Abstract
Solid lipid nanoparticles (SLN) have been reported to be an alternative system to emulsions, liposomes, microparticles and their polymeric counterparts for various application routes since the early 1990s due to their advantages. Various research groups have also increasingly focused on improving their stability in body fluids after administration by coating of particles with hydrophilic molecules such as poly(ethylene)glycol (PEG) derivatives. Altering surface characteristics by coating SLN with hydrophilic molecules improves plasma stability and biodistribution, and subsequent bioavailability of drugs entrapped. Their storage stability is also increased. This paper basicly reviews types of SLN, principles of drug loading and models of drug incorporation. The influence of PEG coating on particle size and surface characteristics is discussed followed by alteration in pharmacokinetics and bioavailability of drugs in order to target the site of action via SLN. The future direction of research and clinical implications of SLN is also considered.
Keywords: solid lipid nanoparticles, nanostructured lipid carriers, drug incorporation models, particle size, surface characteristics, PEG coating, drug release
Introduction
Great progress has been made in the treatment of a variety of diseases by using drug delivery systems including solid lipid nanoparticles (SLN). SLN are colloidal drug carrier systems (Mehnert and Mäder 2001; Müller and Keck 2004; Castelli et al 2005). They are very much like nanoemulsions, differing in lipid nature. The liquid lipid used in emulsions is replaced by a lipid solid at room temperature in SLN including high-melting point glycerides or waxes (Siekmann and Westesen 1992; Müller and Keck 2004; Manjunath and Venkateswarlu 2005). They are increasing in significance as alternative drug carriers to polymeric nanoparticles. Controlled drug delivery, enhancement of bioavailability of entrapped drugs via modification of dissolution rate (Demirel et al 2001) and/or improvement of tissue distribution and targeting of drugs (Göppert and Müller 2005) by using SLN have been reported in various application routes:
– Parenteral (intravenously, intramuscularly or subcutaneously) (Yang et al 1999a; Wissing et al 2004),
– Oral (Yang et al 1999b; Pandey et al 2005),
– Rectal (Sznitowska et al 2001),
– Opthalmic (Cavalli et al 2002; Friedrich et al 2005),
– Topical (in cosmetics and dermatological preparations) (De Vringer and De Ronde 1995; Dingler et al 1998; Lippacher et al 2002; Münster et al 2005).
Indeed, nanoparticles were initially thought to be designed as carriers for vaccines and anticancer drugs when they were first developed in about 1970. In the strategy of drug targeting in order to enhance tumor uptake, researchers focused on the development methods to reduce the uptake of the nanoparticles by the cells of the reticuloendothelial system (RES) as the first important step. As one of those methods, coating of nanoparticles and nanocapsules with hydrophilic substances such as polyoxypropylene block copolymers (poloxamers) (Göppert and Müller 2005), chitosan (Garcia-Fuentes et al 2005a), polyvinyl alcohol (Pandey et al 2005) and PEG (De Campos et al 2003) has a clear benefit in their ability to reduce the phagocytic uptake with a minimal non-specific interaction with other proteins. Increasing attention has also been paid to the coating of SLN to provide receptor mediated drug and gene delivery in recent years (Kakizawa and Kataoka 2002; Garcia-Garcia et al 2005). Coating of colloidal carriers has been demonstrated to improve stability of the particles and to enhance transmucosal transport of the associated compounds following either nasal (Vila et al 2004), oral (Jani et al 1990) or ocular administration (De Campos et al 2001).
Several innovative reviews on solid lipid nanotechnology for drug delivery are available in the literature which describes extensive preparation techniques, characterization and types of SLN, investigation of their structural properties, factors affecting their formation and storage stability, drug loading principles and drug release characteristics (Müller et al 2000; Mehnert and Mäder 2001; Müller et al 2002a; Müller et al 2002b; Heurtault et al 2003; Müller and Keck 2003; Uner 2006). This paper reviews hydrophilic surface coating of SLN among various approaches in order to improve efficiency of drugs incorporated. Brief description of production methods, types of SLN and consequently principles of drug release are given. Alteration in particle size and surface characteristics of SLN by PEG coating is described, followed by the benefits of this surface modification in various administration routes, the effect of coating on bioavailability of drugs incorporated and the future direction of research.
Production methods of SLN
SLN are produced by using several methods extensively described in the literature:
– High pressure homogenization (cold and hot homogenization) (Müller and Runge 1998; Jores et al 2004; Üner et al 2005b),
– Breaking of o/w microemulsion (Gasco 1993; Cavalli et al 1997; Cavalli et al 1999; Igartua et al 2002),
– Solvent emulsification-evaporation (Sjostrom and Bergenståhl 1992; Cortesi et al 2002; Shahgaldian et al 2003) or solvent emulsification–diffusion (Quintanar-Guerrero et al 2005; Hu et al 2005),
– Solvent injection (Schubert and Müller-Goyman 2003),
– Preparation via water-in-oil-in-water double emulsion (w/o/w) (Morel et al 1998; Cortesi et al 2002),
– High shear homogenization (Kržič et al 2001) and/or ultrasound dispersion (Mei et al 2003; Song and Liu 2005),
– Preparation by using membrane contactor as a new reported technique for SLN production (Charcosset et al 2005).
Today, the high pressure homogenization technique has been demonstrated to be the most effective technique due to some advantages such as narrow particle size distribution of the product with a low content of microparticles (> 5 μm is requested for iv injections), higher particle content in the dispersions, avoidance of organic solvents, acceptability of the homogenization equipment by the regulatory authorities (even for parenteral products), scale-up feasibility and the availability of homogenization lines in industry (Müller and Runge 1998; Gohla and Dingler 2001; Mehnert and Mäder 2001). Depending on the size of production-scale homogenizers, a wide production range can be possible (Müller and Keck 2004; Wissing et al 2004).
Drug incorporation models and types of SLN
Factors affecting loading capacity of a drug in lipid are (Müller et al 2000):
– solubility of drug in lipid melt,
– miscibility of drug melt and lipid melt,
– chemical and physical structure of solid matrix lipid,
– polymorphic state of lipid material.
In particular, there is an inverse relationship between solubility of the drug and loading capacity. Enhancement in aqueous solubility of drug leads lower to entrapment efficiency. For this reason, Müller et al reported a cold homogenization technique which is performed at room temperature or below (0 °C) (Müller et al 1995; Müller and Hildebrand 1997). Therefore, solubility of the drug is also an important factor for choosing the production method of SLN. While the hot homogenization technique is much more suitable for lipophilic drugs, the cold homogenization technique is employed for hydrophilic drugs in order to reach the highest payload and to prevent drug partition to the aqueous phase during SLN production.
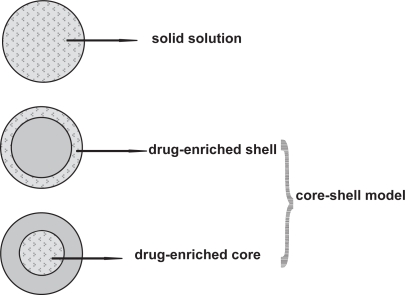
Drug incorporation models of SLN are (Figure 1):
– solid solution model
– core-shell model (drug-enriched shell and drug-enriched core)
Figure 1.
Models of drug incorporation into SLN: homogeneous matrix of solid solution (upper), drug-free core with drug-enriched shell (middle), drug-enriched core with lipid shell (lower).
In the case of the solid solution model, the drug is molecularly dispersed in the lipid matrix when the particles are produced by the cold homogenization technique and using no surfactant or no drug-solubilizing surfactant. The drug has strongly pronounced interactions with the lipid (Schwarz 1995; Zur Mühlen et al 1998).
According to the drug-enriched shell model of drug incorporation, a solid lipid core forms when the recrystallization temperature of the lipid is reached. On reducing the temperature of the dispersion, the drug concentrates in the still liquid outer shell of the SLN (Heiati et al 1997; Müller et al 2002a).
According to the drug-enriched core model of drug incorporation, cooling the nanoemulsion leads to a supersaturation of the drug which is dissolved in the lipid melt at or close to its saturation solubility and the drug precipitates prior to lipid recrystallization. Further cooling finally leads to the recrystallization of the lipid surrounding the drug as a membrane (Müller et al 2000).
