Abstract
Introduction
The in vitro and in vivo behavior of the radiolabeled monoclonal antibody MORAb-003 was investigated as a prelude to a clinical trial.
Methods
The cellular rentention of 111In and 131I labeled MORAb-003 were investigated using IGROV1 and SW620 cells. Biodistribution studies in tumor bearing mice were performed with the more favorable agent.
Results
Five DOTAs were conjugated to MORAb-003 with no apparent loss of immunoreactivity. Radiolabeled MORAb-003 had a high affinity for the folate receptor alpha expressed by both IGROV1 and SW620 cells and was found to bind to around 8 × 105 and 1.3 × 106 sites/cell respectively. Both cancer cell lines were found to internalize both 131I and 111In labeled MORAb-003, but 111In was retained and 131I was released as iodide. In athymic mice 111In-DOTA-MORAb-003 was cleared from the blood with a single exponential biological clearance rate of 110 hours. The uptake in SW620 tumors was 32±5 %ID/g after 4 days. The clearance rate of activity from normal organs such as liver, kidney and spleen was similar to the blood clearance and was 5.36, 4.03 and 4.36 %ID/g at 1 day post injection and 2.14, 1.65 and 3.74 %ID/g after 6 days. In a pilot clinical study the biodistribution and tumor targeting of 111In-MORAb-003 was assessed in 3 patients undergoing treatment with cold MORAb-003.
Conclusion
MORAb-003 is an attractive antibody for radioimmunoscintigraphy and possibly radioioimmunotherapy of FRA expressing cancers in addition to its potential direct therapeutic effects.
Introduction
Folate receptor alpha (FRA) is a 35 kDa cell surface glycoprotein which is elevated in around 90% of ovarian cancers [1]. In addition, FRA levels are high in certain other malignant tumors of epithelial origin, such as cancers of the kidney, lung, mammary gland, brain, endometrium, compared to normal cells, and are positively associated with tumor stage and grade [2].
FRA is distinct from the bulk folate carrier and is not in the pathway of cellular metabolism of folic acid, a vitamin necessary for DNA synthesis and cellular homeostasis. FRA is either absent from normal tissues or inaccessible to circulating drugs so it has frequently been exploited as a target for receptor-directed cancer therapies, including chemotherapies and immunotherapies. The chimeric monoclonal antibody MOv18 has been raised against FRA and used for antibody-dependent cellular cytotoxicity [3]. The same antibody has also been radiolabeled with a variety of radionuclides such as 131I [4-6], 212At [7] and more recently with 90Y [8] with promising results.
In this study, we report on the characterization the binding properties of MORAb-003, a new antibody against FRA and its in vitro and in vivo binding properties prior to clinical evaluation. MORAb-003 is being developed by Morphotek Inc. (Exton, PA) as a therapeutic antibody for FRA-expressing tumors. It is fully humanized and has an affinity of 2 nM for FRA.
Materials and Methods
Cell Lines and Reagents
The human ovarian adenocarcinoma cell line IGROV1 (kindly provided by J. Bernard, Institute G. Roussy, Villejuif, France) was grown in RPMI-1640 containing sodium bicarbonate supplemented with 10% fetal calf serum (FCS), 2 mM glutamine and penicillin/streptomycin. SW620 colon adenocarcinoma was obtained from ATCC and grown in Leibovitz's L-15 medium supplemented with 10% FCS, 2 mM glutamine and penicillin/streptomycin at a temperature of 37°C in an environment containing 5% CO2. Prior to use, the cells were either trypsinized, counted and suspended in serum free medium or seeded into 12 well microtiter plates and were allow to grow until subconfluant.
All reagents were obtained from commercial sources. 111In and 131I were purchased from Nordion (Kanata, Ontario). In order to reduce metallic contamination, all reagents used to modify and purify the monoclonal antibodies were made with deionized water. Ammonium acetate buffer and sodium phosphate buffer were also purified with Chelex 100 (Bio-Rad, Richmond, CA) to remove any metal ions. The monoclonal antibody MORAb-003 [9] was supplied by Morphotek.
