Abstract
Nanotechnology has tremendously influenced gene therapy research in recent years. Nanometer-size systems have been extensively investigated for delivering genes at both local and systemic levels. These systems offer several advantages in terms of tissue penetrability, cellular uptake, systemic circulation, and cell targeting as compared to larger systems. They can protect the polynucleotide from a variety of degradative and destabilizing factors and enhance delivery efficiency to the cells. A variety of polymeric and non-polymeric nanoparticles have been investigated in an effort to maximize the delivery efficiency while minimizing the toxic effects. This article provides a review on the most commonly used nanoparticulate systems for gene delivery. We have discussed frequently used polymers, such as, polyethyleneimine, poly (lactide-co-glycolide), chitosan, as well as non-polymeric materials such as cationic lipids and metallic nanoparticles. The advantages and limitations of each system have been elaborated.
Keywords: polynucleotides, gene delivery, gene therapy, transfection efficiency, polymers, liposomes
Introduction
The field of gene-based medicine has witnessed great progress since the first somatic gene therapy performed in 1990. Several thousand patients have been involved in clinical trials all over the world with majority focusing on cancer (67%), followed by vascular diseases (8.9%) and monogenic diseases (8.6%) (http://www.wiley.co.uk/genetherapy/clinical). Gene therapy works on the basic concept that the delivery of polynucleotides to the cells will alter the expression of a given protein resulting in therapeutic benefit. Gene therapy involves delivering polynucleotides such as DNA, RNA, anti-sense oligonucleotides and small interfering RNA, either locally or systemically. Although Vitravene, an antisense oligonucleotide-based product, is the only gene delivery product approved so far by US-FDA, there are several other products in late stages of clinical trials. Gendicine, an adenovirus encoding tumor suppressor p53 gene, developed by SiBiono GeneTech Co., Ltd., was recently approved by China’s state food and drug administration, for the treatment of head and neck squamous cell carcinoma.
Delivering functional polynucleotides into cells is the first and most critical step towards efficient gene therapy. The administration of naked DNA resulted in local transient expression in skeletal muscle tissue (Wolff et al 1990). Efficient transfection levels have also been obtained on direct application of naked DNA to the liver (Hickman et al 1994). To obtain systemic effect with the injection of naked DNA is difficult, however, as the intravenous injection of naked DNA results in low levels of gene expression in all major organs.
In order to enhance uptake of genes into cells, they have to be delivered using a carriers or vectors. The vectors can be broadly classified as viral and non-viral. Viral vectors account for nearly 75% of all clinical trials conducted so far (http://www.wiley.co.uk/genetherapy/clinical). They are essentially viruses that have been stripped of their gene for replication while preserving their ability to transfect cells. The gene of interest is then incorporated into the viral genome. Most commonly used viral vectors are retrovirus, adenovirus, adeno-associated virus and herpes simplex virus (Robbins and Ghivizzani 1998; Walther and Stein 2000)). Virus based approaches are highly efficient as viruses have a highly evolved and specific mechanism for inserting their genome into that of host cell. Despite the high efficiency of viral vectors, their use has been limited by their pathogenicity, immunogenicity and potential for insertional mutagenesis.
Incidences of severe adverse reactions using viral vectors during clinical trials have caused a gradual shift towards non-viral vectors. Non-viral vectors mostly include use of polymers and lipids to deliver genetic material intracellularly protecting it from extracellular and intracellular degradative enzymes and blood components (De Laporte et al 2006). Non-viral vectors are mostly non-immunogenic, less expensive to produce, relatively safer, and can carry higher amounts of genetic material as compared to viruses. However, transfection efficiency using non-viral vectors remains lower as compared to viral vectors.
Challenges associated with non-viral gene delivery
Non-viral vectors face a multitude of barriers at systemic, tissue and cellular levels that prevent efficient gene delivery to the nucleus of cells. A significant portion of DNA is lost at each step, resulting in a several fold decrease in expression of the encoded protein. Most non-viral delivery vectors display colloidal instability which results in aggregation of the complexes thereby hindering cellular internalization. Systemic delivery of charged vector may result in interaction with blood components which may lead to opsonisation by the reticulo-endothelial system (Liu and Huang 2002). Problems such as steric instability and rapid plasma clearance have been partly overcome by shielding the surface charge of the vectors (Kommareddy et al 2005). Properties of vector such as size, shape, and surface characteristics can also have a major impact on its pharmacokinetic properties and delivery efficiency.
