Abstract
In an attempt to achieve post-inhalation self-regulated insulin release, we constructed a microparticle agglomerate of nano-sized liposomal particles, with the agglomeration facilitated by cross-linkages capable of cleavage by glucose. The particles exhibited a small aerodynamic diameter within the human respirable range, but a large geometric diameter that prevents macrophage uptake and clearance. Upon intratracheal instillation of the “glucose-sensitive” microparticle into the lungs of rats, hyperglycemic events triggered an acceleration of the release of insulin achieving normoglycemia shortly after “sensing” the elevated systemic glucose. This work is a demonstration of an inhalable particle with long residence times in the lungs capable of modulating insulin release based on systemic glucose levels.
Keywords: self-regulated release, inhaled insulin, agglomerated vesicle technology, liposomes, glucose sensitive particle
Introduction
A recent long-term (10+ years) study compared an intensive diabetes therapy (involving 5 or more daily injections or continuous subcutaneous infusion) to conventional insulin therapy. The outcome was that a much tighter control of blood glucose levels closer to normoglycemia achieved by a more frequent insulin administration benefited diabetics, resulting in a significant reduction in cardiovascular diseases, retinopathies and nephropathies (Barnie et al 1999; Nathan et al 2003; Skyler 2004). Therefore, there has been an increased effort to develop an insulin delivery system that will mimic physiological insulin secretion. To date, there has been some progress in the modulation of insulin delivery based on the blood glucose level (Steil et al 2004; Chu 2005; Lapeyre 2006). There has been discussion of coupling of insulin infusion pumps to blood-glucose monitoring systems, to enable feedback control of the insulin delivery resulting in self-regulated delivery system for insulin. Continuous glucose monitoring, however, is problematic (Mastrototaro 2000; Maran et al 2002); shortcomings include fouling (Moussy 2002), the need for constant recalibration and the long time constant of the sensor (Potts et al 2002).
Two novel triggered-release strategies were proposed in the 1980s, both utilizing the blood glucose as the trigger for insulin release from implantable polymeric devices. Langer and co-workers designed a polymeric system enzymatically sensitive to glucose (Fischel-Ghodsian et al 1988) while Brownlee et al (1979) proposed a glucose-sensitive system based on competitive binding. The enzymatically sensitive polymeric system consisted of an enzyme (glucose oxidase) which mediates the conversion of the marker (glucose) to an acid (gluconic acid). The resulting pH drop can modulate the release of insulin utilizing either the pH-dependence of insulin solubility (Brown et al 1986, 1996) or pH-responsive polymers (Kost et al 1985; Goldraich et al 1993; Traitel et al 2000). The second strategy utilized the competitive binding of glucose and glycosylated insulin onto sugar binding moieties. Park and co-workers proposed a copolymer capable of sol-gel phase-reversible transition by changes in the environmental glucose concentration (Lee et al 1996; Obaidat et al 1996). Once the copolymer was exposed to a glucose-binding protein, concanavalin A (con A), a gel was formed due to the cross-linking of the copolymer’s glucose chains via glucose-con A bridges. The hydrogel transitioned to a sol in the presence of free glucose. This transition was reversible and once the free glucose was removed the sol became a gel again. The insulin release exhibited a pulsatile pattern upon stepwise changes of glucose (Kim et al 2001). Kim et al proposed glycosylated insulin molecules complexed to con A (Kim et al 1990, 1994; Pai et al 1992, 1993). The resulting complex released the glycosylated insulin in the presence of glucose, due to preferential binding to glucose. In subsequent developments, Kim and co-workers have enclosed the complexed species in a polymeric membrane pouch that is permeable to glucose and glycosylated insulin, but impermeable to con A (Liu et al 1997). Use of this technology in vivo requires surgical implantation of the pouch in the peritoneal cavity, followed by periodic catheter-based refill procedures.
Invasive methods such as these proposed solutions have the potential to reduce patient compliance, and noninvasive methods are therefore desirable. Among the many alternative noninvasive routes (oral, transdermal, and ocular) to deliver insulin, the respiratory route has attracted the most attention. Insulin has a high permeability through the alveolar membrane (Gannslen et al 1925; Wigley et al 1971) and consequently, the release of free insulin in the region of the alveoli is a suitable method to transport significant amounts of the drug into the blood stream (Patton et al 1999). This has led to the development of numerous clinical trials of inhaled insulin (Patton et al 2004). One of them is close to commercialization in US and European Union (Nektar 2006), but is a short-acting inhaled insulin requiring an injection of a long-acting insulin to control overnight glycemic episodes. Addressing this problem, a breakthrough discovery by Edwards et al (1997) in the late 1990’s showed that large porous particles with aerodynamic diameter suitable for deep lung delivery, but geometric diameter too large for macrophage uptake, can facilitate extended release in the lung.
While these advances in pulmonary delivery permit controlled release of the drug at some pre-programmed rate, there is no possibility of modulating this rate on demand. To address this need, we introduced the concept of an AVT (Agglomerated Vesicle Technology) particle (Bhavane et al 2003). The AVT particles consist of liposomes with spacer arms on their outer leaflet, cross linked via the spacers to create a chemically cross-linked agglomerate of the constituent liposomes. They are capable of encapsulating drug within the constituent vesicles (liposomes), and triggered release of drug when exposed to a suitable trigger. These particles have been shown to be nebulized from suspension, resulting in respirable particles well within the aerodynamic diameter range for deep lung delivery, and exhibit minimal loss of structure upon nebulization, preserving 70%–85% of their contents encapsulated (Bhavane et al 2003). Further, the geometric diameter of the particles was shown to be quite large (>20 μm) while the structure was shown to be porous (Karathanasis et al 2006), resulting in the respirable aerodynamic diameter. The triggered release of drug was shown to be due to the cleavage of inter-liposome links, with the additional possibility of some liposomal membrane disruption. In a subsequent development (Karathanasis et al 2005), a disulfide linkage capable of cleavage by benign levels of free thiols such as cysteine and glutathione was developed. The triggered release of ciprofloxacin from both these particles was demonstrated. It was then shown (Karathanasis et al 2006) that AVT particles with cysteine cleavable links could successfully deliver insulin in vivo, when administered to rats via the lungs. While the untriggered (basal) release rate could maintain normoglycemia in the test animals, hyperglycemic events could be controlled by the triggering. Further, in glucose clamp studies at elevated glucose levels where hepatic glucose secretion is suppressed, it was shown that systemic delivery of insulin was indeed occurring by triggering the AVT particles, and that the triggered release persisted for a short period of time before subsiding to the basal level.