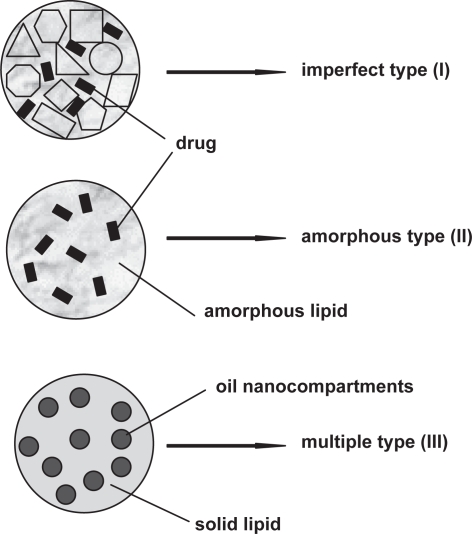
A SLN modified by incorporation of liquid lipid into the solid structure has been proposed as nanostructured lipid carriers (NLC) to overcome some limitations related to old generation SLN. Müller et al described NLC with a special structure for better drug accommodation in order to increase the payload and prevent drug expulsion during storage. NLC combine controlled drug release characteristics with some advantages over SLN. NLC have so far been studied for topical use, but they offer all the advantages and production aims of SLN. Three types of NLC have been described: I) imperfect type, II) amorphous type and III) multiple type (Figure 2) (Radtke and Müller 2001; Müller et al 2002a; Müller et al 2002b).
Imperfect type NLC (imperfectly structured solid matrix): Spatially different lipids are mixed, and thus imperfections in the crystal order of lipid nanoparticles are provided. Large distances between fatty acid chains in the matrix structure of lipid nanoparticles can be increased by using glycerides composed of very different fatty acids. Therefore, the matrix contains imperfections to accommodate the drug in amorphous clusters (Figure 1, upper). Mixing small amounts of chemically very different liquid lipids (oils) with solid lipids in order to achieve the highest incompatibility leads the highest drug payload.
Amorphous type (structureless solid amorphous matrix): This kind of NLC can be achieved by mixing solid lipids with special lipids, eg, hydroxyoctacosanylhydroxystearate, isopropylmyristate or medium chain triglycerides such as Miglyol® 812. Therefore, drug expulsion caused by the process of crystallization to β forms during storage is prevented by the special structure of the lipid matrix since NLC are solids in an amorphous but not crystalline state (Figure 1, middle) (Radtke and Müller 2001).
Multiple type (multiple oil in fat in water (O/F/W) carrier): The solubility of the drug in the lipophilic phase decreases during the cooling process after homogenization and the crystallization process during storage. Continuously reducing drug solubility leads to drug expulsion from the lipid nanoparticles especially when the drug concentration in the formulation is too high. Solubility of many drugs in a liquid lipid is higher than in a solid lipid. When lipids lack appropriate drug solubilities, addition of a higher amount of liquid lipid to the lipophilic phase displays the advantages of the solid matrix which prevented drug leakage while the liquid regions (oily nanocompartments) show comparatively high solubility for lipophilic drugs (Figure 1, lower) (Jenning et al 2000b).
Figure 2.
Types of NLC: (I) imperfect type, (II) amorphous type and (III) multiple type.
Principles of drug release from SLN
The general principles of drug release from lipid nanoparticles are as follows (Zur Mühlen and Mehnert 1998; Müller et al 2002a; Venkateswarlu and Manjunath 2004; Uner 2006);
– There is an inverse relationship between drug release and the partition coefficient of the drug.
– Higher surface area due to smaller particle size in nanometer range gives higher drug release.
– Slow drug release can be achieved when the drug is homogenously dispersed in the lipid matrix. It depends on type and drug entrapment model of SLN.
– Crystallinization behaviour of the lipid carrier and high mobility of the drug lead to fast drug release. There is an inverse relationship between crystallization degree and mobility of drug.
The drug incorporation model of SLN is crucial to the drug release pattern. It is related to the composition and production method of SLN as explained above. For instance, the drug-loaded lipid phase remains mainly in the solid state in the case of production by cold homogenization technique. The solid solution drug incorporation model appears here. Drug release is prolonged over several weeks since mobility of the drug molecularly dispersed in colloidal particles is very limited (Müller et al 1994).
Fast initial drug release (burst effect) exists in the first 5 minutes in the drug-enriched shell model (ie, about 100% within <5 min) as a result of the outer layer of the particles due to the large surface area of drug depositon on the particle surface (Müller et al 1994; Zur Mühlen and Mehnert 1998). The burst release is reduced with increasing particle size and prolonged release could be obtained when the particles were sufficiently large, ie, lipid microparticles (Schwarz et al 1994; Müller et al 1995). The type of surfactant and its concentration, which will interact with the outer shell and affect its structure, should be noted as the other important factor, because a low surfactant concentration leads to a minimal burst and prolonged drug release.
In the drug-enriched core model, the drug release is membrane controlled and is governed by the Fick law diffusion since the lipid surrounds the drug as a membrane (Müller et al 2000).
In the case of NLC – the new generation SLN – the oil content of the particles solves the drug and combines controlled release characteristics with high drug loading capacity. Müller et al (2002b) reported that the imperfect type and amorphous type of NLC, in particular, provide much more flexibility to achieve the desired prolonged release.
The particle size that affects drug release rate directly depends on various parameters such as composition of SLN formulation (such as surfactant/surfactant mixture, amount of drug incorporated, structural properties of lipid and drug), production methods and conditions (such as time, production temperature, equipment, sterilization and lyophilization). All those parameters have been extensively investigated and data have been reported in the literature for years (Siekmann and Westesen 1992; Cavalli et al 1997; Freitas and Müller 1998; Liedtke et al 2000; Mehnert and Mäder 2001; Hou et al 2003; Schubert et al 2006). Additionally, surface modifiers to reduce phagocytic uptake such as polyethylene oxide and PEG may change the particle size. The effect of surface modifiers on particle size and on drug release rate is discussed in upcoming sections.
Coating of solid lipid nanoparticles (SLN) with hydrophilic substances
In case of systemic use, ideal drug delivery is selective uptake by the target organ or at the site of action with a low systemic level of drug. It is very difficult to provide this ideal situation in practice because anatomical barriers limit and govern the distribution of drugs. SLN which are hydrophobic, are exposed to phagocytic uptake by macrophages. Studies on systemic use of SLN have focused on improving their presence in the blood circulation since macrophages in RES recognize them as foreign substances and quickly remove them due to their physicochemical properties, mainly particle size, surface charge and surface hydrophobicity (Illum et al 1987; Müller 1991; Bocca et al 1998). Uptake by phagocytic cells is mediated by blood components which are called opsonins and specific cell receptors on macrophages that operate independently. The opsonic factors include proteins such as immunoglobulin G, Complement C3b and fibronectin. These factors are adsorbed by nanoparticles, then the particles are immediately cleared by the macrophages of the mononuclear phagocytic system (Müller et al 1997). The surface characteristics of the particle determine whether or not opsonization will take place and which component will be involved. As a consequence, the mechanism of particle-cell interaction will also depend on the nature of the opsonic component and the relevant receptor-mediated process (Wright and Illum 2000).
To avoid phagocytic uptake and to modulate biodistribution parameters of drugs for their long blood circulation, surface properties of colloidal drug carriers can be modified by using various techniques. Of the many techniques used to enhance the blood circulation time of particles, surface coating with polyethylene glycol (PEG) polymers has been reported to be successful (Yuda et al 1996; Heiati et al 1998; Dierling et al 2006). Abuchowski et al (1977) decreased immunological properties of bovine serum albumin by using PEG. This technique has started to be used to decrease the immunogenicity of proteins including enzymes such as superoxide dismutase (Gray and Stull 1983), arginase (Savoca et al 1984) and asparaginase (Abuchowski et al 1984; Wright and Illum 2000). A decrease was observed in recognition of PEG-modified proteins by macrophages. The same mechanism was thought to be used for particles to increase their biodistribution. When the particles are coated with PEG, it can favourably modify the surface hydrophobicity of particles and sterically stabilize them, thus suppressing the binding of serum proteins (eg, apoproteins) and other opsonic factors (Heurtault et al 2003; Garcia-Fuentes et al 2005a; Dierling et al 2006). Sterically stabilized SLN have also been called stealth SLN, but conversely non-stealth SLN by various research groups (Bocca et al 1998; Fundaro et al 2000).