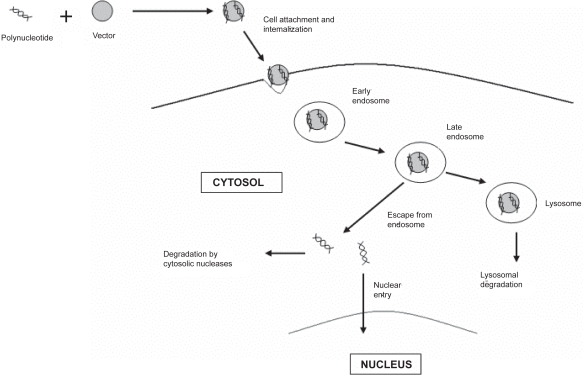
Non-viral vectors, also encounter several extracellular and intracellular barriers before reaching its final destination, the nucleus (Ruponen et al 2003; Wiethoff and Middaugh 2003). Typical pathway of a nanoparticulate vector inside the cell is depicted in Figure 1. The presence of nucleases in the extracellular compartment may lead to extensive degradation of the DNA. Cellular internalization is another major barrier as DNA is a polyanionic molecule with a large hydrodynamic volume which makes its entry through the negatively charged plasma membrane extremely difficult. Following uptake into the cell, the vector may become trapped within the endosomal compartment, from which it must escape. Failure to do so may result in degradation of DNA by lysosomal degradative enzymes. Surviving passage through the cytoplasm is a crucial step, because of the presence of cytosolic endonucleases, which may further lead to DNA fragmentation. Lastly, the DNA has to traverse the nuclear envelope to get transcribed. In order to enhance gene therapy, specialized design features are required for delivery vector to overcome each of these barriers, and ensure efficient DNA delivery to the nucleus.
Figure 1.
Schematic diagram of the entry of non-viral vector inside the nucleus of cell.
Nanoparticulate vectors for polynucleotide delivery
Nanotechnology has been at the forefront of drug and gene delivery in the last few years. Several types of nanometer scale devices such as nanoparticles, nanospheres, nanotubes, nanogels and molecular conjugates are being investigated (Lemieux et al 2000; Bianco 2004; Ravi Kumar et al 2004; Heidel et al 2005; Murakami and Nakashima 2006). Sub-micron size of delivery systems confers distinct advantages as compared to large sized systems such as higher and deeper tissue penetrability, greater cellular uptake, greater ability of cross blood-brain barrier, and greater ability to target specific cell types (Kreuter et al 1995; Davis 1997; Vinogradov et al 2002; Vogt et al 2006). Although this review, for most part, deals with polymeric nanoparticles, commonly used non-polymeric nanovectors have also been discussed briefly. A summary of all the nanoparticulate vectors discussed in the review is presented in Table 1.
Table 1.
Polymeric and non-polymeric nanoparticulate vectors for gene delivery
| Nanoparticles | Properties | References | |
|---|---|---|---|
| Poly lactide-co-glycolide (PLGA) and Poly lactic acid (PLA) | Biocompatible and biodegradable, can provide sustained delivery of polynucleotides. | Kim et al 2005; Ribiero et al 2005 | |
| Polyethyleneimine (PEI) | High transfection efficiency owing to faster endosomal escape. High toxicity reported in vitro and in vivo. | Moghimi et al 2005; Thomas et al 2005 | |
| Polymeric | Polymethacrylate | Lower toxicity as compared to PEI. Endosomal buffering ability similar to PEI. | Dubruel et al 2003; Feng et al 2006 |
| Poly-L-Lysine (PLL) | Biodegradable. Slow endosomal escape resulting in lower transfection efficiency | Merdan et al 2002; Zhang et al 2004 | |
| Poly (β-amino ester) (PBAE) | High transfection efficiency comparable to PEI. Prolonged release of polynucleotide. | Lynn and Langer 2000; Little et al 2005 | |
| Chitosan | Mucoadhesive property desired for oral and nasal delivery. Slow onset of expression. | Ferrari et al 1997; Koping-Hoggard et al 2001 | |
| Cationic liposomes | Extensively used for in vitro transfections. High in vivo toxicity | Felgner et al 1987; Tousignant et al 2000 | |
| Non-polymeric | Gold Nanoparticles | Highly inert and non-toxic. Surface functionalization can be easily performed. | Kawano et al al 2006; Sandhu et al 2002 |
| Magnetic nanoparticles | High transfection efficiency in variety of cell lines. | Huth et al 2004; Scherer et al 2002 |
Polymeric nanoparticles
Polymeric nanoparticles or nanospheres are the most commonly used type of nano-scale delivery systems. They are mostly spherical particles, in the size range of 1–1000 nm, carrying the genetic material of interest. The mechanism of incorporation of polynucleotides into polymeric nanoparticles depends on the nature of the polymer. Most cationic polymers have the ability to condense plasmid DNA into nanometer size complexes (polyplexes). DNA can also be entrapped into the polymeric matrix or can be adsorbed or conjugated on the surface of nanoparticles. A variety of natural and synthetic polymers have been used for gene delivery. In this section, we have discussed the most extensively used polymers for nanoparticle-based gene delivery.
Poly lactide-co-glycolide (PLGA) and Poly lactic acid (PLA)
Biodegradable polyesters, PLGA and PLA are amongst the most commonly used polymers for delivering drugs and biomolecules. They consist of units of lactic acid and glycolic acid connected through ester linkage. These biodegradable polymers undergo bulk hydrolysis thereby providing sustained delivery of the therapeutic agent. The rate of degradation of polymer, and the release rate of drug, can be controlled by changing the polymer molecular weight and copolymer composition. The degradation products, lactic acid and glycolic acid, are removed from the body through citric acid cycle (Shive and Anderson 1997). The release of therapeutic agent from these polymers occurs by diffusion and polymer degradation.
