Abstract
Bioactive coatings are in high demand to increase the functions of cells for numerous medical devices. The objective of this in vitro study was to characterize osteoblast (bone-forming cell) adhesion on several potential orthopedic polymeric materials (specifically, polyetheretherketone, ultra-high molecular weight polyethylene, and polytetrafluoroethylene) coated with either titanium or gold using a novel Ionic Plasma Deposition process which creates a surface-engineered nanostructure (with features below 100 nm). Results demonstrated that compared to currently-used titanium and uncoated polymers, polymers coated with either titanium or gold using Ionic Plasma Deposition significantly increased osteoblast adhesion. Qualitative cell morphology results supported quantitative adhesion results as increased osteoblast cell spreading was observed on coated polymers compared to uncoated polymers. In this manner, this in vitro study strongly suggests that Ionic Plasma Deposition should be further studied for creating nanometer surface features on a wide variety of materials to enhance osteoblast functions necessary for orthopedic applications.
Keywords: Nanostructured metal coatings, medical devices, osteoblast
Introduction
Bioactive coatings are in high demand to increase the functions of cells for numerous medical devices. For example, hydroxyapatite has often been used as a coating to improve the performance of conventional titanium-based materials for orthopedic applications (ie, fabricated by traditional metallurgy techniques and possibly surface-treated by mechanical methods such as grinding and polishing). By simulating the chemical composition of natural bone, hydroxyapatite coatings on titanium (Ti) greatly enhance osseointegration between the implant and juxtaposed bone (Furlong et al 2001; Baker et al 2006). Commercially, hydroxyapatite is coated on Ti-based metals through a high-temperature plasma-spray deposition process which transforms the initial nanocrystalline hydroxyapatite into micron grain size hydroxyapatite containing less crystalline calcium phosphates. Plasma-spray coating processes have, thus, often been criticized since they are not versatile enough to handle a wide range of chemistries and frequently alter the properties of the starting coating materials. Specifically, plasma-spray deposition of hydroxyapatite results in phase transformations which may lead to the formation of highly soluble calcium phosphates that delaminate during clinical use (Furlong et al 2001; Baker et al 2006).
Furthermore, several research groups have provided evidence that materials with nanometer surface features enhance bone formation compared to materials with conventional (or micron-scale) surface features (Karlsson et al 2003; Curtis et al 2004; Webster et al 2004; Popat et al 2005; Sato et al 2005). Although these findings indicate that nanometer surface features are important to increase the cytocompatibility of currently-used implants, traditional coating processes (like the aforementioned plasma-spray due to high heat) cannot uniformly create nanofeatures in orthopedic coatings to more effectively regenerate bone.
To obtain nanoroughness on a metal substrate, researchers have tried several techniques including the use of ultrafine-grained Ti (and other metals) (Valiev et al 2003; Webster et al 2004), anodizing Ti (Yao et al 2005), and chemical etching of Ti (Brunette et al 2001); all have shown promise towards improving bone cell responses. In terms of coatings, in 2004, Ionic Fusion Corp. announced a novel vacuum surface modification process called Ionic Plasma Deposition (IPD), which allows for the accurate control of material properties during coating procedures. Basically, the IPD process creates a surface-engineered nanostructure (with features usually below 100 nm (Tobler et al 2005)) by first using a vacuum to remove all contaminants. High kinetic energies (around 200 eV) then guide charged metallic ions or plasma to the surface of the medical device. The process runs at ambient temperature and can be supercooled when required, enabling a wide range of materials (ie, Ag, Au, Ti, etc.) to be coated on a wide range of underlying materials (for example, metals, polymers, and ceramics). The IPD process has been used to create silver/silver oxide coatings on medical devices to prevent infection (Halford 2006). Beyond silver, IPD may also be helpful to create versatile bioactive coatings on orthopedic implants.