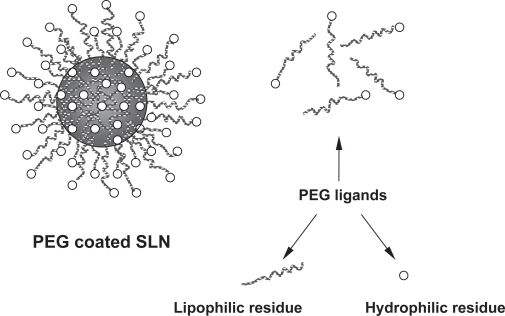
PEG derivatives at various molecular weights which are block copolymers, chemically contain hydrophilic and hydrophobic residues that give them amphiphilic characteristics. In an aqueous PEG coated SLN dispersion, soluble hydrophilic residue of PEG turns to the water phase and insoluble hydrophobic residue is oriented on the lipophilic SLN by formation of a shell around it (Figure 3).
Figure 3.
Schematic representation of SLN coated with PEG and molecular residues of PEG.
Effect of PEG coating on physical properties of SLN and bioavailability of drugs
In general, surface modification of colloidal particles by coating with a hydrophilic substance like PEG reported to bring following benefits
– Providing good physical stability and dispersability of colloids (Acar et al 2005; Zhang and Zhang 2005),
– Improving presence of colloids in blood circulation for systemic use (Moghimi et al 1994; Gref et al 1995),
– Increasing stability of colloids in body fluids such as GI fluids (Garcia-Fuentes et al 2005a),
– Acceleration of colloid transport accross the epithelium (De Campos et al 2003; Prego et al 2005),
– Modulation of interaction of colloids with mucosa for specific delivery requirements and drug targeting (Otsuka et al 2003; Oyewumi et al 2004),
– Increasing biocompatibility and decreasing thrombogenicity of drug carriers (Otsuka et al 2003; Zhang and Zhang 2005).
– Providing reservoir function to colloid particles carrying hydrophobic drugs due to hydrophilic coating around the particles (Lundberg et al 1996; Heiati et al 1998).
Coating materials are mostly adsorbed on the surface of the nanoparticles. Stability of coated particles also depends on the strength of the bond between particle and coating. In case of PEG, it attaches covalently to the nanoparticle surface and provides a hydrophilic steric barrier around SLN (Acar et al 2005). This protective layer prevents agglomeration during the production and/or storage, and subsequently, improves physical stability and dispersability of inner phase. Concentration of stealth agents strongly affects the rate of the phagocytic uptake. There is an inverse relationship between concentration and uptake. Bocca et al (1998) studied the phagocytic uptake of PEG-coated dipalmitoylphosphatidylamine and stearic acid SLNs by murine macrophages. They reported a significant increase in particle size related to the PEG concentration in the formulations and a decrease in murine uptake compared with non-stealth SLN. Optimum particle size for lymphatic uptake was reported to be between 10 and 100 nm and to be selective and slower as the particle size increases (Swartz 2001). In general, increase in particle size by coating nanoparticles with stabilizing hydrophilic molecules leads to extended blood circulation of SLN. Various researchers also indicated that the colloidal carrier systems are required to possess smaller size, additionally hydrophobicity and strong negative surface charge for effective penetration into lymphatic interstitium (Porter 1997; Harivardhan Reddy et al 2005).
Heiati et al (1998) determined that PEG coating did not changed the particle size of azidothymidine loaded trilaurin SLN in their study. The hydrophilic character of coating prolonged the release of lipophilic azidothymidine here. PEG coating further enhanced the blood level of the drug compared to non-stealth SLN and decreased urinary excretion in mice.
Fundaro et al (2000) also observed no change in the particle size of SLN. However, the hydrophilic surface characteristic of the particles which was provided by PEG, reduced the phagocytic recognition in rats. Correlation between hydrophobicity and the rate of phagocytosis of colloidal particles was observed, as reported in various studies (Müller et al 1996a; Harivardhan Reddy et al 2005; Prego et al 2005). Although there was a slight alteration in particle size (from 80 nm to 90 nm), a five-fold enhancement of the doxorubicin peak plasma concentration was obtained by using SLN as a carrier, and a seven-fold enhancement for stealth SLN (Fundaro et al 2000). Highest drug concentration was observed in the lung with stealth SLN. In brain tissues, doxorubicin was determined after administration of stealth SLN while the drug was not determined with drug solution and nonstealth SLN.
Surface charge of a colloid was reported to strongly affect biodistribution and clearance rate of drugs in 1966. Thus it was altered by coating, so that plasma half-life of the colloids could be increased. Half-life of negatively charged colloids was generally found to be higher than that of positively charged colloids (Wilkins and Myers 1966). However, it should be noted that a strong negative charge or a strong positive charge usually leads to phagocytic uptake more rapidly than weak negative charge (Stossel et al 1972). Zeta potential which indicates the surface characteristics, is greatly affected by the nature of coating. A study which investigated the surface modification of PEG-coated SLN, uncoated tripalmitin SLN presented a negative charge due to the anionic ingredients in their composition (Garcia-Fuentes et al 2005a). When they were coated with PEG, the zeta potential showed a less negative value than that of non-stealth SLN. PEG altered zeta potential from −50.3 to −34.8 in the negative range. The presence of PEG on the surface of SLN partially masked the negative charge of the uncoated particles. This effect is common for PEG-coated nanoparticles due to an extension of the plane of shear of the nanoparticles (Gref et al 2000). Similar results were found in various studies on drug carriers (Tröster et al 1992; Lundberg et al 1996; Vila et al 2004). In Bocca’s study, zeta potential of the stealth SLN (between −38.0 and −32.5 mV) was reported to be lower than those of the non-stealth SLN (−47.5 mV) indicating the presence of PEG chains on the surface (Bocca et al 1998).
Administration routes of solid lipid nanoparticles (SLN) and future direction of research
Parenteral administration
Peptide and protein drugs are usually available for parenteral use in the market. Their conventional oral administration is not possible due to enzymatic degradation in GI tract. Repeated parenteral administration is necessary since their half-lives are too short (a few minutes). To solve the problems, improve patient compliance and provide an effective treatment, researchers have studied non-parenteral administration routes such as transdermal and nasal for years. As an alternative, the development of parenteral drug carriers, which will provide controlled drug release over a month or longer, has been attempted. SLN are promising colloidal drug carriers among many other carriers such as their polymeric counterparts and liposomes. Wissing et al (2004) intensively reviewed parenteral use of SLN. SLN are very suitable for systemic delivery because they consist of physiologically well-tolerated ingredients and they have good storage capabilities after lyophilization and/or sterilization. When injected intravenously, SLN are sufficiently small to circulate in the microvascular system and prevent macrophage uptake in case of hydrophilic coating. Therefore, SLN have been suggested for viral and non-viral gene delivery. Cationic SLN has been demonstrated to bind genes directly via electrostatic interactions, and to have potential benefits in targeted gene therapy in treatment of cancer. The charge of particles can also be modulated via the composition, thus allowing binding of oppositely charged molecules (Olbrich et al 2001; Tabatt et al 2004; Pedersen et al 2006). Moreover, coating of SLN with PEG increases stability and plasma half life of SLN in order to decrease phagocytic uptake, and therefore improves the biovailability of drugs.