Modification and radiolabeling of MORAb-003
MORAb-003 was radiolabeled with 131I using the Iodogen method [10]. Briefly, 131I (4-40 MBq, 1-10 μL, 0.01 M NaOH) was added to a 5 mL glass tube, coated with 50 μg of iodogen (Pierce, Rockford, IL), containing 0.1 mg of mAb in 0.1 mL of ice cold phosphate buffer (pH 7.4). This reaction mixture was allowed to react five minutes on ice before being loaded onto a 10 mL Biogel-P6 column (BioRad Laboratories, Hercules, CA) equilibrated with 1% BSA in phosphate buffered saline (PBS). The column was washed with 2 mL of 1% bovine serum albumin (BSA)/PBS before the main 131I-mAb fraction was eluted with 2 mL of 1% BSA PBS. The amount of free iodine in the 131I-mAb preparations was evaluated using instant thin layer chromatography with a silica gel impregnated glass fiber support (Gelman Sciences, Ann Arbor, MI) and a mobile phase of isotonic saline. Under these conditions, the antibody remains at the origin and free iodide moves with the solvent front so the ITLC strip was cut at an Rf of 0.5 and the two halves measured in a gamma counter to determine the radiochemical purity.
MORAb-003 was modified with 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA) and characterized for the number of binding sites attached as reported perviously [11]. Briefly, 312 mg DOTA (0.77 mmoles) and 68.5 mg N-hydroxysuccinimide (0.60 mmoles) were dissolved in 2 ml of 1 M NaOH and pH adjusted to 7.2 with NaOH. This solution was cooled in an ice bath for 30 minutes before adding 72.5 mg of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (0.39 mmoles) and the reaction allowed to proceed for 1 hour on ice to form the active ester. This active ester solution was then added to 450 mg MORAb-003 in 489 mL of 50 mM sodium phosphate (pH 7.4, 4°C) and the mixture left overnight at 4°C. The product was purified by repeated washing with 1 M ammonium acetates in a 30 kDa centrifugal filter (Amicon Centiprep YM30, Milipore MA). The DOTA-MORAb-003 conjugate was finally concentrated to 10 mg/mL and stored at 4°C until required for radiolabeling.
The DOTA-antibody conjugate was assayed for the number of DOTAs attached per antibody molecule [11]. Briefly, the conjugate was mixed with known quantity of non-radioactive indium spiked with 111In in NH4OAc (pH 6.0). After 24 hours at 37°C, a 10 fold excess of DTPA (in relation to [In3+]) was added to the mixtures to chelate any unbound indium. The mixtures were analyzed using ITLC-SG using a solvent of 5 mM DTPA (pH 5.0). Under these conditions, the antibody remains at the origin and unchelated 111In moves with the solvent front as a DTPA chelate. The ITLC strip was cut at an Rf of 0.5 and the two halves were measured in a gamma counter to determine the amount of 111In bound to the antibody and hence the number of DOTAs bound per antibody.
The DOTA-MORAb-003 conjugate was radiolabeled with 111In by adding 10 μL of 111InCl3 (40 MBq, 0.01 M HCl) to 20 μL antibody (10 mg/mL, 1.0 M NH4OAc, pH 7.0) and allowing the mixture to react at 37°C for 20 minutes. The reaction mixture was quenched with 10 μL of 5 mM DTPA (pH 7.0) then separated on a 10 mL Biogel-P6 column. Quality control was performed with the same ITLC-SG method as described earlier.
The stability of the 111In-DOTA-MORAb-003 was examined by studying the transchelation of 111In to DTPA. Briefly, 400 kBq of radiolabeled antibody (ca 1 μg in 100 μL of 1% BSA PBS) was diluted with 100 μL of 50 mM DTPA (pH 7.0) and maintained at 37 °C. Periodically samples were removed and analyzed by ITLC as described earlier.