PLGA and PLA nanoparticles are mostly matrix-type systems prepared by emulsification followed by evaporation of the organic phase (Rosca et al 2004). Several studies have shown higher uptake of nanoparticles into cells as compared to larger size particles (Desai et al 1996; Desai et al 1997; Delie et al 2001; Panyam et al 2002). Nanoparticles have the ability to escape endo-lysosomal compartments and release the therapeutic molecule inside the cell at a sustained rate (Panyam et al 2002; Kim and Martin 2006). The release pattern can be modulated according to the dosing requirement by changing various formulation parameters. The poly-nucleotide can be incorporated in PLGA/PLA nanoparticles by either entrapment into the polymeric matrix (Ribeiro et al 2005) or by surface-adsorption by using a cationic polymer or surfactant (Kim et al 2005). PLGA and PLA nanoparticles have been used for the delivering plasmid DNA (Cohen et al 2000), siRNA (Yuan et al 2006), and aptamers (Cheng et al 2007).
Cationic polymers
Cationic polymers by virtue of their positive charge can efficiently condense the anionic polynucleotides into nano-meter range complexes (polyplexes) thereby masking their negative charge (De Smedt et al 2000; Zhang et al 2004). Polynucleotides are polyanionic molecules with a large hydrodynamic diameter which presents a significant barrier towards efficient cellular uptake. Cationic polymers, apart from condensing it to a several fold smaller size, also provide a net positive charge to the complex which helps in attachment on cellular membrane. Also, most cationic polymers bear amine groups that are protonable at acidic pH. Thus, once inside the endosome, these polymers accept proton thereby resisting a drop in pH. This causes influx of counterions (chloride ions) resulting in osmotic swelling and subsequent rupture of endosome. This phenomenon, first explained by Behr, is known as proton sponge effect (Behr 1997). The efficiency of polyplexes has been found to be dependent on the ratio of nitrogen atoms of the polymer to the phosphate groups present in the polynucleotide (N/P ratio) (Gebhart and Kabanov 2001). A variety of natural and synthetic cationic polymers have been used for gene delivery. Some of the most commonly used cationic polymers have been discussed below.
Polyethylenimine (PEI)
Polyethyleneimine is the most commonly used cationic polymer and is widely regarded as a gold standard, amongst non-viral vectors, in order to compare transfection efficiencies. Transfection efficiency of PEI has been found to be dependent on a multitude of factors such as molecular weight, degree of branching, N/P ratio, complex size etc (Thomas et al 2005). PEI has a high density of protonable amino groups, every third atom being amino nitrogen, which imparts it a high buffering ability at practically any pH. Hence, once inside the endolysosomal compartment, PEI can efficiently destabilize the endosome releasing the polynucleotide in the cytoplasm. PEI can also be used in combination with PLGA/PLA for preparing matrix-type nanoparticles to deliver DNA by adsorption/complexation on the surface (Kim et al 2005). Toxicity of PEI, however, has been a concern. Acute toxicity has been observed in several studies both in vitro and in vivo (Chollet et al 2002; Moghimi et al 2005). Investigators have studied conjugation of polyethylene glycol (PEG) to PEI to form diblock or triblock copolymers to reduce PEI-associated toxicity (Park et al 2005; Zhong et al 2005; Choi et al 2006). PEG also shields the positive charge of the polyplexes, thereby providing steric stability to the complex. Such stabilization prevents non-specific interaction with blood components during systemic delivery (Kursa et al 2003).
Polymethacrylates
Polymethacrylates are cationic vinyl-based polymers that possess the ability to condense polynucleotides into nano-meter size particles. Several polymethacrylates such as poly [2-(dimethylamino) ethyl methacrylate] (DMAEMA) and its co-polymers have been used for polynucleotide delivery. Presence of protonable tertiary amine groups in their structure provides buffering ability similar to that of PEI. A range of Polymethacrylates, differing in molecular weights and chemical structures, have been evaluated for their potential as gene delivery vector (Dubruel et al 2003; Dubruel et al 2004). Polymethacrylates containing only tertiary amine groups were found to be similar to PEI in terms of transfection efficiency while displaying much better biocompatibility profile (Dubruel et al 2004). Nanoparticles with a methacrylate core and PEI shell prepared via graft copolymerization have also been employed lately for gene delivery (Li et al 2002; Feng et al 2006). Such conjugation resulted in nanoparticles with a higher transfection efficiency and lower toxicity as compared to PEI alone. We have recently formulated cationic nanoparticles with commercially available polymethacrylate Eudragit® E100 in combination with PLGA/PLA using cationic surfactant, cetyltrimethyl-ammonium bromide (CTAB), and achieved much improved transfection efficiency as compared to PLA/CTAB and PLGA/CTAB nanoparticles (Unpublished data).