Treatment of central nervous system diseases such as brain tumors, AIDS, neurological and psychiatric disorders is often constrained by the inability of potent drugs to pass blood-brain barrier (BBB), which is formed by the endothelium of the brain vessels, the basal membrane and neurological cells. Hydrophilic coating of colloids improves the transport of these through BBB and tissue distribution (Kreuter 2001; Wang et al 2002). Various researchers also reported that tumor microvessels were more permeable to macromolecules than normal blood vessels, and their lower selectivity to permeability was presumably due to larger pores in the vessel walls (Yuan et al 1995). Fundaro et al (2000) prepared doxorubicin loaded stealth and non-stealth SLN. They used PEG 2000 to stabilize stearic acid SLN and to modify their surface. They observed that the stealth nanoparticles were present in blood at higher concentrations than non-stealth SLN after 24 h following iv administration. Doxorubicin was determined at a detectable concentration in the brain only after administration of stealth SLN. The drug was more easily transported through the BBB in this case. Stealth SLN showed a lower clearance, a higher distribution volume, and a significantly higher AUC than non-stealth SLN. In the same study, doxorubicin concentration in tissues was determined to be higher in the lung and brain with stealth SLN, and in the liver, heart, kidney and spleen with non-stealth SLN. The decreased uptake by RES tissues (such as spleen and liver) of SLN increased the drug bioavailability in non-RES tissue targeting. Researchers have demonstrated that coated SLN with hydrophilic molecules carrying anticancer drugs can reach cancer cells in solid tumors more effectively than healthy tissues.
In general, parenteral application of SLN reduces the possible side effects of drug incorporated with increased bioavailability. These systems are very suitable for drug targeting. Excellent properties of SLN make them attractive drug carrier systems even for pharmaceutical companies. SLN products of several pharmaceutical companies can be given as follows: cationic solid lipid nanoparticles (SLN) for gene transfer namely TransoPlex® was produced by PharmaSol DDS (Germany) (Olbrich et al 2001; www.pharmasol-berlin.de). AlphaRx (USA) is developing vancomycin and gentamicin products with Vansolin™ and Zysolin™ trade names (www.alpharx.com). They are very effective in treatment of life-threatening infectious disease such as pneumonia. The intention of incorporating them into SLN has been to increase their efficacy while reducing their side effects. SkyePharma (UK) also have formulations of nanoparticulate technology which includes nanosuspensions and solid lipid nanoparticles under preclinical development (Powers 2005; www.skyepharma.com).
Oral administration
The use of submicron-size particular systems in oral drug delivery, especially peptide drugs, has attracted considerable pharmaceutical interest. Controlled release behavior of these systems is reported to enable the bypass of gastric and intestinal degradation of the encapsulated drug (Damgé et al 1990; Olbrich et al 2002), and their possible uptake and transport through the intestinal mucosa (Tobio et al 2000). However, the assessment of the stability of colloidal carriers in GI fluids is essential in order to predict their suitability for oral administration. Critical parameters have been widely overlooked in the design of new and efficent colloidal drug carrier systems for oral use: I) their stability upon contact with GI fluids since they are composed of biodegredable materials and particle size in nanorange maximizes the surface area for enzymatic degradation (Müller et al 1996b), II) particle aggregation due to environmental conditions of the GI tract leading decrease in the interaction capability of particles with the intestinal mucosa (Jani et al 1990).
The oral administration of SLN has been performed, but no data has been published so far concerning stability of SLN in the GI tract. SLN were introduced as a novel drug carrier system for oral delivery in the middle of 1990s (Runge et al 1996). The adhesive properties of nanoparticles are reported to increase bioavailability and reduce or minimize erratic absorption (Ponchel et al 1997). Absorption of nanoparticles occurs through mucosa of the intestine by several mechanisms, namely through the Peyer’s patches, by intracellular uptake or by the paracellular pathway. Pinto and Müller (1999) incorporated SLN into spherical pellets and investigated SLN release for oral administration. SLN granulates or powders can be put into capsules, compressed into tablets or incorporated into pellets. For some of these applications, the conversion of the liquid dispersion into a dry product by spray-drying or lyophilization is useful, or often necessary (Freitas and Müller 1998; Pinto and Müller 1999). In an upcoming study, the influence of artificial GI media on the physical stability of SLN formulations was investigated and it was concluded that it was possible to produce stable SLN dispersions by optimizing the ingredients in the formulation (Zimmermann and Müller 2001). Tobramycin which is not absorbed in solution form, was incorporated into SLN (Bargoni et al 2001). SLN changed the pharmacokinetics of the drug after being administered to rats by the duodenal route. AUC was higher than that of tobramycin in SLN or in solution administered by the iv route.
Hydrophilic coating as a successful technique provides protection of biodegradable colloidal carriers from enzymatic degradation and aggregation, favours the interaction with epithelia, thus prolonging the efficiency of drugs. Antitubercular drugs (rifampicin, isoniazid and pyrazinamide) were entrapped into polyvinyl alcohol coated SLN and following a single oral administration to mice, therapeutic drug concentrations were maintained in the plasma for 8 days and in the organs (lungs, liver and spleen) for 10 days (Pandey et al 2005). A recent report of Garcia-Fuentes et al (2002) showed that the surface modification with PEG-stearate led to a significant improvement in the stability and resistance to lipolytic enzymes. Various studies have indicated that PEG coating of colloids leads to increased attention to their oral use, especially in delivery of peptide drugs. For instance, oral calcitonin delivery via SLN was attempted by coating particles with hydrophilic substances including PEG (Garcia-Fuentes et al 2005a and 2005b). The results showed that the nature of the coating might affect the surface association and, hence, the immediate release of the peptide. It could provide a continuous delivery of the associated peptide and improved the drug bioavailability.
Various companies are interested in solid lipid nanotechnology for oral drug delivery. Pharmatec (Italy) developed a cyclosporine SLN formulation for oral administration (Müller et al 1998; Radtke and Müller 2001). Avoidance of high plasma peak and low variability in plasma profile were provided in this case. AlphaRx have also rifampicin-loaded SLN under preclinical phase (Rifamsolin™) (www.alpharx.com). Rifampicin is mainly used to treat tuberculosis, which requires long-term treatment due to poor cellular antibiotic penetration. AlphaRx aims to deliver this drug inside the human cell, to increase its efficacy and as a result to increase patient compliance.
Rectal administration
When rapid pharmacological effect is required, in some circumstances, parenteral or rectal administration is preferred. Conventional rectal delivery of drugs is also very often used for pediatric patients all over the world due to easy application. In the meantime, plasma levels and therapeutic efficacy of rectally administered drugs were reported to be higher compared with those given orally or intramuscularly in the same dose (Kanto 1975; Sznitowska et al 2001). A few reports are available on the rectal drug administration via SLN in the literature (Sznitowska et al 2000). Sznitowska et al (2001) incorporated diazepam into SLN for rectal administration in order to provide a rapid action. They applied SLN dispersions on rabbits and performed bioavailability studies. They found that lipid matrix which is solid at body temperature is not an advantageous system for diazepam rectal delivery. They decided to employ lipids which melt around body temperature in their next experiments. This area seems very open to investigation, especially when the benefits of rectal route are taken into consideration. PEG coating seems to be a promising approach on rectal delivery and consequently, enhancement of bioavailability.
Nasal administration
Nasal administration was a promising alternative non-invasive route of drug administration due to fast absorption and rapid onset of drug action, avoiding degradation of labile drugs (such as peptides and proteins) in the GI tract and insufficient transport across epithelial cell layers (Lee et al 1994). In order to improve drug absorption through the nasal mucosa, approaches such as formulation development and prodrug derivatization have been employed. SLN has been proposed as alternative transmucosal delivery systems of macromolecular therapeutic agents and diagnostics by various research groups (Müller and Keck 2004; Prego et al 2005). Additionally, hydrophilic coating of SLN will permit the interaction and transport of SLN through the nasal mucosa and therefore bring great benefits and compliance as nasal drug carriers especially for vaccines. In a recent report, coating polymeric nanoparticles with PEG gave promising results as vaccine carriers (Vila et al 2004). Hydrophilic particles are preferable to hydrophobic ones in terms of their utility as mucosal carriers. The role of PEG coating of polylactic acid nanoparticles in improving the transmucosal transport of the encapsulated bioactive molecules was reported to be successful by Tobio et al (1998). This concept can be useful for solid lipid nanoparticles.