Immunoreactivity
The immunoreactivities of the 131I and 111In labeled mAb preparations were assessed by the method of Lindmo [12] which extrapolates the binding of the radiolabeled antibody at an infinite excess antigen. Briefly six test solutions were prepared (in triplicate) and contained 20 000 cpm of the radioiodinated antibody and increasing amounts of SW620 or IGROV1 cells, in a total test volume of 500 μL of PBS (0.2 % BSA, pH 7.4). A blank test solution with no cells was also made. The solutions were incubated at 37°C for 45 minutes prior to being filtered through a glass membrane filter and washed with ice cold 10 mM Tris 0.9% NaCl buffer. Filters were counted in a gamma counter with standards representing the total radioactivity added. Data were then plotted as the reciprocal of the cell number (x-axis) against the reciprocal of the fraction bound (y-axis). The data was then fitted according to a least squares linear regression method using Origin software (Microcal, Software Inc., Northampton, MA). The y intercept gave the reciprocal of the immunoreactive fraction.
Saturation Binding Studies
Saturation binding studies were performed with both radiolabeled antibodies using substrates of intact SW620 or IGROV1 cells. Briefly, ten test solutions were prepared (in triplicate) and they contained increasing amounts of the radioiodinated antibodies, 500 000 cells in a total volume of 500 μL of PBS (0.2 % BSA, pH 7.4). The solutions were incubated at ambient temperature for 1 hour, and centrifuged and washed twice with ice cold PBS (0.2 % BSA). For each concentration of radiolabeled antibody, non-specific binding was determined in the presence of 100 nM of the unmodified antibody. The data was analyzed with a least squares regression method (Origin, Microcal, Software Inc., Northampton, MA) to determine the Kd and Bmax values and a Scatchard transformation performed.
Cell internalization of 131I and 111In labeled MORAb-003
SW620 cells were plated in 6 well micro plates and allowed to grow until confluent. An initial study into cellular uptake of radiolabeled antibodies consisted of adding 40 kBq of either the 131I or 111In labeled forms of MORAb-003 (ca 0.1-0.2 μg) to the plated cells and allowed them to incubate for up to two days at 37°C. After various time points, triplicate samples were periodically removed, the media isolated and the cells washed with fresh media. Surface bound activity was then stripped and collected with an ice cold acid wash (100 mM acetic acid 100 mM glycine pH 3.0). The cells were then dissolved with 1 ml 1 M NaOH and collected. At the end of the study all samples were counted with a gamma counter together with standards representing the initial amount of radioactivity added. The solutions were then analyzed by ITLC-SG with mobile phases of either 0.9% NaCl or 5 mM DTPA (pH 7.0) to determine unbound 131I or 111In, respectively.
In a second study the cellular retention of the radiolabeled antibodies was investigated following a preincubation with the radiolabeled antibody. 40 kBq of either the 131I or 111In labeled forms of MORAb-003 (ca 0.1-0.2 μg) were added to the plated cells and then allowed to stand at 37°C for either one or 16 hours. The media was then removed and the cells washed once with fresh media. One milliliter of fresh media was added and the cells incubated, for up to two days, at 37°C. Triplicate samples were periodically removed and the location of the radioactivity determined as described above.
Biodistribution Studies
Eight to nine week old nu/nu athymic female mice were obtained from the National Cancer Institute-Frederick Cancer Center and maintained in ventilated cages. These mice were injected sc in the flank with 10 × 106 SW620 cells in 200 μL of 50% matrigel. The animals were fed and watered ad libertum. Experiments were carried out under an IACUC approved protocol and they followed institutional guidelines for the proper and humane use of animals in research. Tumors, with volumes of 100-200 mg were produced in around 2-3 weeks. These mice were injected, via the tail vein, with 400 KBq of 111In-DOTA-MORAb-003 mAb (400 MBq/mg) in 200 μL of PBS (pH 7.4, 1% BSA). Groups of animals were sacrificed, by CO2 asphyxiation, after 1, 2, 4, 6 or 8 days and the major organs and tumors recovered. These samples were weighed and counted, with appropriate standards in an automatic NaI(Tl) counter. These measured relative activity data (cpm) were background corrected and expressed as a percentage of the injected dose per gram (%ID/g). These data were also fitted with a least squares regression analysis (Microcal Origin, Northampton, MA) to determine the biological clearance of the various agents.