While the administration of a secondary release agent is certainly feasible, it is not as useful as having the release triggered by a product of the disease or condition to be treated. For example, if insulin release were to be triggered by the glucose level, it would enable a versatile, feedback control system for diabetics. In the current work, we have attempted to take this platform to the next level, by creating a glucose-sensitive linkage that forms the AVT particles. Thus, elevated glucose levels trigger insulin release. Fortuitously, the pulmonary glucose level appears to correlate with the systemic glucose level (Philips et al 2003; Wood et al 2004). Above a systemic level of ∼120 mg/dL active transport of glucose via the SGLT transporter (Saumon et al 1996) results in a pulmonary level of ∼60 mg/dL, which then rises commensurately with the systemic level. Intrapulmonary delivery of insulin-containing, glucose-sensitive AVT particles therefore has the potential to continuously maintain normoglycemia in otherwise hyperglycemic subjects. To test this hypothesis, we constructed AVT particles using a Concanavalin A (Con A) linkage to sugars as the linker (as in Figure 1). Con A is well known to be highly inflammatory, and yet has been extensively studied as a possible component of a glucose sensing device. In fact, triggered insulin release systems based on Con A have previously been constructed (Lee et al 1996; Kim et al 2001). In our current work therefore we used Con A as a representative linker, fully recognizing that its long term in vivo use is not advisable, yet seeking to demonstrate the feasibility of intrapulmonary glucose-sensing systemic insulin delivery. The ability of these AVT particles, when administered intrapulmonarily, to modulate systemic glucose levels in a rat model was tested. Hyperglycemia was induced in the rats by suppressing pancreatic activity temporarily using ketamine/xylazine (Abdel el Motal et al 1985; Hindlycke et al 1992; Saha et al 2005), and simultaneously injecting glucose i.v. To our knowledge, this is the first demonstration of an intrapulmonary, glucose-sensing, insulin delivery system.
Figure 1.
Con A based AVT particles: Liposomes bearing PEG-sugar conjugates on their external surface are agglomerated by free con A.
Materials and methods
Materials
1,2-Dipalmitoyl-sn-Glycero-3-Phosphatidylcholine (DPPC) and 1,2-Distearoyl-sn-Glycero-3-Phosphatidylethanol-amine (DSPE) were purchased from Genzyme Pharmaceuticals (Cambridge, MA). Cholesterol, concanavalin A, D-mannosamine, D-galactosamine, and D-mannosamine were purchased from Sigma-Aldrich (St. Louis, MO). DSPE-PEG-COOH was obtained from Nektar (San Carlos, CA). Dithiobis(succinimidylpropionate) (DTSSP), 1-Ethyl-3-(3-Dimethylaminopropyl) carbodiimide Hydrochloride (EDC) and N-hydroxysulfosuccinimide (sulfo-NHS) were purchased from Pierce (Rockford, IL). Insulin USP (human recombinant-crystalline) was purchased from Serologicals (Milford, MA). Ketamine and Xylazine were purchased from Butler Animal Health Supply (Richmond, VA). All other materials were purchased from Fisher Scientific (Houston, TX).
Synthesis of glycosylated PEG conjugates
Carbodiimide coupling of DSPE-PEG-COOH to galactosamine was performed following a published procedure (Hermanson 1996). DSPE-PEG-COOH (200 mg, 0.07 mmol) was first micellized in 10 mL of MES buffer at pH 4.9. EDC (20 mg, 0.1 mmol) was then added and the unstable amine reactive intermediate was stabilized using sulfo-NHS (22 mg, 0.1 mmol). After 15 minutes, galactosamine (19 mg, 0.1 mmol) was added. The pH of the mixture was increased to 7.3 and maintained for 3 hours. Unreacted EDC, sulfo-NHS and galactosamine were removed by dialyzing the reaction mixture against PBS at pH 7.3 overnight. The suspension was then lyophilized, yielding a white powder. Buffer salts and any remaining unreacted sugar were then removed by suspending the powder in ethanol at 60 °C, filtration of the ethanolic mixture, followed by evaporation. TLC in CHCl3/MeOH = 85/15 yielded a single spot with Rf = 0.41. The spot of the parent molecule DPSE-PEG-COOH was not present. NMR analysis of the final product was performed on a Bruker Avance 600 MHz NMR Spectrometer, with the following results: 1HNMR (DMSO-d6 solvent): δ 0.9 (t, CH3 of lipid, 6H), 1.3 (m, (CH2)n of lipid, 56H), 1.6 (m, CH2CH2C = 0, 4H), 2.3 (m, CH2CO, 4H), 2.6 (t, CH2CH2CONH, 4H), 3.6 (s, PEG, ∼190H), 3.7–3.8 (overlapping sugar and CH2PO4CH2, 9H), 3.9 (t, Gal, 1H), 4.1 (t, Gal, 2H), 4.2 (m, Gal, 2H), 4.1–4.2 (m, Gal, 4H), 4.4 (dd, Gal, 1H), 5.1 (d, POCH2CHCH2, 1H).
An identical procedure that used glucosamine or man-nosamine instead of galactosamine was followed to prepare the DPSE-PEG-glucose, and DPSE-PEG-mannose conjugates respectively.
Preparation and characterization of AVT particles
Preparation of core liposomes
Human recombinant insulin was dissolved in citrate buffer (100 mM) at pH 2.5. Lipids (56 mole% DPPC, 40 mole% Cholesterol, 0.4 mole% DSPE-PEG-NH2 conjugate, 1.2 mole% each of DSPE-PEG-galactose, DSPE-PEG-glucose, and DSPE-PEG-mannose) were dissolved in ethanol at 52 °C and hydrated with the insulin solution, the lipid concentration in the final mixture being 50 mM. The suspension was then passed 5 times through a 400 nm Nucleopore track-etch membrane at 52 °C and a pressure of approximately 100 psi. MnCl2 and CaCl2 solutions, which are known to enhance binding of sugars to con A, were added to a final concentration of 3 mM, and the overall suspension was made isotonic by the addition of NaCl. The liposomal suspension was dialyzed against citrate buffer at pH 2.5 for 2 hrs to remove ethanol.
Size determination of liposomes
The size distribution of the liposomes was measured by Dynamic Light Scattering (DLS) using a Brookhaven Instruments BI-9000AT Digital Autocorrelator, a BI-200SM goniometer and a Hamamatsu photomultiplier. The light source was a 532 nm, Ti-sapphire, frequency doubled laser. For the measurement, the liposomal suspension was appropriately diluted in citrate buffer. No settling of particles is observed in this practically neutrally-buoyant system, and permits long data acquisitions.
Preparation of AVT particles
The pH of the liposomal suspension was raised to 6.6, optimal for the cross-linking by Con A. A solution of Con A at 5 mg/ml in citrate buffer at pH 6.6 in the presence of 1 M NaCl, 3 mM CaCl2 and 3 mM MnCl2 and then filtered through a 0.8 μm syringe filter in order to remove a small number of undissolved Con A aggregates. An aliquot of con A solution, sufficient to provide 2:1 mole ratio of Con A to glycosyl species on the external leaflet of the liposomes, was added. The agglomeration process was complete within 10 min, indicated by unchanging size of agglomerates as measured by Fraunhofer Diffraction. 30 min after the addition of con A, traces of the cross-linker DTSSP reactive towards the amine-PEG were added, to create a small number of permanent links between the liposomes. The encapsulated fraction was estimated by testing an identical preparation where the pH of the liposo-mal suspension was raised to 5.3, causing external insulin to precipitate, followed by extensive dialysis. The insulin was estimated by HPLC, after lysis of the agglomerates by ethanol. The USP insulin assay (official monograph USP 24, 2000) was modified to use a Phenomenex Luna 5 μ C18(2) column (150 × 2.0 mm) at a column temperature of 40 °C, flow rate 0.25 mL/minute; mobile phase acetonitrile: aqueous sodium sulfate (28.4 mg/mL) 27:73 v/v and sample injection volume 20 μL. The AVT insulin preparation was thus estimated to contain a total of 184.9 IU/ml insulin (1 mg ∼ 27.6 IU), of which 41 IU/ml was encapsulated within the constituent liposomes and the remaining was unencapsulated.