Poly-L-Lysine (PLL)
Poly-l-lysine is amongst the first cationic polymers investigated for gene delivery. It is a biodegradable polymer synthesized by polymerization of N-carboxy-anhydride of lysine (Zhang et al 2004). Biodegradability is a highly desirable property for gene delivery applications in vivo. Poly-l-lysine is able to form nanometer size complexes with polynucleotides owing to the presence of protonable amine groups on the lysine moiety. Complexation of PLL with polynucleotide results in formation of toroidal or rod-shaped polyplexes less than 100 nm in size (Kwoh et al 1999). Although PLL polyplexes have shown good cellular uptake, their transfection efficiency is several folds lower than PEI polyplexes (Merdan et al 2002). Possible reason for this may be reduced endosomal escape due to lack of amine groups that can be protonated at acidic pH (Akinc and Langer 2002). Resultant lack of buffering ability leads to degradation of polyplexes by lysosomal enzymes. Conjugation of PLL with poly (ethylene glycol (PEG) has been performed to shield charge on the surface thereby providing in vivo stability, delaying body clearance, and protecting DNA from nuclease degradation (Katayose et al 1998; Kwoh et al 1999). Enhanced transfection efficiency has been observed by conjugating the PLL polyplexes with membrane disruptive peptides and fusion peptides (Wagner et al 1992; Lee et al 2002). Delivery of siRNA using PLL has also been reported (Moriguchi et al 2005).
Poly (β-amino ester)s
Poly (β-amino ester) (PBAE) is a novel, charge inducible, biodegradable, and non-toxic polymer. PBAE polyplexes have been prepared and optimized in terms of polymer molecular weight, polymer end groups, complex size, and DNA/polymer ratio (Lynn and Langer 2000; Akinc et al 2003). The polyplexes displayed transfection efficiencies comparable to PEI and commercial lipid-based transfection reagents and were able to transfect a variety of cell types (Akinc et al 2003). The complexes displayed significantly low cytotoxicity as compared to PEI polyplexes in vitro (Lynn and Langer 2000). PBAE in combination with PLGA was used for DNA vaccination and led to significant enhancement in transfection efficiency (Little et al 2004; Little et al 2005). PBAE/PLGA microparticles showed high immunogenic potential and led to a significant reduction of tumor size in mice (Little et al 2004). PBAE/PLGA particles were also able to delay the release of plasmid DNA for several days similar to PLGA particles thereby prolonging bioavailability (Little et al 2005).
Chitosan
Chitosan, a naturally derived polycation, is amongst most widely investigated polymers for gene delivery. Mucoadhesive property of Chitosan makes it suitable for oral and nasal delivery of DNA (Ferrari et al 1997). Chitosan can deliver polynucleotides, both, by encapsulation and by complexation, forming positively charged particles (Bozkir and Saka 2004a; Koping-Hoggard et al 2004). Chitosan nanoparticles efficiently protect DNA from nuclease degradation (Bozkir and Saka 2004b). Chitosan nanoparticles have also been evaluated for siRNA delivery (Howard et al 2006; Katas and Alpar 2006). Transfection efficiency of chitosan polyplexes is found to be dependent on charge ratio, pH, cell type, molecular weight of chitosan, and degree of deacetylation (Sato et al 2001; Koping-Hoggard et al 2004; Lavertu et al 2006). Transfection efficiency of optimized chitosan polyplexes was similar to PEI but the onset of expression was found to be slower than PEI which may be due to slower endosomal escape of chitosan nanoparticles (Koping-Hoggard et al 2001). Recently, oligosaccharides derived from chitosan were evaluated for gene delivery after conjugation with stearic acid (Hu et al 2006). These nanoparticles displayed transfection efficiency comparable to that of commercial transfection reagent, Lipofectamine™ 2000, at higher time points post-transfection, while showing low cytotoxicity. Chitosan polyplexes physically combined with PLGA microparticles have also been evaluated for sustained gene delivery (Yun et al 2005).
Cationic liposomes
Liposomes are spherical vesicles made of phospholipids used to deliver drugs or genes inside the cells. They can range in size from 20 nm to a few microns. The use of cationic liposomes to deliver DNA into cells was first reported in 1987 (Felgner et al 1987). Since then, liposomes have been routinely used in gene delivery research. Positively charged liposomes combine with negatively charged polynucleotides to form liposome/polynucleotide complexes (lipoplexes). Although the positive charge of liposomes is a requirement for preparing lipoplexes, neutral lipids dioleoylphosphatidyl-ethanolamine is also commonly incorporated. It facilitates endosomal destabilization apart from providing structural stability to the liposome. Liposomes destabilize the lipid bilayer membranes by promoting the formation of non-bilayer lipid structures (Hafez et al 2001). Cellular entry of lipoplexes is shown to be primarily via clathrin-mediated endocytosis (Dass and Burton 2003). Liposomes have also been employed for cell-targeting using a variety of targeting ligands (Talsma et al 2006; Torchilin 2006). Although a variety of high-efficiency liposomes are commercially available for transfecting cells in vitro and in vivo, their toxicity is still a concern. Several lipoplex formulations have caused moderate to severe toxicities in animal models (Stewart et al 1992; Tousignant et al 2000). At cellular level, lipoplexes have been reported to cause cell shrinking, reduced mitoses, and vacuolization of cytoplasm (Lappalainen et al 1994).