Clinical Studies
The biodistribution of 111In MORAb-003 was examined in three patients who were participating in an ongoing clinical study of cold MORAb-003. This phase I clinical trial featured escalating doses of MORAb-003 in patients with recurrent epithelial ovarian cancer and was conducted under the auspices of Memorial Sloan Kettering Institutional Review Board (IRB# 05-055). Sequential cohorts of patients received four weekly infusions of MORAb-003 at dose levels from 12.5 mg/m2 to 400 mg/m2. Three patients, receiving MORab-003 as part of this study, were co-administered 7 mCi (range 6.9-7.7) of 111In DOTA MORAb-003 antibody on the first and fourth infusion cycles. The MORab-003 antibody dose levels were 100, 200 and 400mg/m2 for the three patients (absolute dose range 142-674mg).
Planar whole body gamma camera images were acquired on the day of infusion and on two subsequent occasions (2-3 days post-injection and 5-6 days post-injection). SPECT-CT images of involved regions were also acquired on at least one occasion. Blood samples were taken at 0.5, 1, 2, 4 hours post-injection and at the times of subsequent images (nominally 44 and 120 hours post–injection).
Whole body clearance and organ distribution were derived from planar gamma camera images. Regions of interest (ROI) corresponding to whole body, liver, spleen, index lesions and appropriate tissue backgrounds were drawn on geometric mean images. Tissue background-subtracted counts were normalized to the initial whole body count, corrected for physical decay and expressed as a percentage of the administered dose (%ID). Lesion volumes were estimated from CT scan and used to determine lesion percentage administered dose per unit volume (%ID/ml).
Aliquots of serum were counted in a gamma well counter together with appropriate 111In standards. Decay-corrected serum activity concentrations were expressed in terms of percentage administered dose per liter (%ID/L). Biexponential clearance curves were fit to the data and pharmacokinetic parameters estimated.
Paired t-tests were used to examine differences in pharmacokinetic and biodistribution parameters between the administrations of 111In DOTA MORAb-003
Results
Radiolabeling and quality control
The radioiodination yield for MORAb-003 was typically around 95 % and the amount of free iodine in the purified mAb was below 0.3%. Specific activities of up to 350 MBq/mg were routinely achieved. The immunoreactivities of the 131I labeled mAb, as determined by extrapolating the binding of a fixed amount of 131I labeled mAb to infinite amount of FRA antigen on SW620 cells, were 85% regardless of the specific activity of the radiolabeled antibody indicating that the radiolabeling process has little or no impact on the immunoreactivity of the antibody.
Up to five DOTA molecules were conjugated to MORAb-003, with little apparent loss of immunoreactivity. A 97% incorporation of 111In could be achieved within 5 minutes and specific activities of 350 MBq 111In/mg DOTA-MORAb-003 could be achieved. The resultant radiolabeled MORAb-003 was stable against transchelation of 111In with around 1.5% being lost over a ten day period. The radiochemical purity of the 111In-DOTA-MORAb-003 used in all in vitro and in vivo studies was >99.7%. PAGE and HPLC size exclusion chromatography analysis of the radiolabeled antibodies showed no aggregates (data not shown).
Saturation binding Studies
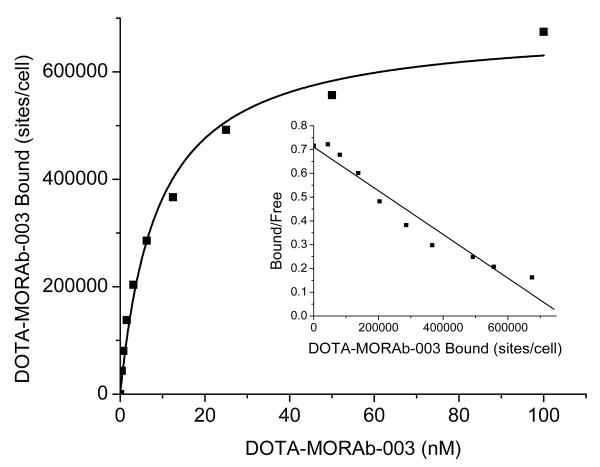
The saturation binding curves generated were characteristic of high affinity binding of an antibody to a single class of antigen. These studies, performed with intact SW620 cells (figure 1) and IGROV1 cells (data not shown), demonstrated that MORAb-003 had a 10-20 nM affinity to the folate receptor alpha and around 8.3 × 105 sites per cell were found for IGROV1 and 6.9 × 105 sites for SW620.
