore: ), pp. 464–471. [Google Scholar]
- Michaelis, C. E., Gehr, D. D., Deingruber, K., Arnold, W., and Lamm, K. (2004). “Optimum primary tone level setting for measuring high amplitude DPOAEs in guinea pigs,” Hear. Res. 189, 58–62. [DOI] [PubMed] [Google Scholar]
- Mills, D. M. (1997). “Interpretation of distortion product otoacoutic emission measurements. I. Two stimulus tones,” J. Acoust. Soc. Am. 10.1121/1.419763 102, 413–429. [DOI] [PubMed] [Google Scholar]
- Mills, D. M. (2002). “Interpretation of standard distortion product otoacoutic emission measurements in light of the complete parametric response,” J. Acoust. Soc. Am. 10.1121/1.1505021 112, 1545–1560. [DOI] [PubMed] [Google Scholar]
- Mills, D. M., Feeney, M. P., Drake, E. J., Folsom, R. C., Sheppard, L., and Seixas, N. S. (2007). “Developing standards for distortion product otoacoutic emission measurements,” J. Acoust. Soc. Am. 10.1121/1.2770543 122, 2203–2214. [DOI] [PubMed] [Google Scholar]
- Moulin, A. (2000). “Influence of primary frequency ratio on distortion product otoacoustic emissions amplitude. I. Intersubject variability and consequences on the DPOAE-gram,” J. Acoust. Soc. Am. 10.1121/1.428433 107, 1460–1470. [DOI] [PubMed] [Google Scholar]
- Neely, S. T., Johnson, T. A., and Gorga, M. P. (2005). “Distortion-product otoacoustic emission measured with continuously varying stimulus level,” J. Acoust. Soc. Am. 10.1121/1.1853253 117, 1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall, A. L., and Dolan, D. F. (1993). “Intermodulation distortion (F2−F1) in inner hair cell and basilar membrane responses,” J. Acoust. Soc. Am. 10.1121/1.406692 93, 2061–2068. [DOI] [PubMed] [Google Scholar]
- Patuzzi, R., and Moleirinho, A. (1998). “Automatic monitoring of mechano-electrical transduction in the guinea pig cochlea,” Hear. Res. 10.1016/S0378-5955(98)00125-7 125, 1–16. [DOI] [PubMed] [Google Scholar]
- Pibal, I., Drexl, M., and Kössl, M. (2002). “Level dependence of optimal stimulus difference for evoking DPOAEs in the gerbil,” Hear. Res. 10.1016/S0378-5955(02)00700-1 174, 260–263. [DOI] [PubMed] [Google Scholar]
- Rhode, W. S. (2007). “Distortion product otoacoustic emissions and basilar membrane vibration in the 6–9 kHz region of sensitive chinchilla cochleae,” J. Acoust. Soc. Am. 10.1121/1.2785034 122, 2725–2737. [DOI] [PubMed] [Google Scholar]
- Robles, L., and Ruggero, M. A. (2001). “Mechanics of the mammalian cochlea,” Physiol. Rev. 81, 1305–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera, C. A. (2004) “Mechanisms of mammalian otoacoustic emission and their implications for the clinical utility of otoacoustic emissions,” Ear Hear. 10.1097/01.AUD.0000121200.90211.83 25, 86–97. [DOI] [PubMed] [Google Scholar]
- Stover, L. J., Neely, S. T., and Gorga, M. P. (1999). “Cochlear generation of intermodulation distortion revealed by DPOAE frequency functions in normal and impaired ears,” J. Acoust. Soc. Am. 10.1121/1.428097 106, 2669–2678. [DOI] [PubMed] [Google Scholar]
- Takahashi, S., and Santos-Sacchi, J. (1999). “Distortion component analysis of outer hair cell motility-related gating charge,” J. Membr. Biol. 10.1007/s002329900531 169, 199–207. [DOI] [PubMed] [Google Scholar]
- Tubach, M., McGee, J. A., and Walsh, E. J. (1996). “Distortion generated by the ear: Its emergence and evolution during development,” Laryngoscope 106, 822–830. [DOI] [PubMed] [Google Scholar]
- Vento, B. A., Durrant, J. D., and Sabo, D. L. (2004). “Development of f2∕f1 ratio functions in humans,” J. Acoust. Soc. Am. 10.1121/1.1675819 115, 2138–2147. [DOI] [PubMed] [Google Scholar]
- Whitehead, M. L., Stagner, B. B., McCoy, M. J., Lonsbury-Martin, B. L., and Martin, G. K. (1995). “Dependence of distortion-product otoacoustic emissions on primary levels in normal and impaired ears. II. Asymmetry in L1,L2 space,” J. Acoust. Soc. Am. 10.1121/1.411960 97, 2359–2377. [DOI] [PubMed] [Google Scholar]