Geometric size distribution of AVT particles
The size distribution of the AVT particles was determined using Fraunhofer Diffraction Pattern Analysis (Malvern Mastersizer with 100 mm lens). The particles were measured at different time points in a period of three weeks, and showed no change over this period.
Aerodynamic diameter of AVT particles
The aerodynamic diameter of the agglomerated liposomes containing insulin was measured by nebulizing 3 ml of the liposomal formulation over a period of 10 minutes into an Andersen Cascade Impactor (Series 20–800, 1 ACFM non-viable sampler). A Parijet LC nebulizer was used for the cascade impaction studies. The impactor was operated under ambient conditions (24 ± 1 °C and ∼55% relative humidity) at constant flow rate of 28.3 l/min. These operating conditions are consistent with the official method described in USP (The United States Pharmacopeia, USP 23, NF 18, 1995). The filter papers from each plate of the impactor, and the filter paper at the end of the last stage were lyophilized. The impactor elbow was washed with citrate buffer that was collected and lyophilized. The amount of dry material collected on each stage was then assigned to the 50% cutoff size for each stage to determine the size distribution. The weight of the drug preparation in the nebulizer was recorded before and after nebulization in order to complete a mass balance calculation and determine any losses upon nebulization. In all cases, over 95% of the initial charge was nebulized and collected in the impactor. Mass mean aerodynamic diameter was estimated from the measured distribution.
Pharmacodynamic studies in rats
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas at Houston. Male Sprague-Dawley rats (Charles River Laboratories) weighing 330–350 g were housed in a 12 hour light /12 hour dark cycle and a constant temperature environment of 21 °C. A standard rat diet with water ad libi-tum was supplied during the 3-day acclimatization period. After initial dose-finding studies, 3 groups of 6 rats each were used to evaluate the pharmacodynamics of the AVT particles: (1) untreated rats with hyperglycemic events (control), (2) treated rats with hyperglycemic events, and (3) treated rats without hyperglycemic events.
Blood glucose concentration was measured using a Hypo-guard Assure 3 glucose meter which required 20 μL of blood and 10 seconds to provide the reading. The small volume of blood required for each reading allowed very frequent sampling (every 5–10 min). Each blood sample was analyzed for glucose 2–4 times and the average was recorded.
Upon restraining the rat using a Plexiglas restrainer, it was anesthetized with a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg) administered i.p. (at t = −40 min). The rat was then placed on a surgical table while temperature was maintained stable by placing a temperature-controlled pad below the animal. On the establishment of anesthesia indicated by the absence of reflexes, the rat was intratracheally intubated via an i.v. catheter (16G × 2 in). The intratracheal tube was connected to the ventilator and the rat was under forced ventilation while being still anesthetized. Successful intubation was verified if the chest was expanding with the same rate as the frequency of the ventilator. 2.7 μL of AVT particle suspension containing 0.5 IU was diluted to 25 μL and then instilled into the intratracheal tube while the animal was placed vertically. A syringe was then used to push the instilled plug to the end of the intratracheal tube, thus insuring instillation at or beyond the tracheal bifurcation. The animal was then placed prone on the surgical table and the intratracheal tube was connected to the ventilator (Harvard model 683, Holliston, MA) operating at 2.5 mL tidal volume and 90 strokes/min frequency. Forced ventilation was used to ensure the particles’ suspension reached into the lungs and was well mixed. After 5 minutes, the forced ventilation was terminated and the animal was allowed to breathe freely. The duration of the entire process of insertion and instillation was ∼10 minutes. 100 minutes after the initial cocktail of anesthetic drugs, the animal was injected i.p. once more with a cocktail of ketamine (40 mg/kg) and xylazine (4 mg/kg). The intratracheal tube was then removed. Blood samples were taken from the tail veins under anesthesia or restrain to monitor the glucose concentration in the blood. A dose of ketamine (50 mg/kg) and xylazine (10 mg/kg) was injected i.p. (at t = 90 and 225 min) followed by an injection of a glucose solution via the tail vein. The animal was euthanized after the end of the 24-hr study by a pentobarbital overdose (100 mg/kg) administered i.p. Upon euthanasia, no further analysis was performed.
The procedures followed in the case of the untreated (control) group were similar to the one of the treated animals with few modifications. The intratracheal intubation was followed by instillation of 25 μL of an isotonic saline solution. The third group was similar to the treated group described above with the exception of absence of the i.p. injections of anesthetics and i.v. injections of glucose solution at t = 90 and 225 min.
Lung histology
Sprague-Dawley rats were anesthetized and intratracheally intubated following the procedures described previously. The animals were euthanized 24 hrs after intratracheal instillation of (1) 20 μl of saline, (2) 40 μl of con A solution containing 2.4 mg con A/kg body weight, (3) 20 μl of liposomes containing 0.41 mg of lipids/kg body weight, and (4) 20 μL of AVT particles containing 0.41 mg of lipids/kg body weight and 0.51 mg con A/kg body weight. The dosage of the liposomal formulations was chosen based on the pharmacodynamic studies. After each animal was euthanized, the lungs were gently inflated with 10% neutral buffered formalin until resistance is appreciated, and then immersed in the formalin overnight. The next day, the lungs were serially sectioned and submitted for routine histological processing and paraffin embedding. Five micron-thick sections were cut from each paraffin block and placed on glass slides, which were stained with a standard hematoxylineosin stain. These slides were reviewed by a practicing pathologist and evaluated semi-quantitatively for changes of edema, inflammation and other histological abnormalities. The inflammation triggered by the preparations was rank-ordered by the pathologist.
Data analysis
Blood glucose concentrations (pharmacodynamic data) are expressed as mean ± SD (Figure 4, n = 3–4). Pharmacodynamic parameters are expressed also as mean ± SD (Table 1, n = 3–4). Each data point was the mean and its standard deviation of the group (3–4 rats per group). Data were compared by variance analysis (Student’s t-test). A p-value less than 0.05 was used to confirm significant differences at the 95% confidence level.