Figure 1.
Saturation binding curve for 111In-DOTA-MORAb-003 and SW620 cells (inset Scatchard transformation of data)
Internalization and Cellular processing of 111In and 131I labeled MORAb-003
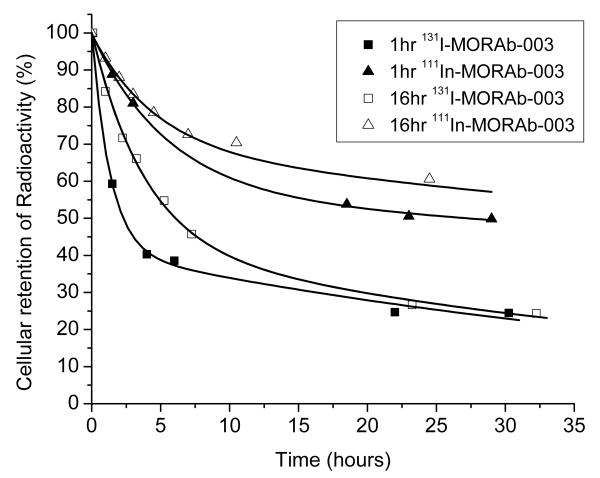
The overall retention of radioactivity, after the 1 or 16 hour incubation of IGROV1 cells with either 111In or 131I labeled MORAb-003 is shown in figure 2. 131I was rapidly lost from IGROV1 cells after either a 1 or 16 hour preincubation with 131I-MORAb-003. After 24 hours only around 25 % of the initial activity was still bound to the IGROV1 cells. By comparison the cells treated with 111In-DOTA-MORAb-003 retained more 111In over the 24 hour study period and had around 50 to 60% of the 111In still associated with the cells.
Figure 2.
Cellular retention (cell surface and internalized) of 131I-MORAb-003 and 111In-DOTA-MORAb-003 following a 1 or 16 hour preincubation with IGROV1 cells at 37°C.
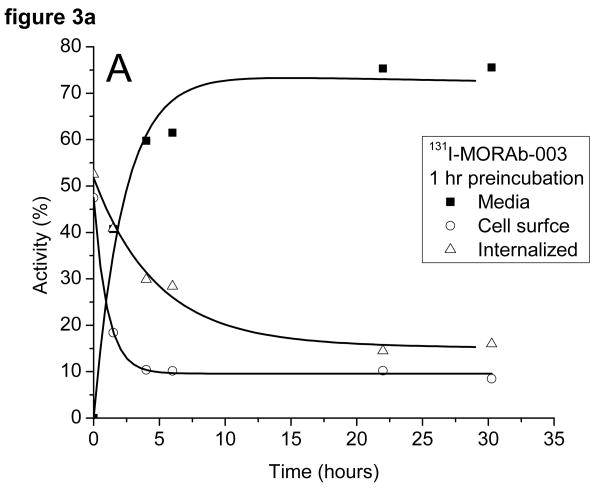
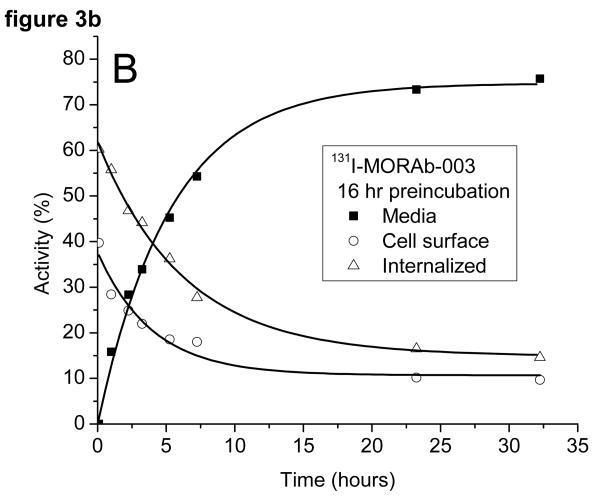
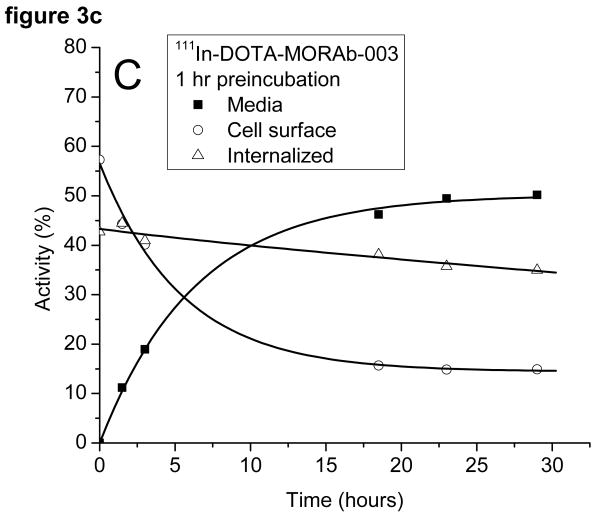
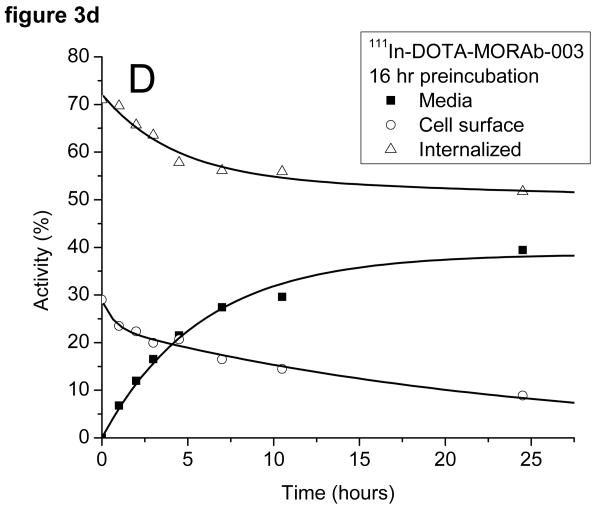
The radioactivity associated with the cells was examined to see if it was bound to the outside of the cell and could be removed by washing the cells with a low pH buffer or internalized (acid wash resistant). The cells preincubated with 131I-MORAb-003 had around 50% of the activity bound to the cell surface and 50% internalized at the