Gold nanoparticles
Gold nanoparticles have lately been investigated as an alternative to lipid-mediated and polymer-mediated gene delivery. Gold nanoparticles can be easily prepared, display low toxicity, and the surface can be modified using various chemical techniques. The gold nanoparticles functionalized using quarternary ammonium chains can efficiently transfect mammalian cells (Sandhu et al 2002). Up to 8 times higher transfection efficiency compared to PEI was observed using optimized formulations. Systemic delivery of plasmid DNA using PEG-modified gold nanoparticles resulted in improved stability and increased circulation time of DNA in the blood (Kawano et al 2006). Surface functionalization of gold nanoparticles using a PEG spacer also resulted in rapid cellular uptake and internalization (Shenoy et al 2006).
Magnetic nanoparticles
Delivery of polynucleotides using magnetic nanoparticles, also known as magnetofection, has been reported (Scherer et al 2002). In this technique, superparamagnetic iron oxide nanoparticles are prepared, and are coated with a polyelectrolyte such as PEI, to produce particles in size range of 400-1000 nm. Surface coating of PEI facilitates complexation of DNA on the surface. These nanoparticles are then delivered to the cells under the influence of a magnetic field. The mechanism of cellular uptake of magnetofectins was shown to be analogous to PEI polyplexes (Huth et al 2004). Magnetofection for 10 minutes resulted in up to several hundred-fold increase in protein expression, compared to standard transfection, using a variety of transfection vectors in different cell lines (Scherer et al 2002). Another study reported an increase in transfection efficiency in primary airway epithelial cells using megetofection (Gersting et al 2004).
A recent study, investigating non-viral gene transfer in vivo has, however, reported a decrease in transfection efficiency using magnetic nanoparticles as compared to non-magnetic particles (Xenariou et al 2006). The versatility and potential applications of this technique thus remain to be seen.
Conclusions
The ultimate goal of gene therapy is to deliver genes with high transfection efficiency to specific cells without causing any adverse effects. Viral vectors are efficient at delivering genes but are inherently unsafe. Nanotechnology-based gene delivery vectors have shown tremendous potential at overcoming physiological and biochemical barriers towards efficient gene delivery. Vector modifications at molecular level have enabled scientists to develop a variety of nanosystems with high efficiency, high specificity and low toxicity. With increase in the information available about cellular and molecular mechanisms involved in the uptake and transport of these carriers inside the cells, there is great hope for the future of nanoparticulate systems for gene delivery.
References
- Akinc A, Langer R. Measuring the pH environment of DNA delivered using nonviral vectors: implications for lysosomal trafficking. Biotechnol Bioeng. 2002;78(5):503–8. doi: 10.1002/bit.20215. [DOI] [PubMed] [Google Scholar]
- Akinc A, Anderson DG, Lynn DM, et al. Synthesis of poly (beta-amino ester)s optimized for highly effective gene delivery. Bioconjug Chem. 2003;14:979–88. doi: 10.1021/bc034067y. [DOI] [PubMed] [Google Scholar]
- Behr JP. The Proton Sponge -A Trick to Enter Cells the Viruses Did Not Exploit. CHIMIA. 1997;51:34–6. [Google Scholar]
- Bianco A. Carbon nanotubes for the delivery of therapeutic molecules. Expert Opin Drug Deliv. 2004;1:57–65. doi: 10.1517/17425247.1.1.57. [DOI] [PubMed] [Google Scholar]
- Bozkir A, Saka OM. Chitosan nanoparticles for plasmid DNA delivery: effect of chitosan molecular structure on formulation and release characteristics. Drug Deliv. 2004a;11:107–12. doi: 10.1080/10717540490280705. [DOI] [PubMed] [Google Scholar]
- Bozkir A, Saka OM. Chitosan-DNA nanoparticles: effect on DNA integrity, bacterial transformation and transfection efficiency. J Drug Target. 2004b;12:281–8. doi: 10.1080/10611860410001714162. [DOI] [PubMed] [Google Scholar]
- Cheng J, Teply BA, Sherifi I, et al. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28:869–76. doi: 10.1016/j.biomaterials.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Ooya T, Yui N. One-pot synthesis of a polyrotaxane via selective threading of a PEI-b-PEG-b-PEI copolymer. Macromol Biosci. 2006;6:420–4. doi: 10.1002/mabi.200600024. [DOI] [PubMed] [Google Scholar]
- Chollet P, Favrot MC, Hurbin A, et al. Side-effects of a systemic injection of linear polyethylenimine-DNA complexes. J Gene Med. 2002;4:84–91. doi: 10.1002/jgm.237. [DOI] [PubMed] [Google Scholar]
- Cohen H, Levy RJ, Gao J, et al. Sustained delivery and expression of DNA encapsulated in polymeric nanoparticles. Gene Ther. 2000;7:1896–905. doi: 10.1038/sj.gt.3301318. [DOI] [PubMed] [Google Scholar]