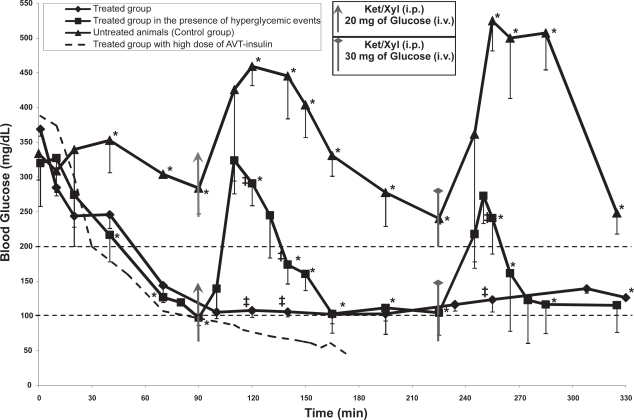
Figure 4.
Blood glucose concentration over the period of ~6 hrs of the untreated group (n = 3), treated group with lung instillation of the AVT particle containing 1.4 IU/kg (n = 3), and the treated group with lung instillation of the AVT particle containing 1.4 IU/kg followed by hyperglycemic events (n = 4). The asterisk and the double cross indicate significant statistical difference based on two-tailed student’s t-test (p < 0.05, a = 0.05) between the treated group in the presence of hyperglycemic events and the control group and between the two treated groups respectively.
Table 1.
Pharmacodynamic parameters of the untreated and the treated group during the three glucose excursions
| Parameters(mean ± SD) | Excursion 1 (t = 0 ± 90) Control | Excursion 2 (t = 90 ± 195) Treated | Excursion 3 (t = 225 ± 325) Control | Treated | Control | Treated |
|---|---|---|---|---|---|---|
| Cmax (mg dL–1) | 353 ± 46 | 320 ± 62 | 459 ± 27 | 324 ± 48 | 525 ± 43 | 273 ± 39 |
| tmax (min) | 40 | 0 | 120 | 110 | 255 | 250 |
| Cbasal (mg dL–1) | 145 ± 27 | 145 ± 27 | 284 ± 37 | 98 ± 11 | 240 ± 7 | 105 ± 32 |
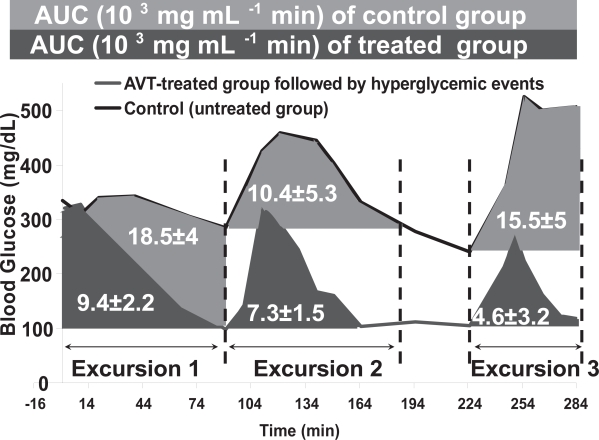
| AUCe (103 mg dL–1 min) | 18.5 ± 4 | 9.4 ± 2.2 | 10.4 ± 5.3 | 7.3 ± 1.5 | 15.5 ± 5 | 4.6 ± 3.2 |
Results and discussion
Characterization of AVT particles
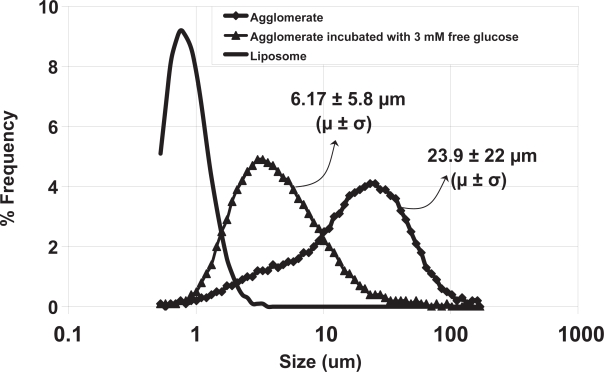
Dynamic light scattering measurements of the parent liposomes indicated a diameter of 228 ± 175 nm. This broad size distribution is commensurate with the single extrusion filter size (0.4 μ). The formation of liposomes with widely distributed diameters when saturated lipid multilamellar vesicles are extruded through such large pore size membranes has previously been demonstrated (Sood 1999). Approximately 23% of the total insulin was found to be encapsulated in these liposomes. The theoretical limit of encapsulation can be calculated if both a size distribution and a lamellarity distribution are assumed. The lamellarity was not measured in this work, but 23% encapsulation at the diameter of liposomes used in this work implies a lamellarity of between one and two bilayers per liposome, consistent with that observed in previous work on AVT particles (Karathanasis et al 2005). Size distributions before and after agglomeration by Con A were also measured by Fraunhofer diffraction. The size distribution of the parent liposomes as measured by Fraunhofer diffraction is shown as a solid line in Figure 2. Note that the Fraunhofer technique is not accurate in the submicron region, and therefore this size distribution is not to be relied on for accurate sizing of the parent liposomes, but its presence in the submicron range can be taken as an indicator of the presence of submicron particles in the sample. After agglomeration by the addition of Con A, the size distribution as measured by Fraunhofer Diffraction indicated that the agglomerates were roughly 23.9 μm ± 22 μm (mean ± SD). This distribution is shown in Figure 2 (data plotted as filled diamonds). Interestingly, the distribution shows a peak at 23 μm and a distinct shoulder around 6 μm, where the submicron particles have completely disappeared. The reasons for this shoulder are not clear, but one can speculate that the 6 μm size particles constitute an intermediate agglomerate that then participates in further agglomeration events as an independent species, and results in the formation of larger particles. A similar shoulder was also observed in previous preparations of AVT particles (Karathanasis 2005), agglomerated by a variety of different linkers, over the same time scale. The mechanism of agglomeration and the structure of the agglomerates formed is clearly dependent on the kinetics of the cross linking steps. Since the time scales for agglomeration for each of the particles that displayed this shoulder were roughly the same, it suggests that their mechanisms were also similar, and thus, similar structures also resulted. The encapsulation fraction after agglomeration was measured to be 21%, practically equivalent to that before agglomeration, suggesting that no significant leakage of insulin occurred during the agglomeration process.
Figure 2.
Size distributions measured by Fraunhofer Diffraction of con A-linked liposomes before and upon cleavage by free glucose.
The agglomerates were readily cleaved upon exposure to 3 mM free glucose. The size distribution of agglomerates 10 minutes after addition of the glucose is shown in Figure 2 (data plotted as filled triangles). It is interesting to note that upon glucose-induced cleavage, the agglomerate peak at 23 μm completely disappears, and a new peak appears at ∼6 μm, close to the shoulder observed in the agglomerate size distribution. Further, there is no evidence for the release of parent liposomes by glucose-induced cleavage of AVT particles, such release would have resulted in the observation of an increased number of particles in the submicron range. In previous work on AVT particles, we have consistently observed this phenomenon, where the predominant daughter particles formed by cleavage of the AVT particles are intermediate clusters, and not the parent liposomes. If the speculated agglomeration process, with the formation of intermediate size clusters which then further agglomerate to form larger particles, is indeed occurring, then the formation of these intermediate clusters upon cleavage would again be consistent with the cleavage of just the most recent bonds formed in the history of a large cluster, thus releasing the intermediate clusters. The degree of glucose-induced fragmentation of the AVT particle can be adjusted by choosing the appropriate types or combination of carbohydrates on the liposome. The binding constants of con A to a number of glycosyl ligands are available in the literature (Mansouri 1983). Ligands that bind far stronger than glucose (eg, mannose), as well as weaker than glucose (eg, galactose) are known. Therefore, one can fine-tune the glucose-sensitivity of the AVT particles by using different combinations of ligands. Indeed, we have fabricated a variety of AVT particles with a range of numbers and types of sugar-con A bridges and tested their response to different levels of free glucose. We were capable of achieving different size reduction rates as well as different resulted sizes ranging from small size reduction to complete dissociation to the constituent liposomes (Karathanasis 2005).