start of the study (cf figure 3a). The surface bound activity was rapidly lost from the cells as was the internalized activity. There was little difference in the amounts of 131I on the cell surface or internalized when the cells were preincubated for 1 or 16 hours (c.f. figure 3b) with the radiolabeled antibody. The same experiment, when performed with 111In-DOTA-MORAb-003 showed a pronounced difference in the initial amounts of cell surface bound and internalized activity (cf figures 3c and 3d). After a 1 hour preincubation approximately 40% of the activity was internalized and after a 16 hour preincubation approximately 70% was internalized. The various fractions of the 131I studies were analyzed for protein bound 131I and 131I-iodide. Cell bound activity was >98% protein bound whereas increasing amounts of 131I-Iodide were found in the media and at 24 hours represented 35% of all activity. Consequently the amounts of protein associated radioactivity in the cell media was around 50% for both 131I and 111In labeled MORAb-003 at 24 hours regardless of how long the cells were preincubated with radiolabeled antibody. The reason for this lower cellular retention of 131I is due to the cellular metabolism and release of 131I-iodide.
Figure 3.
Distribution of radioactivity in IGROV1 cells following incubation at 37°C with either 131I-MORAb-003 (A: 1 hour and B: 16 hours) or 111I-DOTA-MORAb-003 (C: 1 hour and D: 16 hours).