This is important since numerous types of materials (metals, polymers, and ceramics) have been used in traditional orthopedic implants. In particular, polymers have certain advantages for orthopedic applications in terms of mechanical properties (such as wear, fatigue, biomimicking strength, etc.), especially in avoiding the mismatch of modulus between bone and metal implants that could lead to detrimental stress shielding (Green 2005). However, currently used polymers in orthopedic implants (such as ultra-high molecular weight polyethylene as the acetabular cup) have unsolved problems such as a lack of cytocompatibility properties for promoting bone cell growth. For these reasons, these polymers are often coated with bioactive ceramics, ie, hydroxyapatite, before use.
The hypothesis of the present study is that nano-engineered Ti (and other metals) deposited by IPD onto polymers may enhance bone cell functions due to changes in both chemistry and surface roughness. As a first step in this direction, the objective of this in vitro study was to characterize osteoblast (bone-forming cell) adhesion on several polymer orthopedic implant materials coated with metals using the IPD process. Osteoblast adhesion is a necessary step before the deposition of calcium-containing mineral on bone implants can occur.
Materials and methods
Substrates
Substrates (specifically, PEEK or polyetheretherketone; UHMWPE or ultra-high molecular weight polyethylene; and PTFE or polytetrafluoroethylene) were modified using the IPD deposition process. Raw materials were purchased off the shelf from McMaster-Carr. This process, performed in a vacuum, creates controllable nanometer surface features to promote cell attachment. Energy levels of 150eV to 500eV were controlled depending on the properties of the depositing materials (specifically, Ti and Au) allowing for low temperature (~30 °C) deposition onto the various substrates. Ti 6Al-4V and 4N Au were obtained from Process Materials Inc. The deposited particles (Ti and Au) were mostly less than 100 nm in diameter. Uncoated samples were used as controls. Moreover, commercially obtained micron-grain size Ti (Osteonics) was used as a reference.
After the coatings were completed, all samples were cleaned with deionized water in an aqua-sonicator with 70% ethanol for 10 minutes. These cleaned substrates were dried in an oven at 65 °C and exposed to UV light for 1 h.
Some of the original and as-deposited samples were used for qualitative surface characterization by Field Emission Scanning Electron Microscopy (LEO 1530 VP).
Cytocompatibility tests
Human osteoblasts (CRL-11372, American Type Culture Collection, population numbers 2–4) were used in the cell adhesion experiments in this study. All substrates of interest were rinsed with phosphate buffered saline (PBS) (1X strength) before seeding the cells. The cells were cultured on the substrates in Dulbecco’s Modified Eagle Medium (Hyclone) supplemented with 10% fetal bovine serum (Hyclone) and 1% penicillin/streptomycin (Hyclone) with an initial seeding density of 3500 cells/cm2 of substrate surface area according to previous protocols (Yao et al 2005). Cells were then allowed to adhere on the substrates under standard cell culture conditions (37 °C temperature, 5% CO2 and 95% humidified air) for 4 hours. After the prescribed time period, the cell culture medium was aspirated from the wells and the substrates were gently rinsed with PBS three times to remove any non-adherent cells. The adherent cells were then fixed with a 4% formaldehyde solution (Fisher) and the cell nuclei were stained with Hoescht 33258 dye (Sigma). The cell numbers in five random selected areas were counted under a fluorescence microscope (Swiss) in transmission mode. All experiments were carried out at four different times each in triplicate.
Lastly, at the conclusion of the adhesion assay, each sample was dehydrated through a series of ethanol solutions (30%, 50%, 70%, 90%, and 100%), sputter-coated with AuPd, and osteoblast morphology characterized by Field Emission Scanning Electron Microscopy (LEO 1530 VP).