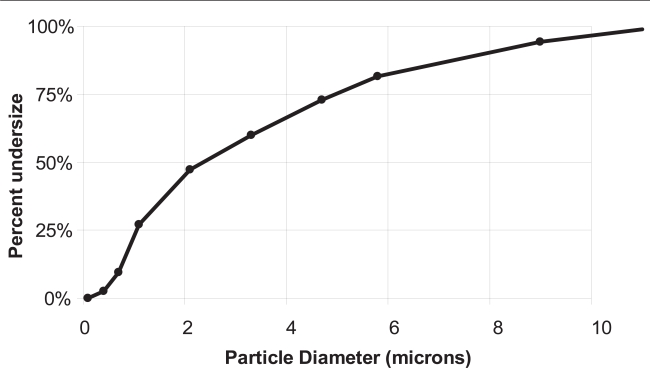
Nebulization of the AVT particles results in the aerosolization of particles with ∼75% in the respirable range with aerodynamic diameter smaller than 5 μm. Figure 3 shows the results of cascade impactor measurements of the nebulizate from such an experiment. Once again, this data is consistent with that observed in past experiments on the nebulization of AVT particles. Previous work (Bhavane et al 2003) also showed that AVT particles thus nebulized (1) did not significantly break or lose their structure upon nebulization, and (2) did not leak their contents, ie, their encapsulation capacity was maintained. Electron microscopy (Karathanasis et al 2006) has also confirmed the porous nature of AVT particles, consistent with their small aerodynamic diameter as measured by cascade impaction.
Figure 3.
Aerodynamic characterization of the agglomerated liposomes by cascade impactor.
A key question that arises however, is how a nebulized (albeit porous) particle can exhibit such a low aerodynamic diameter, since one would expect the pores to be filled with the aqueous suspending medium, thus resulting in an essentially nonporous particle. While there is an obvious concern that the nebulization process is fragmenting the AVT particle, or somehow selecting the smaller particles alone, this is not consistent with the previous observation that the size distribution and encapsulation fraction of nebulized AVT particles is practically the same as that pre-nebulization. We therefore speculate that since the nebulization process and cascade impactor measurement, per USP guidelines (The United States Pharmacopeia, USP 23, NF 18, 1995), are conducted using air at 55% R.H., that much of the aqueous medium filling the pores of the AVT particles is evaporating prior to entry into the impactor. Such evaporation would then result in essentially porous particles entering the impactor, exhibiting aerodynamic diameters substantially lower than the indicated geometric diameter, and consistent with the current measurements. Indeed, in preliminary simulations of droplet trajectories and evaporation in nebulizers under similar circumstances, we have estimated the evaporation to be sufficient to reduce 10+ μm particles to respirable diameters. We therefore believe such evaporation is taking place in the current experiments, yielding AVT particles with aerodynamic diameters in the respirable range <5 μm.
Pharmacodynamic studies in rats
In the rest of the present work, however, in vivo administration of the AVT particles was accomplished by intratracheal instillation. While the predominantly respirable aerodynamic diameters of the particles, consistent with past measurements of AVT particles, suggests that their inhalation would be feasible, the airflow in rats is so different from that in humans that the deposition profiles in rats are not representative of that in humans. We therefore chose not to pursue inhaled delivery of the AVT particles, but relied on instillation. We recognize that instillation causes a somewhat more central-airway weighted delivery of drug, rather than the deep lung delivery achievable by inhalation. We attempted to reduce this effect by forcing ventilation for 5 minutes post instillation, thus forcing distribution of the instilled drug. While this is not very likely to be completely equivalent to inhalation in humans, it is highly unlikely that the current preparation will be used in humans in any case: Con A, the key glucose-sensitive component of the current formulation is highly inflammatory, and unsuitable for human use. We therefore satisfied ourselves with aerodynamic characterization, which assured us that the particles were indeed in the respirable range for humans, and instillation in rat lungs as a surrogate for inhalation in humans. The search for a replacement for Con A, leading to human-usable glucose-sensitive AVT particles is the subject of ongoing work, and a more complete characterization of the glucose-sensitive AVT particles with more representative aerosol delivery is the subject of future work in this laboratory.
Figure 4 shows the blood levels of glucose resulting from the instillation of various preparations to the lungs of rats as described. 4 curves are shown in Figure 4, the circles representing one animal that had 1 IU of insulin in AVT particles administered to its lungs, the triangles representing the untreated control (3 animals), and the other two curves representing the treated animals with 0.5 IU insulin in AVT particles delivered (4 and 3 animals with and without hyperglycemic episodes respectively). Initially, upon observing that the 1 IU dose caused hypoglycemia in the one test animal, the dose was reduced to 0.5 IU for the remaining experiments. All animals began (at time zero) with elevated blood glucose caused by anesthetic-induced suppression of pancreatic activity (Abdel el Motal et al 1985; Hindlycke et al 1992; Saha et al 2005). Normal glucose levels in this species are ∼100 mg/dL (Harkness et al 1995). The untreated control animals exhibited continued hyperglycemia (300 mg/dL) for at least the first 90 minutes, at which point further anesthesia and an intravenous glucose bolus (20 mg) was injected, causing a rapid rise in the blood glucose levels to above 450 mg/dL. This hyperglycemic episode slowly subsided over the next 120 minutes, to a level of about 250 mg/dL and appeared to be dropping further towards normoglycemic levels (the normoglycemic level of 100–200 mg/dL for rats is marked by the straight dotted lines on Figure 4). At this point, a further dose of anesthesia and a glucose bolus (30 mg) caused an even higher increase in the blood glucose, to over 500 mg/dL, which was then maintained for about one hour before dropping back to around 250 mg/dL. On the other hand, the treated animals (with 0.5 IU insulin in AVT particles instilled in the lungs) performed much better. Their blood glucose level dropped rapidly in the first 90 minutes to the low end of the normoglycemic range (without dropping into the hypoglycemic range as did the one animal with a higher dose of 1 IU). Subsequent doses of anesthetic and i.v. bolus glucose (20 mg at 90 minutes, 30 mg at 220 minutes) (shown by the filled squares on Figure 4) caused elevations in the blood glucose levels, but the peak levels were far lower than the untreated control animals, and the elevation also subsided very quickly compared to the untreated controls. Treated animals with no subsequent anesthetic or i.v. bolus glucose (the filled diamonds on Figure 4) showed no further deviations from normoglycemia, and maintained blood glucose levels unchanged for the remainder of the 6 hour experiment.