Respiratory delivery
The lungs offer a high surface area for drug absorption by avoiding first-pass effects. Rapid drug absorption by aerosolization of drugs (in the 1–3 μm size range) occurs since the walls of alveoli in the deep lung are extremely thin (Agu et al 2001; Banga 2003). The nebulization of SLN is a new and upcoming area of research. Lymphatic drainage plays an important role in the uptake of particulates in the respiratory system. SLN can be proposed as carriers of anticancer drugs in lung cancer treatment or peptide drugs to improve their bioavailability. Assessment of inhaled radio-labelled SLN biodistribution has been described and the data showed an important and significant uptake of the radio-labelled SLN into the lymphatics after inhalation (Videira et al 2002). A high rate of distribution in periaortic, axillar and inguinal lymph nodes was observed indicating that SLN could be effective colloidal carriers for lymphoscintigraphy or therapy upon pulmonary delivery. In a recent study, antitubercular drugs (rifampicin, isoniazid and pyrazinamide) were incorporated into various formulations of solid lipid particles ranged from 1.1–2.1 μm and formulations were nebulized to guinea pigs by mouth for direct pulmonar delivery (Pandey and Khuller 2005). Nebulization of solid lipid particles carrying antitubercular drugs was observed to be successful in improving drug bioavailability and reducing the dosing frequency for better management of pulmonary tuberculosis.
Ocular administration
Colloidal drug delivery systems are considered to enhance the ocular bioavailability of drugs (De Campos 2001; Barbault-Foucher et al 2002; Ludwig 2005). Polymeric nanoparticles have been prepared for this aim, even benefits of PEG coating on the bioavailability has been investigated. The importance of surface characteristics of nanoparticles in their interaction with ocular mucosa has been discussed (De Campos et al 2003). Ocular drug administration via SLN has been reported several times (Cavalli et al 1995; Friedrich et al 2005). Biocompatibility and muco-adhesive properties of SLN improve their interaction with ocular mucosa and prolong corneal residence time of the drug, with the aim of ocular drug targeting. Cavalli et al (2002) evaluated SLN as carriers for ocular delivery of tobramycin in rabbit eyes. Drug concentration in the aqueous humor was determined up to six hours. As a result SLN significantly enhanced the drug bioavalability in the aqueous humor. Cavalli et al (1995) also studied pilocarpin delivery via SLN which is commonly used in glaucoma treatment, earlier. They reported very similar results in order to enhance the ocular bioavailability of drug.
Another research group incorporated poorly water-soluble drugs (hydrocortisone, estradiol hemihydrate and pilocarpine base) into SLN and performed in vitro drug permeation study through human organotypical cornea construct (Friedrich et al 2005). They observed high loading capacity, because drugs were nearly completely incorporated within the nanoparticles due to their high lipophilic character. Consequently, permeation studies indicated prolonged drug release in all the formulations.
In industrial fields, the incorporation of several antibiotics has been attempted in SLN, due to their broad antimicrobial spectrum. For an instance, Ocusolin™ from AlphaRx is a gentamicin loaded-SLN product in the form of ophthalmic solution. It is still under preclinical development (www.alpharx.com).
Topical application
SLN and NLC are very attractive colloidal carrier systems for skin applications due to their various desirable effects on skin besides the characteristics of a colloidal carrier system. They are well suited for use on damaged or inflamed skin because they are based on non-irritant and non-toxic lipids (Wissing and Müller 2003). Researchers have reported intensively on the topical application of SLN. During the last few years, SLN and NLC have been studied with active compounds such as vitamin E (Dingler et al 1999), tocopherol acetate (Wissing and Müller 2001), retinol (Jenning et al 2000a), ascorbyl palmitate (Üner et al 2005a and 2005b), clotrimazole (Souto et al 2004), triptolide (Mei et al 2003), phodphyllotoxin (Chen et al 2006) and a nonsteroidal antiandrogen RU 58841 (Münster et al 2005) for topical application.
Chemisches Laboratorium Dr. Kurt Richter (Germany) introduced a NCL formulation containing black currant seed oil for regenerative care of scaly and aged skin (NanoLipid Restore™) in the German market (www.clr-berlin.de).
Conclusion
SLN constitute an attractive colloidal drug carrier system due to successful incorporation of active compounds and their related benefits. Their benefits are still being demonstrated and new approaches are introduced. SLN offer an economical and patient-friendly device for administration of drugs by various routes. Coating of SLN with hydrophilic substances is very promising in the treatment of various diseases such as cancer and tuberculosis. Reports on surface modification of SLN by PEG coating have distinctly increased attention of various research groups with the aim of improving drug bioavailability. The concept of surface modification is further increasing the importance of SLN among traditional colloidal drug carrier systems.
References
- Abuchowski A, Van Es T, Palczuk NC, et al. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977;252:3578–81. [PubMed] [Google Scholar]
- Abuchowski A, Kazo GM, Verhoest CR, et al. Cancer therapy with chemically modified enzymes. I. Antitumor properties of poly-ethylene glycol asparaginase conjugates. Cancer Biochem Biophys. 1984;7:175–86. [PubMed] [Google Scholar]
- Acar HY, Garaas RS, Syud F, et al. Superparamagnetic nanoparticles stabilized by polimerized PEGylated coatings. J Magnet Magnet Mater. 2005;293:1–7. [Google Scholar]
- Agu RU, Ugwoke MI, Armand M, et al. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respir Res. 2001;2:198–209. doi: 10.1186/rr58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga AK. Deivery of protein therapeutics. Business Briefing: Pharmatech. 2003:198–201. [Google Scholar]
- Barbault-Foucher S, Gref R, Russo P, et al. Design of poly-ɛ-caprolactone nanospheres coated with bioadhesive hyaluronic acid for ocular delivery. J Control Rel. 2002;83:365–75. doi: 10.1016/s0168-3659(02)00207-9. [DOI] [PubMed] [Google Scholar]
- Bargoni A, Cavalli R, Zara GP, et al. Transmucosal transport of tobramycin incorporated in solid lipid nanoparticles (SLN) after duodenal administration to rats. Part II – Tissue distribution. Pharm Res. 2001;43:497–502. doi: 10.1006/phrs.2001.0813. [DOI] [PubMed] [Google Scholar]
- Bocca C, Caputo O, Cavalli R, et al. Phagocytic uptake of fluorescent stealth and non-stealth solid lipid nanoparticles. Int J Pharm. 1998;175:185–93. [Google Scholar]
- Castelli F, Puglia C, Sarpietro MG, et al. Characterization of indomethacin-loaded lipid nanoparticles by differential scanning calorimetry. Int J Pharm. 2005;304:231–38. doi: 10.1016/j.ijpharm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Cavalli R, Morel S, Gasco MR, et al. Preparation and evaluation of colloidal lipospheres containing pilocarpine as ion pair. Int J Pharm. 1995;117:243–6. [Google Scholar]
- Cavalli R, Caputo O, Carlotti ME, et al. Sterilization and freeze-drying of drug-free and drug-loaded solid lipid nanoparticles. Int J Pharm. 1997;148:47–54. doi: 10.1016/s0378-5173(97)00222-6. [DOI] [PubMed] [Google Scholar]
- Cavalli R, Peira E, Caputo O, et al. Solid lipid nanoparticles as carriers of hydrocortisone and progesterone complexes with β-cyclodextrins. Int J Pharm. 1999;182:59–69. doi: 10.1016/s0378-5173(99)00066-6. [DOI] [PubMed] [Google Scholar]
- Cavalli R, Gasco MR, Chetoni P, et al. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm. 2002;238:241–5. doi: 10.1016/s0378-5173(02)00080-7. [DOI] [PubMed] [Google Scholar]