- Dass CR, Burton MA. Modified microplex vector enhances transfection of cells in culture while maintaining tumour-selective gene delivery in-vivo. J Pharm Pharmacol. 2003;55:19–25. doi: 10.1111/j.2042-7158.2003.tb02429.x. [DOI] [PubMed] [Google Scholar]
- Davis SS. Biomedical applications of nanotechnology—implications for drug targeting and gene therapy. Trends Biotechnol. 1997;15:217–24. doi: 10.1016/S0167-7799(97)01036-6. [DOI] [PubMed] [Google Scholar]
- De Laporte L, Cruz Rea J, Shea LD. Design of modular non-viral gene therapy vectors. Biomaterials. 2006;27:947–54. doi: 10.1016/j.biomaterials.2005.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delie F, Berton M, Allemann E, et al. Comparison of two methods of encapsulation of an oligonucleotide into poly(D, L-lactic acid) particles. Int J Pharm. 2001;214:25–30. doi: 10.1016/s0378-5173(00)00627-x. [DOI] [PubMed] [Google Scholar]
- Desai MP, Labhasetwar V, Amidon GL, et al. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996;13:1838–45. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- Desai MP, Labhasetwar V, Walter E, Levy RJ, et al. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm Res. 1997;14:1568–73. doi: 10.1023/a:1012126301290. [DOI] [PubMed] [Google Scholar]
- De Smedt SC, Demeester J, Hennink WE. Cationic polymer based gene delivery systems. Pharm Res. 2000;17:113–26. doi: 10.1023/a:1007548826495. [DOI] [PubMed] [Google Scholar]
- Dubruel P, Christiaens B, Vanloo B, et al. Physicochemical and biological evaluation of cationic polymethacrylates as vectors for gene delivery. Eur J Pharm Sci. 2003;18:211–20. doi: 10.1016/s0928-0987(02)00280-4. [DOI] [PubMed] [Google Scholar]
- Dubruel P, Christiaens B, Rosseneu M, et al. Buffering properties of cationic polymethacrylates are not the only key to successful gene delivery. Biomacromolecules. 2004;5:379–88. doi: 10.1021/bm034438d. [DOI] [PubMed] [Google Scholar]
- Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–17. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Lee D, Li P. Intracellular uptake and release of poly(ethyleneimine)-co-poly(methyl methacrylate) nanoparticle/pDNA complexes for gene delivery. Int J Pharm. 2006;311:209–14. doi: 10.1016/j.ijpharm.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Rossi S, Bonferoni MC, et al. Characterization of rheological and mucoadhesive properties of three grades of chitosan hydrochloride. Farmaco. 1997;52:493–7. [PubMed] [Google Scholar]
- Gebhart CL, Kabanov AV. Evaluation of polyplexes as gene transfer agents. J Control Release. 2001;73:401–16. doi: 10.1016/s0168-3659(01)00357-1. [DOI] [PubMed] [Google Scholar]
- Gersting SW, Schillinger U, Lausier J, et al. Gene delivery to respiratory epithelial cells by magnetofection. J Gene Med. 2004;6:913–22. doi: 10.1002/jgm.569. [DOI] [PubMed] [Google Scholar]
- Hafez IM, Maurer N, Cullis PR. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001;8:1188–96. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- Heidel J, Mishra S, Davis ME. Molecular conjugates. Adv Biochem Eng Biotechnol. 2005;99:7–39. doi: 10.1007/10_002. [DOI] [PubMed] [Google Scholar]
- Hickman MA, Malone RW, Lehmann-Bruinsma K, et al. Gene expression following direct injection of DNA into liver. Hum Gene Ther. 1994;5:1477–83. doi: 10.1089/hum.1994.5.12-1477. [DOI] [PubMed] [Google Scholar]
- Howard KA, Rahbek UL, Liu X, Damgaard CK, et al. RNA Interference in Vitro and in Vivo Using a Novel Chitosan/siRNA Nanoparticle System. Mol Ther. 2006;14:476–84. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Hu FQ, Zhao MD, Yuan H, et al. A novel chitosan oligosaccharide-stearic acid micelles for gene delivery: properties and in vitro transfection studies. Int J Pharm. 2006;315:158–66. doi: 10.1016/j.ijpharm.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Huth S, Lausier J, Gersting SW, et al. Insights into the mechanism of magnetofection using PEI-based magnetofectins for gene transfer. J Gene Med. 2004;6:923–36. doi: 10.1002/jgm.577. [DOI] [PubMed] [Google Scholar]
- Katas H, Alpar HO. Development and characterisation of chitosan nanoparticles for siRNA delivery. J Control Release. 2006 2006 Jul 25; doi: 10.1016/j.jconrel.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Katayose S, Kataoka K. Remarkable increase in nuclease resistance of plasmid DNA through supramolecular assembly with poly(ethylene glycol)-poly(L-lysine) block copolymer. J Pharm Sci. 1998;87:160–3. doi: 10.1021/js970304s. [DOI] [PubMed] [Google Scholar]