Results
Materials characterization
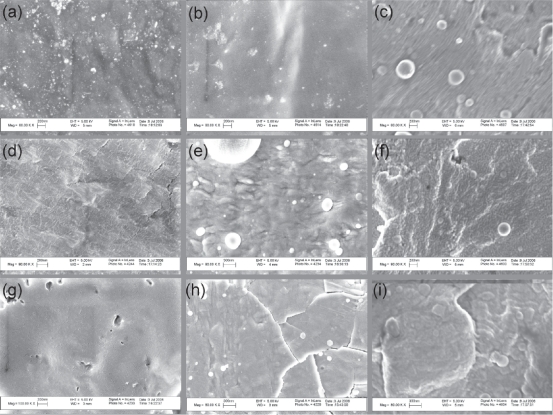
The substrates of interest were observed using Field Emission Scanning Electron Microscopy and their surfaces are shown in Figures 1 and 2. The uncoated polymers showed different roughness (Figure 1). The Ti coatings on the three polymer substrates varied in the degree of micron roughness while all Au coatings had qualitatively greater micron surface roughness compared to Ti coatings (Figure 1). Some of the UHMWPE and PTFE samples showed micro-cracks (Figures 1 and 2) which might be due to the cleaning procedure. Further studies are needed to determine the source of the cracking during sample preparation.
Figure 1.
Low magnification (1000x) SEM images of surfaces of (a-c) PEEK, (d-f) UHMWPE, and (g-i) PTFE before and after coating with either Ti or Au by IPD. (a)(d)(g) are original polymer surfaces while (b)(e)(h) are respective surfaces coated with Ti and (c)(f)(i) with Au.
Figure 2.
High magnification (80,000× or 100,000×) SEM images of surfaces of (a-c) PEEK, (d-f) UHMWPE, and (g-i) PTFE before and after coating with either Ti or Au by IPD. (a)(d)(g) are original polymer surfaces while (b)(e)(h) are respective surfaces coated with Ti and (c)(f)(i) with Au.
Most importantly, for all three types of polymer substrates, IPD coated Ti and Au possessed significantly more nano-scale surface features (Figure 2). Within the nanoscale, Ti coatings on UHMWPE appeared more rough than the other two (PTFE and PEEK) while Au coatings on PTFE appeared more rough than the other two (UHMWPE and PEEK) (Figure 2).
Cytocompatibility characterization
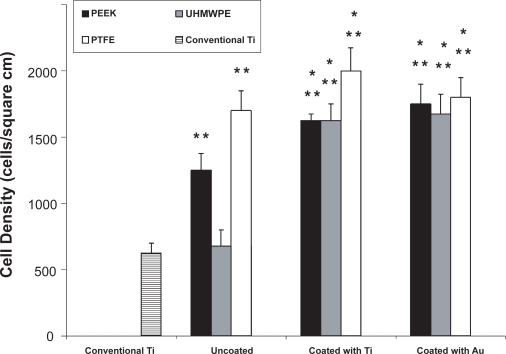
Results of this in vitro study showed for the first time that compared to the respective uncoated polymer samples, osteoblast adhesion increased on the three polymer substrates (PEEK, UHMWPE, and PTFE) coated with either nanoparticulate Ti or Au (Figure 3). Osteoblast adhesion was greater on all samples coated with nanoparticulate Ti compared to currently-used, micron-grain size Ti. Interestingly, PTFE coated with either nanoparticulate Ti or Au outperformed respective PEEK and UHMWPE samples. The sample which enhanced osteoblast adhesion the most was PTFE coated with Ti.
Figure 3.
Osteoblast adhesion on PEEK (uncoated, coated with Ti and coated with Au), UHMWPE (uncoated, coated with Ti and coated with Au), and PTFE (uncoated, coated with Ti and coated with Au). Data = mean ± STDEV; n = 4; * p < 0.01 (compared to respective uncoated sample) and ** p < 0.01 (compared to conventional Ti).
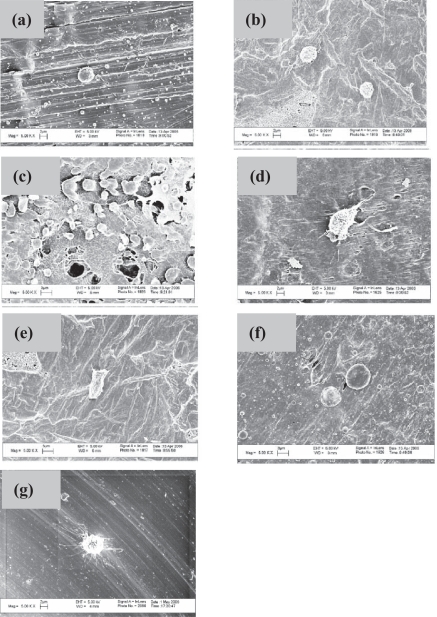
A majority of osteoblasts had a round shape on the three uncoated polymer substrates while they were typically more well-spread on polymers coated with either Ti or Au (Figure 4). Other reports in the literature have correlated increased cell spreading with greater cell adhesion numbers (Valiev et al 2003), which matches the results of the present study. Further studies revealing the cytoskeleton and focal adhesion contacts would be useful to elucidate the effects of IPD coatings on osteoblast spreading.