- Charcosset C, El-Harati A, Fessi H. Preparation of solid lipid nanoparticles using a membrane contactor. J Control Release. 2005;108:112–20. doi: 10.1016/j.jconrel.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Chen H, Chang X, Du D, et al. Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J Control Release. 2006;110:296–306. doi: 10.1016/j.jconrel.2005.09.052. [DOI] [PubMed] [Google Scholar]
- Cortesi R, Esposito E, Luca G, et al. Production of lipospheres as carriers for bioactive compounds. Biomaterials. 2002;23:2283–94. doi: 10.1016/s0142-9612(01)00362-3. [DOI] [PubMed] [Google Scholar]
- Damgé C, Michel C, Aprahamian M, et al. Nanocapsules as carriers for oral peptide delivery. J Control Release. 1990;13:233–9. [Google Scholar]
- De Campos A, Sánchez A, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the ocular retention of drugs. Application to cyclosporin A. Int J Pharm. 2001;224:159–68. doi: 10.1016/s0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- De Campos AM, Sánchez A, Gref R, et al. The effect of a PEG versus a chitosan coating on the interaction of drug colloidal carriers with the ocular mucosa. Eur J Pharm Sci. 2003;20:73–81. doi: 10.1016/s0928-0987(03)00178-7. [DOI] [PubMed] [Google Scholar]
- De Vringer T, De Ronde HAG. Preparation and structure of a water-in-oil cream containing lipid nanoparticles. J Pharm Sci. 1995;84:466–72. doi: 10.1002/jps.2600840415. [DOI] [PubMed] [Google Scholar]
- Demirel M, Yazan Y, Müller RH, et al. Formulation and in vitro-in vivo evaluation of piribedil solid lipid micro- and nanoparticles. J Microencapsul. 2001;18:359–71. doi: 10.1080/02652040010018119. [DOI] [PubMed] [Google Scholar]
- Dierling AM, Sloat BR, Cui Z. Gadolinium incorporated reconstituted chylomicron emulsion for potential application in tumor neutron capture therapy. Eur J Pharm Biopharm. 2006;62:275–81. doi: 10.1016/j.ejpb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Dingler A.1998Feste Lipid-nanopartikel als kolloidale Wirkstoffträgersysteme zur dermalen ApplikationPhD thesis,Freie Universität Berlin [Google Scholar]
- Dingler A, Blum RP, Niehus H, et al. Solid lipid nanoparticles (SLN™/Lipopearls™) – a pharmaceutical and cosmetic carrier for the application of vitamin E in dermal products. J Microencapsul. 1999;16:751–67. doi: 10.1080/026520499288690. [DOI] [PubMed] [Google Scholar]
- Freitas C, Müller RH. Spray-drying of solid lipid nanoparticles (SLN™) Eur J Pharm Biopharm. 1998;46:145–51. doi: 10.1016/s0939-6411(97)00172-0. [DOI] [PubMed] [Google Scholar]
- Friedrich I, Reichl S, Müller-Goymann CC. Drug release and permeation studies of nanosuspensions based on solidified reverse micellar solutions (SRMS) Int J Pharm. 2005;305:167–75. doi: 10.1016/j.ijpharm.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Fundaro A, Cavalli R, Bargoni A, et al. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: pharmacokinetics and tissue distribution after iv administration to rats. Pharm Res. 2000;42:337–43. doi: 10.1006/phrs.2000.0695. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuentes M, Torres D, Alonso MJ. Design of lipid nanoparticles for the oral delivery of hydrophilic macromolecules. Coll Surf B: Biointerf. 2002;27:159–68. [Google Scholar]
- Garcia-Fuentes M, Prego C, Torres D, et al. A comparative study of the potential of solid glyceride nanostructures coated with chitosan or poly(ethylene glycol) as carriers for oral calcitonin delivery. Eur J Pharm Sci. 2005a;25:133–43. doi: 10.1016/j.ejps.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuentes M, Torres D, Alonso MJ. New surface-modified lipid nanoparticles as delivery vehicles for salmon calcitonin. Int J Pharm. 2005b;296:122–32. doi: 10.1016/j.ijpharm.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia E, Andrieux K, Gil S, et al. A methodology to study intracellular distribution of nanoparticles in brain endothelial cells. Int J Pharm. 2005;298:310–4. doi: 10.1016/j.ijpharm.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Gasco MR.1993Method for producing solid lipid microspheres having a narrow size distributionUS Patent 5 250 236.
- Gohla SH, Dingler A. Scaling up feasibility of the production of solid lipid nanoparticles (SLN™) Pharmazie. 2001;56:61–3. [PubMed] [Google Scholar]
- Göppert TM, Müller RH. Adsorption kinetics of plasma proteins on solid lipid nanoparticles for drug targeting. Int J Pharm. 2005;302:172–86. doi: 10.1016/j.ijpharm.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Gray BH, Stull RW. Radioprotection by polyethylene glycol-protein complexes in mice. Radiat Res. 1983;93:581–7. [PubMed] [Google Scholar]
- Gref R, Domb A, Quellec P, et al. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv Drug Del Rev. 1995;16:215–33. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gref R, Lück M, Quellec P, et al. ‘Stealth’ corona-core nanoparticle surface modified by polyethylene glycol (PEG): influence of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Coll Surf B: Biointerf. 2000;18:301–13. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- Harivardhan Reddy L, Sharma RK, Chuttani K, et al. Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice. J Control Release. 2005;105:185–98. doi: 10.1016/j.jconrel.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Heiati H, Tawashi R, Shivers RR, et al. Solid lipid nanoparticles as drug carriers I. Incorporation and retention of the lipophilic prodrug 3′-azido-3′-deoxythymidine palmitate. Int J Pharm. 1997;146:123–31. [Google Scholar]
- Heiati H, Tawashi R, Phillips NC. Solid lipid nanoparticles as drug carriers II. Plasma stability and biodistribution of solid lipid nanoparticles containing the lipophilic prodrug 3′-azido-3′-deoxythymidine palmitate in mice. Int J Pharm. 1998;174:71–80. [Google Scholar]
- Heurtault B, Saulnier P, Pech B, et al. Physico-chemical stability of colloidal lipid particles. Biomaterials. 2003;24:4283–300. doi: 10.1016/s0142-9612(03)00331-4. [DOI] [PubMed] [Google Scholar]
- Hou DZ, Xie CS, Huang K, et al. The production and characteristics of solid lipid nanoparticles (SLNs) Biomaterials. 2003;24:1781–85. doi: 10.1016/s0142-9612(02)00578-1. [DOI] [PubMed] [Google Scholar]
- Hu FQ, Jiang SP, Du YZ, et al. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Coll Surf B: Biointerf. 2005;45:167–73. doi: 10.1016/j.colsurfb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Igartua M, Saulnier P, Heurtault B, et al. Development and characterization of solid lipid nanoparticles loaded with magnetite. Int J Pharm. 2002;233:149–57. doi: 10.1016/s0378-5173(01)00936-x. [DOI] [PubMed] [Google Scholar]
- Illum L, Davis SS, Müller RH, et al. The organ distribution and circulation time of intravenously injected colloidal carriers sterically stabilized with a block copolymer poloxamine 908. Life Sci. 1987;40:367–70. doi: 10.1016/0024-3205(87)90138-x. [DOI] [PubMed] [Google Scholar]
- Jani PU, Halbert GW, Langridge J, et al. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol. 1990;42:821–6. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- Jenning V, Gysler A, Schäfer-Korting M, et al. Vitamin A-loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm. 2000a;49:211–8. doi: 10.1016/s0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- Jenning V, Mäder K, Gohla SH. Solid lipid nanoparticles (SLN™) based on binary mixtures of liquid and solid lipids: a 1H-NMR study. Int J Pharm. 2000b;205:15–21. doi: 10.1016/s0378-5173(00)00462-2. [DOI] [PubMed] [Google Scholar]
- Jores K, Mehnert W, Dreschler M, et al. Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J Control Release. 2004;95:217–7. doi: 10.1016/j.jconrel.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Kakizawa Y, Kataoka K. Block copolymer micelles for delivery of gene and related compounds. Adv Drug Deliv Rev. 2002;54:203–2. doi: 10.1016/s0169-409x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Kanto J. Plasma concentrations of diazepam and its metabolites after peroral, intramuscular and rectal administration. Int J Clin Pharmacol. 1975;12:419–26. [PubMed] [Google Scholar]
- Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- Kržič M, Šentjurc M, Kristl J. Improved skin oxygenation after benzyl nicotinate application in different carriers as measured by EPR oximetry in vivo. J Control Release. 2001;70:203–11. doi: 10.1016/s0168-3659(00)00351-5. [DOI] [PubMed] [Google Scholar]
- Lee WA, Ennis RD, Longenecker JP, et al. The bioavailability of intranasal salmon calcitonin in healthy volunteers with and without permeation enhancer. Pharm Res. 1994;11:747–50. doi: 10.1023/a:1018992716621. [DOI] [PubMed] [Google Scholar]
- Liedtke S, Wissing SA, Müller RH, et al. Inflence of high pressure homogenisation equipment on nanodispersions characteristics. Int J Pharm. 2000;196:183–5. doi: 10.1016/s0378-5173(99)00417-2. [DOI] [PubMed] [Google Scholar]
- Lippacher A, Müller RH, Mäder K. Semisolid SLN™ dispersions for topical application: influence of formulation and production parameters on viscoelastic properties. Eur J Pharm Biopharm. 2002;53:155–60. doi: 10.1016/s0939-6411(01)00233-8. [DOI] [PubMed] [Google Scholar]
- Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Del Rev. 2005;57:1595–639. doi: 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Lundberg BB, Mortimer BC, Redgrave TG. Submicron lipid emulsions containing amphipathic poly(ethylene glycol) for use as drug carriers with prolonged circulation time. Int J Pharm. 1996;134:119–27. [Google Scholar]
- Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J Control Release. 2005;107:215–28. doi: 10.1016/j.jconrel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Mehnert W, Mäder K. Solid lipid nanoparticles. Production, characterization and applications. Adv Drug Del Rev. 2001;47:165–96. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Mei Z, Chen H, Weng T, et al. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur J Pharm Biopharm. 2003;56:189–96. doi: 10.1016/s0939-6411(03)00067-5. [DOI] [PubMed] [Google Scholar]
- Moghimi SH, Hawley AE, Christy NM, et al. Surface engineered nanospheres with enhanced drainage into lymphatics and uptake by macrophages of the lymph nodes. FEBS Lett. 1994;344:25–30. doi: 10.1016/0014-5793(94)00351-3. [DOI] [PubMed] [Google Scholar]
- Morel S, Terreno E, Ugazio E, et al. NMR relaxometric investigations of solid lipid nanoparticles (SLN) containing gadolinium (III) complexes. Eur J Pharm Biopharm. 1998;45:157–63. doi: 10.1016/s0939-6411(97)00107-0. [DOI] [PubMed] [Google Scholar]
- Müller RH. Colloidal carriers for controlled drug delivery and targeting-modification, characterization and in vivo distribution. Wissenschaftliche Verlagsgesellschaft Stuttgart, CRC Press Boca Raton; 1991. [Google Scholar]
- Müller RH, Schwarz C, Zur Mühlen A, et al. Incorporation of lipo-philic drugs and drug release profiles of solid lipid nanoparticles (SLN) Proc Int Symp Control Rel Bioact Mater. 1994;21:146–7. [Google Scholar]
- Müller RH, Mehnert W, Lucks JS, et al. Solid lipid nanoparticles (SLN) – An alternative colloidal carrier system for controlled drug delivery. Eur J Pharm Biopharm. 1995;41:62–9. [Google Scholar]
- Müller RH, Maaβen S, Weyhers H, et al. Phagocytic uptake and cytotoxicity of solid lipid nanoparticles (SLN) sterically stabilized with Poloxamine 908 and Poloxamer 407. J Drug Target. 1996a;4:161–70. doi: 10.3109/10611869609015973. [DOI] [PubMed] [Google Scholar]
- Müller RH, Rühl D, Runge S. Biodegradation of solid lipid nanoparticles as a function of lipase incubation time. Int J Pharm. 1996b;144:115–21. [Google Scholar]
- Müller RH, Hildebrand GE. Feste Lipidnanopartikel (SLN) In: Müller RH, editor. Pharmazeutische Technologie: Moderne Arzneiformen. Wissenschaftliche Verlagsgesellschaft Stuttgart; 1997. pp. 265–72. [Google Scholar]
- Müller RH, Rühl D, Lück M, et al. Influence of fluorescent labelling of polystyrene particles on phagocytic uptake, surface hydrophobicity, and plasma protein adsorption. Pharm Res. 1997;14:18–24. doi: 10.1023/a:1012043131081. [DOI] [PubMed] [Google Scholar]
- Müller RH, Runge SA. Solid lipid nanoparticles (SLN®) for controlled drug delivery. In: Benita S, editor. Submicron emulsion in drug targeting and delivery. The Netherlands: Harwood Academic Publishers; 1998. pp. 219–34. [Google Scholar]
- Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–77. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002a;54(Suppl. 1):S131–55. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- Müller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002b;242:121–8. doi: 10.1016/s0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- Müller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs – a review of drug nanocrystal technology and lipid nanoparticles. J Biotech. 2004;113:151–70. doi: 10.1016/j.jbiotec.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Münster U, Nakamura C, Haberland A, et al. RU 58841-myristate – prodrug development for topical treatment of acne and androgenic alopecia. Pharmazie. 2005;60:8–12. [PubMed] [Google Scholar]
- Olbrich C, Bakowski U, Lehr CM, et al. Cationic solid-lipid nanoparticles can efficiently bind and transfect plasmid DNA. J Control Release. 2001;77:345–55. doi: 10.1016/s0168-3659(01)00506-5. [DOI] [PubMed] [Google Scholar]
- Olbrich C, Kayser O, Müller RH. Lipase degradation of Dynasan 114 and 116 solid lipid nanoparticles (SLN) – effect of surfactants, storage time and crystallinity. Int J Pharm. 2002;237:119–28. doi: 10.1016/s0378-5173(02)00035-2. [DOI] [PubMed] [Google Scholar]
- Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55:403–19. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- Oyewumi MO, Yokel RA, Jay M, et al. Comparison of cell uptake, biodistribution and tumor retention of folate-coated and PEG-coated gadolinium nanoparticles in tumor-bearing mice. J Control Release. 2004;95:613–26. doi: 10.1016/j.jconrel.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Pandey R, Khuller GK. Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis. 2005;85:227–34. doi: 10.1016/j.tube.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Pandey R, Sharma S, Khuller GK. Oral solid lipid nanoparticle-based antitubercular chemotherapy. Tuberculosis. 2005;85:415–20. doi: 10.1016/j.tube.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Pedersen N, Hansen S, Heydenreich AV, et al. Solid lipid nanoparticles can effectively bind DNA, streptavidin and biotinylated ligands. Eur J Pharm Biopharm. 2006;62:155–62. doi: 10.1016/j.ejpb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Pinto JF, Müller RH. Pellets as carriers of solid lipid nanoparticles (SLN) for oral administration of drugs. Pharmazie. 1999;54:506–9. [Google Scholar]
- Ponchel G, Montisci MJ, Dembri A, et al. Mucoadhesion of colloidal particulate systems in the gastro-intestinal tract. Eur J Pharm Biopharm. 1997;44:25–31. [Google Scholar]
- Porter CJH. Drug delivery to the lymphatic system. Crit Rev Ther Drug Carr Syst. 1997;14:333–93. [PubMed] [Google Scholar]
- Powers M. Innovata emerges as first suitor in acquisition play for SkyePharma. Nanobiotech News. 2005;3(45):1. [Google Scholar]
- Prego C, García M, Torres D, et al. Transmucosal macromolecular drug delivery. J Control Release. 2005;101:151–62. doi: 10.1016/j.jconrel.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Quintanar-Guerrero D, Tamayo-Esquivel D, Ganem-Quintanar A, et al. Adaptation and optimization of the emulsification-diffusion technique to prepare lipidic nanospheres. Eur J Pharm Sci. 2005;26:211–8. doi: 10.1016/j.ejps.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Radtke M, Müller RH. Nanostructured lipid carriers: the new generation of lipid drug carriers. New Drugs. 2001;2:48–52. [Google Scholar]
- Runge S, Mehnert W, Müller RH. SLN (solid lipid nanoparticles), a novel formulation for the oral administration of drugs. Eur J Pharm Sci. 1996;4(Suppl 1):S132. [Google Scholar]