- Kawano T, Yamagata M, Takahashi H, et al. Stabilizing of plasmid DNA in vivo by PEG-modified cationic gold nanoparticles and the gene expression assisted with electrical pulses. J Control Release. 2006;111:382–9. doi: 10.1016/j.jconrel.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Kim IS, Lee SK, Park YM, et al. Physicochemical characterization of poly(L-lactic acid) and poly(D,L-lactide-co-glycolide) nanoparticles with polyethylenimine as gene delivery carrier. Int J Pharm. 2005;298:255–62. doi: 10.1016/j.ijpharm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Kim DH, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27:3031–7. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Kommareddy S, Tiwari SB, Amiji MM. Long-circulating polymeric nanovectors for tumor-selective gene delivery. Technol Cancer Res Treat. 2005;4:615–25. doi: 10.1177/153303460500400605. [DOI] [PubMed] [Google Scholar]
- Koping-Hoggard M, Tubulekas I, Guan H, et al. Chitosan as a nonviral gene delivery system. Structure-property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene Ther. 2001;8:1108–21. doi: 10.1038/sj.gt.3301492. [DOI] [PubMed] [Google Scholar]
- Koping-Hoggard M, Varum KM, Issa M, et al. Improved chitosan-mediated gene delivery based on easily dissociated chitosan polyplexes of highly defined chitosan oligomers. Gene Ther. 2004;11:1441–52. doi: 10.1038/sj.gt.3302312. [DOI] [PubMed] [Google Scholar]
- Kreuter J, Alyautdin RN, Kharkevich DA, et al. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles) Brain Res. 1995;674:171–4. doi: 10.1016/0006-8993(95)00023-j. [DOI] [PubMed] [Google Scholar]
- Kursa M, Walker GF, Roessler V, et al. Novel shielded transferrin-polyethylene glycol-polyethylenimine/DNA complexes for systemic tumor-targeted gene transfer. Bioconjug Chem. 2003;14:222–31. doi: 10.1021/bc0256087. [DOI] [PubMed] [Google Scholar]
- Kwoh DY, Coffin CC, Lollo CP, et al. Stabilization of poly-L-lysine/ DNA polyplexes for in vivo gene delivery to the liver. Biochim Biophys Acta. 1999;1444:171–90. doi: 10.1016/s0167-4781(98)00274-7. [DOI] [PubMed] [Google Scholar]
- Lappalainen K, Jaaskelainen I, Syrjanen K, et al. Comparison of cell proliferation and toxicity assays using two cationic liposomes. Pharm Res. 1994;11:1127–31. doi: 10.1023/a:1018932714745. [DOI] [PubMed] [Google Scholar]
- Lavertu M, Methot S, Tran-Khanh N, et al. High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials. 2006;27:4815–24. doi: 10.1016/j.biomaterials.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Lee H, Jeong JH, Park TG. PEG grafted polylysine with fusogenic peptide for gene delivery: high transfection efficiency with low cytotoxicity. J Control Release. 2002;79:283–91. doi: 10.1016/s0168-3659(02)00002-0. [DOI] [PubMed] [Google Scholar]
- Lemieux P, Vinogradov SV, Gebhart CL, et al. Block and graft copolymers and NanoGel copolymer networks for DNA delivery into cell. J Drug Target. 2000;8:91–105. doi: 10.3109/10611860008996855. [DOI] [PubMed] [Google Scholar]
- Li P, Zhu JM, Sunintaboon P, et al. New route to amphiphilic core-shell polymer nanospheres: graft copolymerization of methyl methacrylate from water-soluble polymer chains containing amino groups. Langmuir. 2002;18:8641–6. [Google Scholar]
- Little SR, Lynn DM, Ge Q, et al. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc Natl Acad Sci U S A. 2004;101:9534–9. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SR, Lynn DM, Puram SV, et al. Formulation and characterization of poly (beta amino ester) microparticles for genetic vaccine delivery. J Control Release. 2005;107:449–62. doi: 10.1016/j.jconrel.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Liu F, Huang L. Development of non-viral vectors for systemic gene delivery. J Control Release. 2002;78:259–66. doi: 10.1016/s0168-3659(01)00494-1. [DOI] [PubMed] [Google Scholar]
- Lynn D, Langer R. Degradable Poly(β-amino esters): Synthesis, Characterization, and Self-Assembly with Plasmid DNA. J Am Chem Soc. 2000;122:10761–8. [Google Scholar]
- Merdan T, Kunath K, Fischer D, et al. Intracellular processing of poly(ethylene imine)/ribozyme complexes can be observed in living cells by using confocal laser scanning microscopy and inhibitor experiments. Pharm Res. 2002;19:140–6. doi: 10.1023/a:1014212630566. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Symonds P, Murray JC, et al. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther. 2005;11:990–5. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Moriguchi R, Kogure K, Akita H, et al. A multifunctional envelope-type nano device for novel gene delivery of siRNA plasmids. Int J Pharm. 2005;301:277–85. doi: 10.1016/j.ijpharm.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Murakami H, Nakashima N. Soluble carbon nanotubes and their applications. J Nanosci Nanotechnol. 2006;6:16–27. [PubMed] [Google Scholar]