Figure 4.
Selective osteoblast morphologies after the 4 h adhesion tests on: (a) uncoated PEEK, (b) uncoated UHMWPE, (c) uncoated PTFE, (d) PEEK coated with Ti, (e) UHMWPE coated with Ti, (f) PEEK coated with Au and (g) uncoated Ti. Scale bars = 2 μm in (a), (b), (d), and (g) and 3 μm in (c), (e), and (f). Note that these regions were chosen to highlight individual cell morphology and do not necessarily correspond to cell densities presented in Figure 3.
Discussion
Ionic Plasma Deposition (IPD) is a versatile technique that can be used to coat different medical devices of and with diverse chemistries. Using conventional deposition methods (such as plasma-spray), numerous problems exist such as poor adhesion strength, inability to maintain starting nanoparticle size, change of coating material crystallinity, etc. (Furlong et al 2001). However, in the IPD coating process, ions of the depositing material are accelerated to ensure that they have proper energy to coat the specific medical device at room temperature. As a result, properties of the coatings are improved.
Previous studies (Karlsson et al 2003; Curtis et al 2004; Webster et al 2004; Popat et al 2005; Sato et al 2005) have highlighted that one important property in material coatings desirable to create to improve cell performance is nanometer surface features. That is, due to the importance of nanometer features in promoting bone cell functions, another key advantage of IPD is that the original coating particle size, chemistry, and crystallinity can be retained due to the low heat presented during the coating application. Clearly, this allows IPD to create nanotopographies on conventional materials to improve their bioactivity properties, as this study demonstrated.
Specifically, to test whether such bioactive properties resulting from surface nanometer features are still present in the IPD coated materials, osteoblast adhesion assays were conducted here. These results showed that compared to the uncoated materials, three polymer chemistries popular to the medical device field when coated with either Ti or Au enhanced osteoblast attachment after 4 h of culture. At this time, though, it is unclear what properties of the coatings enhanced osteoblast adhesion: such as a change in wettability, chemistry, and/or nanometer surface features. For example, UHMWPE, PEEK, PTFE are all hydrophobic materials whose surface energy was changed through Ti and Au coatings. However, when compared to conventional Ti (or micron grain size Ti) which possessed the same chemistry as the polymers coated with nanoparticulate Ti, increased osteoblast adhesion was still measured; this suggests the possibility that nanometer surface roughness alone increased osteoblast adhesion on the polymer samples coated with Ti using IPD.
However, a change in nanometer roughness is also related to changes in wettability since previous studies have shown lower aqueous contact angles on Ti composed of nanometer compared to micron grain sizes (Webster et al 1999). The authors speculated in those studies that hydrophilicity is enhanced on Ti with nanometer surface features due to the increased presence of surface defects compared to conventional Ti (Webster et al 1999). Those studies continued to show greater initial adsorption of hydrophilic proteins (specifically, vitronectin) and subsequent osteo-blast functions from adhesion to the deposition of calcium containing mineral on nanograined Ti (Webster et al 2001; Webster et al 2000). More studies, though, are needed for the presently described IPD process to determine specifically what properties enhanced osteoblast adhesion on the coated materials. None-the-less, this study provides strong evidence for the continued investigation of IPD for improved orthopedic applications.