Glucose, therefore, seems to be a suitable marker that can be used to trigger the release of insulin from inhaled AVT particles, since it appears to be rapidly transported into the lungs from the blood. Philips et al (2003) and Wood et al (2004) have studied the effect of hyperglycemia on the glucose concentration in tracheobronchial lung fluids. Their study, in volunteer humans, showed: (1) Glucose is not present in detectable quantities in the lung fluids of non diabetic normoglycemic individuals, (2) upon application of blood glucose clamps at increasing levels of glucose, no pulmonary glucose is observed until the blood levels reach 12–14 mM (∼140 mg/dL, the threshold value for a diagnosis of diabetes). At this point, the pulmonary glucose level rises rapidly, and reaches a level of about 4–5 mM. This led to the conclusion that an active transport mechanism for glucose between the blood and the lung possibly existed, which is consistent with studies in rats (Saumon et al 1996). In the same study Wood et al. (2004) showed that once the threshold for active transport is crossed, the transport of glucose is very fast, and equilibration takes place in a matter of minutes. Therefore, it appears that the transport rate of glucose is sufficiently fast to allow it to be used as a lung reporter of blood glucose.
Since the AVT preparation contained both unencapsulated (free) and encapsulated insulin, it was expected to have short term action, long-term action, and triggered action as evidenced in Figure 4. The effects of intratracheally instilled free insulin in rats have been studied previously (Hussain et al 2005, 2006). In both these studies, it was observed that free insulin in the rat lung had a very short duration of action, and that the duration was dose-dependent. At free insulin doses 3–5 times higher (1–1.5 IU) than those in our experiments (∼0.3 IU) the blood levels of insulin had dropped to normal levels after 90 minutes, with corresponding relaxation of the blood glucose level as well. At lower doses (0.375 IU) comparable to those in our experiment, the blood levels of insulin hardly changed, and the glycemic effect relaxed extremely quickly, in less than 60 minutes. Thus, the initial drop in glycemic levels in our experiments (at times less than 60 minutes after initiation of therapy) can be attributed to the free insulin contribution, while all subsequent effects can be attributed to the encapsulated insulin alone.
The effects of i.v. glucose bolus can be quantified by integrating the area under the blood glucose curve, with appropriate baselines that isolate the glycemic excursion. The results of such integration are shown in Figure 5, showing that in each time interval considered, the treated animals had far lower glycemic exposure than the untreated animals. This is consistent with the hypothesized mechanism of systemic glucose being transported into the lung via the SGLT transporter (Saumon 1996), and triggering cleavage of Con A links in AVT particles, thus releasing insulin into the lung, which in turn is transported into the blood stream and systemically distributed, causing a reduction in systemic glucose levels. It is also interesting to note that this dose of AVT particles did not result in hypoglycemic events, while a larger dose did result in hypoglycemia shortly after initial administration. We hypothesize that at the lower insulin dose, hepatic glucose secretion is sufficient to restore normoglycemia, in the manner that would be normal in an animal with normal pancreatic function and insulin secretion.
Figure 5.
A comparison of the control and the AVT-treated group during the 3 hyperglycemic events. The shaded areas indicate the areas under the curve (AUCe) for each excursion.
The remainder of the pharmacodynamic parameters extracted from the data in Figures 4 and 5 (Cmax, tmax, Cdelta, AUC and Cbasal) are all summarized in Table 1. It is apparent that in addition to lowering of the AUC, the basal levels in the treated cases are also in the normoglycemic range, and the Cmax achieved is similarly reduced. Other parameters are not distinguishable within the ranges of statistical variation observed in these experiments. We however conclude from these data that the level of hyperglycemia induced by glucose bolus is significantly reduced by the treatment with insulin-containing AVT particles.
Lung histology
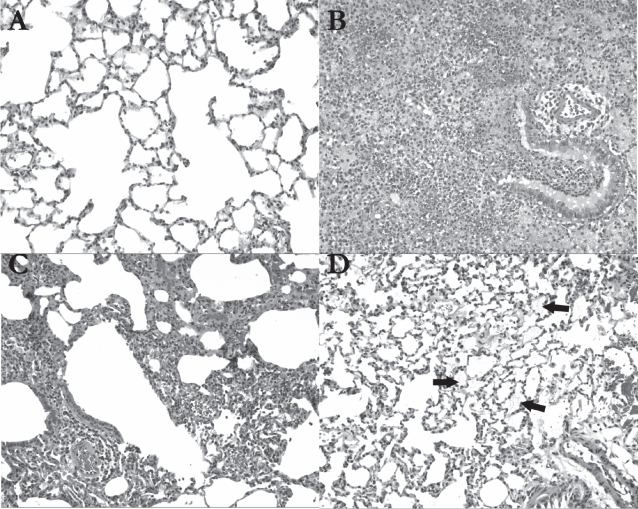
AVT particles in their current form however, are not suitable for human use. Con A is known to be highly inflammatory, and cause macrophage activation. In pursuing this work however, we hypothesized that the level of Con A we used would be very low, substantially below the known toxic level (The Registry of Toxic Effects of Chemical Substances 2006). Further little information is available in the literature on the toxicity of Con A upon pulmonary delivery. Since automodulation of insulin delivery was demonstrated using Con A AVT particles, we decided to examine the possible inflammatory effects of these particles in the lung. Figure 6 shows hematoxylin-eosin stained sections of representative portions of the lungs of rats treated with (A) Saline (B) Con A (C) insulin containing liposomes and (D) AVT particles. Clearly, saline treatment had the smallest effect on the lungs, with the tissue appearing practically normal, wide open alveolar spaces with no sign of inflammation. The Con A positive control in (B) shows extensive pneumonic changes, with mixed inflammatory cell infiltrates and alveolar hemorrhage. Liposome treatment showed patchy lymphocytic infiltrates in one of the animals (C) but not in the other. AVT on the other hand (D) showed the remnants of deposited material in the alveoli consistent with the projected long residence time of the porous AVT particles in the lung, and small signs of inflammation as demonstrated by constriction of the alveolar spaces. While in an acute treatment, requiring a small number of doses, this response may have been acceptable, the long-term effects of a chronic treatment are not known and have to be further investigated.
Figure 6.
Histological analysis of hematoxylin-eosin stained lung tissue 24 hrs after treatment (original magnification 100X); (A) In saline-treated animals (n = 2), the lung tissue was almost entirely normal, as shown in this view; (B) The con A-treated rats (n = 2) demonstrated extensive pneumonic changes in their lungs. A bronchiole and adjacent alveoli show mixed inflammatory cell infiltrates including neutrophils and lymphocytes, accompanied by alveolar hemorrhage; (C) In liposome-treated animals (n = 2), patchy lymphocytic infiltrates were noted around a bronchiole, blood vessels, and in the interstitium in the left upper lobe of one of the rats receiving the liposomes; (D) In AVT-treated animals (n = 2), alveolar material is focally visible (see arrows) in the lungs, and may represent remnants of the particles (original magnification 100X).