- Panyam J, Zhou WZ, Prabha S, et al. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16:1217–26. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- Park MR, Han KO, Han IK, et al. Degradable polyethylenimine-alt-poly(ethylene glycol) copolymers as novel gene carriers. J Control Release. 2005;105:367–80. doi: 10.1016/j.jconrel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Ravi Kumar M, Hellermann G, Lockey RF, et al. Nanoparticle-mediated gene delivery: state of the art. Expert Opin Biol Ther. 2004;4:1213–24. doi: 10.1517/14712598.4.8.1213. [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Hussain N, Florence AT. Release of DNA from dendriplexes encapsulated in PLGA nanoparticles. Int J Pharm. 2005;298:354–60. doi: 10.1016/j.ijpharm.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80:35–47. [PubMed] [Google Scholar]
- Rosca ID, Watari F, Uo M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J Control Release. 2004;99:271–80. doi: 10.1016/j.jconrel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Ruponen M, Honkakoski P, Ronkko S, et al. Extracellular and intracellular barriers in non-viral gene delivery. J Control Release. 2003;93:213–17. doi: 10.1016/j.jconrel.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Sandhu KK, McIntosh CM, Simard JM, et al. Gold nanoparticle-mediated transfection of mammalian cells. Bioconjug Chem. 2002;13:3–6. doi: 10.1021/bc015545c. [DOI] [PubMed] [Google Scholar]
- Sato T, Ishii T, Okahata Y. In vitro gene delivery mediated by chitosan. effect of pH, serum, and molecular mass of chitosan on the transfection efficiency. Biomaterials. 2001;22:2075–80. doi: 10.1016/s0142-9612(00)00385-9. [DOI] [PubMed] [Google Scholar]
- Scherer F, Anton M, Schillinger U, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–9. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- Shenoy D, Fu W, Li J, et al. Surface functionalization of gold nanoparticles using hetero-bifunctional Poly (ethylene glycol) spacer for intracellular tracking and delivery. Int J Nanomedicine. 2006;1:51–8. doi: 10.2147/nano.2006.1.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- Stewart MJ, Plautz GE, Del Buono L, et al. Gene transfer in vivo with DNA-liposome complexes: safety and acute toxicity in mice. Hum Gene Ther. 1992;3:267–75. doi: 10.1089/hum.1992.3.3-267. [DOI] [PubMed] [Google Scholar]
- Talsma SS, Babensee JE, Murthy N, et al. Development and in vitro validation of a targeted delivery vehicle for DNA vaccines. J Control Release. 2006;112:271–9. doi: 10.1016/j.jconrel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Thomas M, Ge Q, Lu JJ, et al. Cross-linked small polyethylenimines: while still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharm Res. 2005;22:373–80. doi: 10.1007/s11095-004-1874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng. 2006;8:343–75. doi: 10.1146/annurev.bioeng.8.061505.095735. [DOI] [PubMed] [Google Scholar]
- Tousignant JD, Gates AL, Ingram LA, et al. Comprehensive analysis of the acute toxicities induced by systemic administration of cationic lipid:plasmid DNA complexes in mice. Hum Gene Ther. 2000;11:2493–513. doi: 10.1089/10430340050207984. [DOI] [PubMed] [Google Scholar]
- Vinogradov SV, Bronich TK, Kabanov AV. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev. 2002;54:135–47. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- Vogt A, Combadiere B, Hadam S, et al. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J Invest Dermatol. 2006;126:1316–22. doi: 10.1038/sj.jid.5700226. [DOI] [PubMed] [Google Scholar]
- Wagner E, Plank C, Zatloukal K, et al. Influenza virus hemagglutinin HA–2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc Natl Acad Sci U S A. 1992;89:7934–8. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther W, Stein U. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs. 2000;60:249–71. doi: 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- Wiethoff CM, Middaugh CR. Barriers to nonviral gene delivery. J Pharm Sci. 2003;92:203–17. doi: 10.1002/jps.10286. [DOI] [PubMed] [Google Scholar]
- Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–8. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Xenariou S, Griesenbach U, Ferrari S, et al. 2006Using magnetic forces to enhance non-viral gene transfer to airway epithelium in vivo Gene Ther2006 Jun 1; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Yuan X, Li L, Rathinavelu A, et al. SiRNA drug delivery by biodegradable polymeric nanoparticles. J Nanosci Nanotechnol. 2006;6:2821–8. doi: 10.1166/jnn.2006.436. [DOI] [PubMed] [Google Scholar]
- Yun YH, Jiang H, Chan R, et al. Sustained release of PEG-g-chitosan complexed DNA from poly(lactide-co-glycolide) J Biomater Sci Polym Ed. 2005;16:1359–78. doi: 10.1163/156856205774472281. [DOI] [PubMed] [Google Scholar]
- Zhang S, Xu Y, Wang B, et al. Cationic compounds used in lipoplexes and polyplexes for gene delivery. J Control Release. 2004;100:165–80. doi: 10.1016/j.jconrel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Feijen J, Lok MC, et al. Low molecular weight linear polyethylenimine-b-poly(ethylene glycol)-b-polyethylenimine triblock copolymers: synthesis, characterization, and in vitro gene transfer properties. Biomacromolecules. 2005;6:3440–8. doi: 10.1021/bm050505n. [DOI] [PubMed] [Google Scholar]