- Savoca KV, Davis FF, Van Es T, et al. Cancer therapy with chemically modified enzymes. II. The therapeutic effectiveness of arginase, and arginase modified by the covalent attachment of polyethylene glycol, on the taper liver tumor and the L5178Y murine leukemia. Cancer Biochem Biophys. 1984;7:261–8. [PubMed] [Google Scholar]
- Schubert MA, Müller-Goyman CC. Solvent injection as a new approach for manufacturing lipid nanoparticles – evaluation of the method and process parameters. Eur J Pharm Biopharm. 2003;55:125–31. doi: 10.1016/s0939-6411(02)00130-3. [DOI] [PubMed] [Google Scholar]
- Schubert MA, Harms M, Müller-Goyman CC. Sructural investigations on lipid nanoparticles containing high amounts of lecitin. Eur J Pharm Sci. 2006;27:226–36. doi: 10.1016/j.ejps.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Schwarz C, Mehnert W, Lucks JS, et al. Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J Control Release. 1994;30:83–96. [Google Scholar]
- Schwarz C.1995Feste Lipidnanopartikel: Herstellung, Charakterisierung, Arzneistoffinkorporation und –freisetzung, Sterilisation und LyophilisationPhD thesis,Freie Universität Berlin [Google Scholar]
- Shahgaldian P, Da Silva E, Coleman AW, et al. Para-acyl-calix-arene based solid lipid nanoparticles (SLNs): a detailed study of preparation and stability parameters. Int J Pharm. 2003;253:23–38. doi: 10.1016/s0378-5173(02)00639-7. [DOI] [PubMed] [Google Scholar]
- Siekmann B, Westesen K. Submicron-sized parenteral carrier systems based on solid lipids. Pharm Pharmacol Lett. 1992;1:123–6. [Google Scholar]
- Sjostrom B, Bergenståhl B. Preparation of submicron drug particles in lecithin-stabilized o/w emulsions I. Model studies of the precipitation of cholesterylacetate. Int J Pharm. 1992;88:53–62. [Google Scholar]
- Song C, Liu S. A new healthy sunscreen system for human: Solid lipid nanoparticles as carrier for 3,4,5-trimethoxybenzoylchitin and the improvement by adding Vitamin E. Int J Biol Macromolecules. 2005;36:116–9. doi: 10.1016/j.ijbiomac.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Souto EB, Wissing SA, Barbosa CM, et al. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;278:71–7. doi: 10.1016/j.ijpharm.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Stossel TP, Mason RJ, Hartwig J, et al. Quantitative studies on phagocytosis by polymorphonuclear leucocyctes: use of paraffin oil emulsions to measure the rate of phagocytosis. J Clin Invest. 1972;51:615–24. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- Sznitowska M, Janicki S, Gajewska M, et al. Investigation of diazepam lipospheres based on Witepsol and lecithin for oral or rectal delivery. Acta Polon Pharm. 2000;57:61–4. [PubMed] [Google Scholar]
- Sznitowska M, Gajewska M, Janicki S, et al. Bioavailability of diazepam from aqueous-organic solution, submicron emulsion and solid lipid nanoparticles after rectal administration in rabbits. Eur J Pharm Biopharm. 2001;52:159–63. doi: 10.1016/s0939-6411(01)00157-6. [DOI] [PubMed] [Google Scholar]
- Tabatt K, Sameti M, Olbrich C, et al. Effect of cationic lipid and matrix lipid composition on solid lipid nanoparticle-mediated gene transfer. Eur J Pharm Biopharm. 2004;57:155–62. doi: 10.1016/j.ejpb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Tobio M, Gref R, Sánchez A, et al. Stealth PLA-PEG nanoparticles as protein carriers for nasal administration. Pharm Res. 1998;15:270–6. doi: 10.1023/a:1011922819926. [DOI] [PubMed] [Google Scholar]
- Tobio M, Sánchez A, Vila A, et al. The role of PEG on the stability in digestive fluids and in vivo fate of PEG-PLA nanoparticles following oral administration. Colloid Surf B: Biointerf. 2000;18:315–23. doi: 10.1016/s0927-7765(99)00157-5. [DOI] [PubMed] [Google Scholar]
- Tröster SD, Wallis KH, Müller RH, et al. Correlation of surface hydrophobicity of 14C-poly(methyl methacrylate) nanoparticles to the body distribution. J Control Release. 1992;20:247–53. [Google Scholar]
- Üner M, Wissing SA, Yener G, et al. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for application of ascorbyl palmitate. Pharmazie. 2005a;60:577–82. [PubMed] [Google Scholar]
- Üner M, Wissing SA, Yener G, et al. Investigation of skin moisturizing effect and skin penetration of ascorbyl palmitate entrapped in solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) incorporated into hydrogel. Pharmazie. 2005b;60:751–5. [PubMed] [Google Scholar]
- Üner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Pharmazie. 2006;61:375–86. [PubMed] [Google Scholar]
- Venkateswarlu V, Manjunath K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J Control Release. 2004;95:627–38. doi: 10.1016/j.jconrel.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Videira MA, Botelho MF, Santos AC, et al. Lymphatic uptake of pulmonary delivered solid lipid nanoparticles. J Drug Target. 2002;10:607–13. doi: 10.1080/1061186021000054933. [DOI] [PubMed] [Google Scholar]
- Vila A, Gill H, McCallion O, Alonso MJ. Transport of PLA-PEG particles across the nasal mucosa: effect of particle size and PEG coating density. J Control Release. 2004;98:231–44. doi: 10.1016/j.jconrel.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Wang JX, Sun X, Zhang ZR. Enhanced brain targeting by synthesis of 3′,5′-dioctanoyl-5-fluoro-2′-deoxyuridine and incorporation into solid lipid nanoparticles. Eur J Pharm Biopharm. 2002;54:285–90. doi: 10.1016/s0939-6411(02)00083-8. [DOI] [PubMed] [Google Scholar]
- Wilkins DJ, Myers PA. Studies on the relationship between the electrophoretic properties of colloids and their blood clearance and organ distribution in the rat. Br J Exp Pathol. 1966;47:568–76. [PMC free article] [PubMed] [Google Scholar]
- Wissing SA, Müller RH. A novel sunscreen system based on tocopherol acetate incorporated into solid lipid nanoparticles. Int J Cosm Sci. 2001;23:233–43. doi: 10.1046/j.1467-2494.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- Wissing SA, Müller RH. Cosmetic applications for solid lipid nanoparticles (SLN) Int J Pharm. 2003;254:65–8. doi: 10.1016/s0378-5173(02)00684-1. [DOI] [PubMed] [Google Scholar]
- Wissing SA, Kayser O, Müller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56:1257–72. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Wright JJ, Illum L. Active targeting of microcapsules and microspheres to specific regions. In: Donbrow M, editor. Microcapsules and nanoparticles in medicine and pharmacy. The United States of America: CRC Press, Inc; 2000. pp. 281–93. [Google Scholar]
- Yang SC, Lu LF, Cai Y, et al. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J Control Release. 1999a;59:299–307. doi: 10.1016/s0168-3659(99)00007-3. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhu J, Lu Y, et al. Body distribution of camptothecin solid lipid nanoparticles after oral administration. Pharm Res. 1999b;16:751–57. doi: 10.1023/a:1018888927852. [DOI] [PubMed] [Google Scholar]
- Yuan F, Dllian M, Fukumura D, et al. Vascular permeability in human tumor xenograft: molecular size dependence and cutoff size. J Cancer Res. 1995;55:3752–5. [PubMed] [Google Scholar]
- Yuda T, Maruyama K, Iwatsuru M. Prolongation of liposome circulation time by various derivatives of polyethyleneglycols. Biol Pharm Bull. 1996;19:1347–51. doi: 10.1248/bpb.19.1347. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang J. Surface modification of monodisperse magnetite nanoparticles for improved intracellular uptake to breast cancer cells. J Coll Interf Sci. 2005;283:352–57. doi: 10.1016/j.jcis.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Müller RH. Electrolyte- and pH-stabilities of aqueous solid lipid nanoparticle (SLN™) dispersions in artificial gastrointestinal media. Eur J Pharm Biopharm. 2001;52:203–10. doi: 10.1016/s0939-6411(01)00167-9. [DOI] [PubMed] [Google Scholar]
- Zur Mühlen A, Mehnert W. Drug release and release mechanisms of prednisolone loaded solid lipid nanoparticles. Pharmazie. 1998;53:552–5. [Google Scholar]
- Zur Mühlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery – Drug release and release mechanism. Eur J Pharm Biopharm. 1998;45:149–55. doi: 10.1016/s0939-6411(97)00150-1. [DOI] [PubMed] [Google Scholar]