Conclusion
In this work, we have demonstrated what is to our knowledge, the first inhaled insulin delivery system capable of glucose-sensing insulin release. The AVT particle constructed in this work uses a Con A linkage, binding to sugars, to create a glucose-sensitive cleavage mechanism, and is based on 3 previous generation AVT particles which had other means of cleavage.
The AVT particles constructed in this work are shown to have large geometric diameters with a fairly broad distribution of sizes (∼23.9 ± 22 μ). Cleavage of these particles in vitro by the presence of 3mM glucose causes a rapid shift to lower particle sizes (6.2 ± 5.8 μ). The aerodynamic diameter of the uncleaved AVT particles is substantially lower than the geometric diameter, with over 75% of the encapsulated insulin being carried by particles in the respirable range of 5 μ. This is consistent with previous investigations of AVT particles, where it has also been shown that nebulization of the particles causes minimal loss of encapsulation and change in particle structure. We therefore believe that the current AVT particles will also be suitable for inhaled drug delivery. However, the current work only tests their performance in a rat model after intratracheal instillation. Future work involving nebulization and true aerosol deposition of these particles in vivo, and their performance after aerosol delivery is required before they can be considered as candidates for inhalation delivery of insulin.
The AVT particles after instillation to the lung, however, do demonstrate the ability to modulate delivery of insulin based on the systemic glucose levels. We hypothesized that the systemic glucose levels would be reflected in the lung, thus enabling cleavage and accelerated release of insulin from AVT particles. This has been shown to be the case. At post-administration times well beyond the window for activity of free insulin in the lung, the AVT particles still respond to systemic glucose levels, and the triggered insulin release is able to restore normoglycemia rapidly in the test animals. A comparison of the glucose pharmacodynamics in these test animals to that in untreated control animals indicates a substantial improvement in both the AUC and Cmax achieved. Lung-instilled AVT thus improves glycemic control substantially over the untreated control. At the same time, dose adjustment of the AVT prevents the occurrence of hypoglycemia. While a high dose of AVT insulin can result in hypoglycemia immediately following administration (possibly because of the free insulin present in the preparation) a reduced dose does not do so. More importantly, subsequent activations of the AVT particles by systemic glucose bolus do not cause any hypoglycemic episodes, while successfully controlling the hyperglycemia induced by the bolus.
AVT particles however, in their current form, may cause inflammation in the lungs, thus limiting the utility of the current preparations. Histological analysis of the lungs upon a 24-hr treatment with AVT particles containing con A revealed minimal inflammation. These were only acute studies, however, and the effect of chronic exposure to this level of con A will probably not be acceptable. The replacement of Con A by a suitable non inflammatory, yet glucose-binding molecule is the subject of ongoing work. Upon identification of such a molecule, a more complete characterization of the aerodynamic behavior, aerosolization fate, and inflammatory potential of AVT particles is indicated. Preliminary indications from this and previous work is that the particles will indeed be successful as “smart” pulmonary delivery devices.
Acknowledgments
This work was financially supported by Whitaker Foundation Bioengineering Research Grant RG-00451. We thank Professor Jerome Schultz for useful discussions and Professor Dani Zander for performing the histological analysis.
References
- Abdel el Motal SM, Sharp GW. Inhibition of glucose-induced insulin release by xylazine. Endocrinology. 1985;116:2337–40. doi: 10.1210/endo-116-6-2337. [DOI] [PubMed] [Google Scholar]
- Barnie A, Cleary P, Genuth S, et al. Epidemiology of Diabetes Interventions and Complications (EDIC): Design and Implementation of a Long-Term Follow-up of the Diabetes Control and Complications Trial Cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavane R, Karathanasis E, Annapragada AV. Agglomerated Vesicle Technology: A new class of particles for controlled and modulated pulmonary drug delivery. J Control Release. 2003;93:15–28. doi: 10.1016/s0168-3659(03)00359-6. [DOI] [PubMed] [Google Scholar]
- Bhavane R, Karathanasis E, Annapragada AV. Triggered release of ciprofloxacin from agglomerated vesicles after instillation in rabbit lungs. Int J Nanomed. 2007;2:xxx–xxx. [PMC free article] [PubMed] [Google Scholar]
- Brown L, Munoz C, Siemer L, et al. Controlled release of insulin from polymer matrices: Control of diabetes in rats. Diabetes. 1986;35:692–7. doi: 10.2337/diab.35.6.692. [DOI] [PubMed] [Google Scholar]
- Brown LR, Edelman ER, Fischel-Ghodsian F, et al. Characterization of Glucose-Mediated Insulin Release from Implantable Polymers. J Pharm Sci-US. 1996;85:1341–45. doi: 10.1021/js9600686. [DOI] [PubMed] [Google Scholar]
- Brownlee M, Cerami A. A glucose-controlled insulin delivery system: semisynthetic insulin bound to lectin. Science. 1979;206:1190–1. doi: 10.1126/science.505005. [DOI] [PubMed] [Google Scholar]
- Chu LY. Controlled release systems for insulin delivery. Expert Opinion on Therapeutic Patents. 2005;15:1147–55. [Google Scholar]
- Edwards D, Hanes J, Caponetti G, et al. Large porous particles for pulmonary drug delivery. Science. 1997;276:1868–71. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- Fischel-Ghodsian F, Brown L, Mathiowitz E, et al. Enzymatically controlled drug delivery. Proc Natl Acad Sci USA. 1988;85:2403–06. doi: 10.1073/pnas.85.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannslen M. Uber inhalation von insulin. Klin Wochenschr. 1925:4–71. [Google Scholar]
- Goldraich M, Kost J. Glucose-sensitive polymeric matrices for controlled drug delivery. Clin Mater. 1993;13:135–42. doi: 10.1016/0267-6605(93)90100-l. [DOI] [PubMed] [Google Scholar]
- Harkness JE, Wagner DJ. The Biology and Medicine of Rabbits and Rodents. Baltimore: Williams and Willkins; 1995. [Google Scholar]
- Hermanson G. Bioconjugate Techniques. New York: Academic Press; 1996. [Google Scholar]
- Hussain A, Ahsan F. State of insulin self-association does not affect its absorption from the pulmonary route. Eur J Pharm Sci. 2005;25:289–98. doi: 10.1016/j.ejps.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Hussain A, Majumder QH, Ahsan F. Inhaled insulin is better absorbed when administered as a dry powder compared to solution in the presence or absence of alkylglycosides. Pharm Res. 2006;23:138–47. doi: 10.1007/s11095-005-8926-9. [DOI] [PubMed] [Google Scholar]
- Hindlycke M, Jansson L. Glucose tolerance and pancreatic islet blood flow in rats after intraperitoneal administration of different anesthetic drugs. Ups J Med Sci. 1992;97:27–35. doi: 10.3109/03009739209179279. [DOI] [PubMed] [Google Scholar]
- Karathanasis E, Ayyagari A, Bhavane R, et al. Preparation of in vivo cleavable agglomerated liposomes suitable for modulated pulmonary drug delivery. J Control Release. 2005;103:159–75. doi: 10.1016/j.jconrel.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Karathanasis E.2005. Triggered and autoregulated pulmonary delivery of insulin, PhD Dissertation at Univ of Houston.