Conclusions
Nanotopography or nanoroughness of an implant surface is desirable to improve initial cell-implant interactions. With respect to implant coatings, Ionic Plasma Deposition (IPD) is an efficient method to deposit numerous nanostructured coatings onto versatile materials, including metals and polymers. The current study represents the first which demonstrated increased osteoblast attachment on various polymers coated with either Ti or Au by IPD; thus, demonstrating the strong potential IPD has in converting versatile conventional medical devices to possess bioactive coatings for orthopedic applications.
References
- Baker KC, Anderson MA, Oehlke SA, Astashkina AI, Haikio DC, Drelich J, Donahue SW. Growth, characterization and biocompatibility of bone-like calcium phosphate layers biomimetically deposited on metallic substrata. Materials Science and Engineering C. 2006;26:1351–60. [Google Scholar]
- Brunette DM, Tengvall P, Textor M, Thomsen P. Mechanical, thermal, chemical and electrochemical surface treatment of titanium. In: Titanium in Medicine, Material Science, Surface Science, Engineering, Biological Responses and Medical Applications. Germany 1st ed. Springer. 2001:232–66. [Google Scholar]
- Curtis ASG, Gadegaard N, Dalby MJ, Riehle MO, Wilkinson CDW, Aitchison G. Cells react to nanoscale order and symmetry in their surroundings. IEEE Trans. Nanobiosci. 2004;3:61–5. doi: 10.1109/tnb.2004.824276. [DOI] [PubMed] [Google Scholar]
- Furlong R, Osborn JF. Fixation of hip prostheses by hydroxyapatite ceramic coatings. J Bone Joint Surg (Br) 2001;73B:741–5. doi: 10.1302/0301-620X.73B5.1654336. [DOI] [PubMed] [Google Scholar]
- Green S. Using implantable-grade PEEK for in vivo devices. Medical Device & Diagnose Industry. 2005;27:104–11. [Google Scholar]
- Halford B. A silver bullet for infections. Science & Technology. 2006;84:34–6. [Google Scholar]
- Karlsson M, Palsgard E, Wilshaw PR, Silvio LD. Initial in vitro interaction of osteoblasts with nanoporous alumina. Biomaterials. 2003;24:3039–46. doi: 10.1016/s0142-9612(03)00146-7. [DOI] [PubMed] [Google Scholar]
- Popat KC, Swan EEL, Mukhatyar V, Chatvanichkul KI, Mor GK, Grimes CA, Desai TA. Influence of nanoporous alumina membranes on long-term osteoblast response. Biomaterials. 2005;26:4516–22. doi: 10.1016/j.biomaterials.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Sato M, Slamovich EB, Webster TJ. Enhanced osteoblast adhesion on hydrothermally treated hydroxyapatite/titania/poly(lactide-co-glycolide) sol-gel titanium coatings. Biomaterials. 2005;26:1349–57. doi: 10.1016/j.biomaterials.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Tobler D, Warner L. Nanotech silver fights microbes in medical devices. Medical Device & Diagnose Industry. 2005;27:164–9. [Google Scholar]
- Valiev RZ, Stolyarov VV, Rack HJ, Lowe TC. SPD-processed ultrafine grained Ti materials for medical applications. Advanced Material & Processes. 2003;Dec:33–4. [Google Scholar]
- Webster TJ, Siegel RW, Bizios R. Osteoblast adhesion on nanophase ceramics. Biomaterials. 1999;20:1221–7. doi: 10.1016/s0142-9612(99)00020-4. [DOI] [PubMed] [Google Scholar]
- Webster TJ, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21:1803–6. doi: 10.1016/s0142-9612(00)00075-2. [DOI] [PubMed] [Google Scholar]
- Webster TJ, Schadler LS, Siegel RW, Rizios R. Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Engineering. 2001;7:291–301. doi: 10.1089/10763270152044152. [DOI] [PubMed] [Google Scholar]
- Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, CoCrMo. Biomaterials. 2004;25:4731–9. doi: 10.1016/j.biomaterials.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Yao C, Perla V, McKenzie JL, Slamovich EB, Webster TJ. Anodized Ti and Ti6Al4V possessing nanometer surface features enhances osteo-blast adhesion. Journal of Biomedical Nanotechnology. 2005;1:68–77. [Google Scholar]