- Karathanasis E, Bhavane R, Annapragada AV. Triggered release of inhaled insulin from the agglomerated vesicles: pharmacodynamic studies in rats. J Control Release. 2006;113:117–27. doi: 10.1016/j.jconrel.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Park K. Modulated insulin delivery from glucose-sensitive hydrogel dosage forms. J Control Release. 2001;77:39–47. doi: 10.1016/s0168-3659(01)00447-3. [DOI] [PubMed] [Google Scholar]
- Kim SW, Jacobs HA. Self-regulated insulin delivery-Artificial pancreas. Drug Dev Ind Pharm. 1994;20:575–80. [Google Scholar]
- Kim SW, Pai CM, Makino K, et al. Self-regulated glycosylated insulin delivery. J Control Release. 1992;11:193–201. [Google Scholar]
- Kost J, Horbett TA, Ratner BD, et al. Glucose-sensitive membranes containing glucose oxidase: activity, swelling, and permeability studies. J Biomed Mater Res. 1985;19:1117–33. doi: 10.1002/jbm.820190920. [DOI] [PubMed] [Google Scholar]
- Lapeyre V, Gosse I, Chevreux S, et al. Monodispersed Glucose-Responsive Microgels Operating at Physiological Salinity. Biomacromolecules. 2006;7:3356–63. doi: 10.1021/bm060588n. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Park K. Synthesis and Characterization of Sol-Gel Phase-reversible Hydrogels Sensitive to Glucose. J Mol Recognit. 1996;9:549–57. doi: 10.1002/(sici)1099-1352(199634/12)9:5/6<549::aid-jmr299>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Liu F, Song SC, Mix D, et al. Glucose-Induced Release of Glycosylpoly(ethylene glycol) Insulin Bound to a Soluble Conjugate of Concanavalin A. Bioconjugate Chem. 1997;8:664–72. doi: 10.1021/bc970128e. [DOI] [PubMed] [Google Scholar]
- Mansouri S.1983. Optical glucose sensor based on affinity binding. PhD Dissertation at Univ of Michigan.
- Maran A, Poscia A. Continuous subcutaneous glucose monitoring: the GlucoDay system. Diabetes Nutr Metab. 2002;15:429–33. [PubMed] [Google Scholar]
- Mastrototaro JJ. The MiniMed Continuous Glucose Monitoring System. Diabetes Technol Ther. 2000;2:S13–S18. doi: 10.1089/15209150050214078. [DOI] [PubMed] [Google Scholar]
- Moussy F. Implantable glucose sensor: progress and problems. Proceedings of IEEE. 2002:270–3. [Google Scholar]
- Nathan DM, Lachin JM, Cleary P, et al. Intensive Diabetes Therapy and Carotid Intima – Media Thickness in Type 1 Diabetes Mellitus. N Engl J Med. 2003;348:2294–303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nektar Press Release 2006Pfizer Receives FDA Approval for Exubera [online]Accessed 15 December 2006. URL: http://www.nektar.com
- Obaidat AA, Park K. Characterization of glucose dependent gel-sol phase transition of the polymeric glucose-concanavalin A hydrogel system. Pharm Res. 1996;13:989–95. doi: 10.1023/a:1016090103979. [DOI] [PubMed] [Google Scholar]
- Pai CM, Bae YH, Mack EJ, et al. Concanavalin A micropheres for a self-regulating insulin delivery system. J Pharm Sci. 1992;81:532–6. doi: 10.1002/jps.2600810612. [DOI] [PubMed] [Google Scholar]
- Pai CM, Jacobs H, Bae YH, et al. Synthesis and characterization of soluble concanavalin A oligomer. Biotechnol Bioeng. 1993;41:957–63. doi: 10.1002/bit.260411006. [DOI] [PubMed] [Google Scholar]
- Patton J, Bukar J, Nagarajan S. Inhaled insulin. Adv Drug Deliv Rev. 1999;35:235–47. doi: 10.1016/s0169-409x(98)00074-x. [DOI] [PubMed] [Google Scholar]
- Patton JS, Bukar JG, Eldon MA. Clinical pharmacokinetics and phar-macodynamics of inhaled insulin. Clin Pharmacokinet. 2004;43:781–801. doi: 10.2165/00003088-200443120-00002. [DOI] [PubMed] [Google Scholar]
- Philips BJ, Meguer JX, Redman J, et al. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003;29:2204–10. doi: 10.1007/s00134-003-1961-2. [DOI] [PubMed] [Google Scholar]
- Potts RO, Tamada JA, Tierney MJ. Glucose monitoring by reverse iontophoresis. Diabetes-Metab Res. 2002;18:S49–S53. doi: 10.1002/dmrr.210. [DOI] [PubMed] [Google Scholar]
- Saha JK, Xia J, Grondin JM, et al. Acute huperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp Biol Med. 2005;230:777–84. doi: 10.1177/153537020523001012. [DOI] [PubMed] [Google Scholar]
- Saumon G, Martet G, Loiseau P. Glucose transport and equilibrium across alveolar-airway barrier of rat. Am Physiol Soc. 1996;270:L183–L190. doi: 10.1152/ajplung.1996.270.2.L183. [DOI] [PubMed] [Google Scholar]
- Skyler JS. Effects of glycemic control on diabetes complications and on the prevention of diabetes. Clin Diabetes. 2004;22:162–6. [Google Scholar]
- Steil GM, Panteleon AE, Rebrin K. Closed-loop insulin delivery-the path to physiological glucose control. Adv Drug Deliv Rev. 2004;56:125–144. doi: 10.1016/j.addr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Sood S.1999. Characterization of liposome manufacturing using extrusion. Master’s Thesis, San Jose State University.
- Traitel T, Cohen Y, Kost J. Characterization of glucose-sensitive insulin release systems in simulated in vivo conditions. Biomaterials. 2000;21:1679–87. doi: 10.1016/s0142-9612(00)00050-8. [DOI] [PubMed] [Google Scholar]
- The Registry of Toxic Effects of Chemical Substances 2006Concanavalin A [online]Accessed 15 December 2006. URL: http://www.cdc.gov/niosh/rtecs/gk692210.html
- Wigley FM, Londono JH, Wood JH. Insulin across respiratory mucosa by aerosol delivery. Diabetes. 1971;20:552–6. doi: 10.2337/diab.20.8.552. [DOI] [PubMed] [Google Scholar]
- Wood DM, Brennan AL, Philips BJ, et al. Effect of hyperglycaemia on glucose concentration of human nasal secretions. Clin Sci. 2004;106:527–33. doi: 10.1042/CS20030333. [DOI] [PubMed] [Google Scholar]