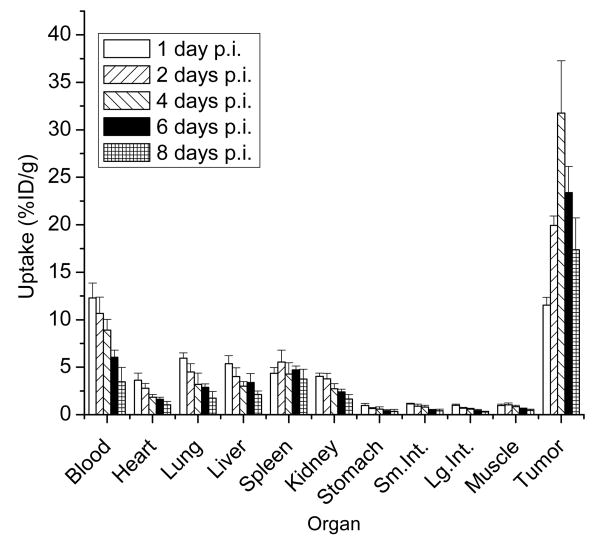
Animal biodistribution studies
The eight day biodistribution of 111In-DOTA-MORAb-003 in athymic mice bearing SW620 tumors is shown in figures 4 and tabulated in table 1. For most normal organs there was a progressive washout of radioactivity which mimicked the clearance of the radioactivity from the blood. The blood clearance data could be fitted to a mono exponential function which gave a biological half-life of 112 ±23 days. The tumor uptake peaked at 4 days post injection
Figure 4.
Biodistribution of 111In-DOTA-MORAb-003 in athymic mice with SW620 tumors.
Table 1.
Biodistribution of 111In-DOTA-MORAb-003 in athymic mice with SW620 tumors (%ID/g ±stdev).
| Time post injection | |||||
|---|---|---|---|---|---|
| Organ | Day 1 | Day 2 | Day 4 | Day 6 | Day 8 |
| Blood | 12.29 ±1.57 | 10.67 ±1.72 | 8.89 ±1.14 | 6.04 ±0.75 | 3.45 ±1.54 |
| Heart | 3.63 ±0.74 | 2.79 ±0.51 | 1.85 ±0.29 | 1.61 ±0.24 | 1.05 ±0.35 |
| Lung | 5.96 ±0.54 | 4.50 ±0.86 | 3.19 ±1.19 | 2.88 ±0.37 | 1.75 ±0.70 |
| Liver | 5.36 ±0.85 | 4.01 ±0.92 | 3.00 ±0.48 | 3.39 ±0.94 | 2.14 ±0.35 |
| Spleen | 4.36 ±0.60 | 5.55 ±1.25 | 4.27 ±1.19 | 4.71 ±0.42 | 3.74 ±1.05 |
| Kidney | 4.03 ±0.34 | 3.78 ±0.57 | 2.74 ±0.52 | 2.40 ±0.28 | 1.65 ±0.49 |
| Stomach | 0.96 ±0.23 | 0.66 ±0.08 | 0.57 ±0.25 | 0.41 ±0.13 | 0.35 ±0.20 |
| Sm Intestine | 1.13 ±0.08 | 0.90 ±0.21 | 0.79 ±0.21 | 0.49 ±0.07 | 0.40 ±0.17 |
| Lg intestine | 0.98 ±0.14 | 0.68 ±0.10 | 0.57 ±0.11 | 0.43 ±0.12 | 0.28 ±0.09 |
| Muscle | 0.96 ±0.16 | 1.03 ±0.21 | 0.85 ±0.15 | 0.61 ±0.08 | 0.45 ±0.12 |
| Tumor | 11.54 ±0.83 | 19.93 ±0.99 | 31.76 ±5.52 | 23.38 ±2.78 | 17.36 ±3.36 |
Clinical Studies
Images were assessed visually for distribution and tumor targeting. Initial images on the day of administration showed accumulation of tracer in the blood pool of heart and vasculature in the body. This decreased steadily through delayed images. Lesion targeting was seen in all three patients and was seen best in the later images done between 4-6 days post injection (figure 5 and 6).
Figure 5.

SPECT-CT for a patient with metastatic ovarian cancer injected with 7.23 mCi of In-111 MORAb-003 co-infused with a total 352 mg of MORAb-003 (200mg/m2). Images were obtained 5 days after antibody administration with a 360 degree acquisition lasting 32 minute, using a SPECT/CT scanner. Localization of antibody is seen in a metastatic soft tissue mass lying against the abdominal rectus muscle (arrow), with corresponding CT (left panel), In-111 Morab-003 images (middle panel) and fused images (right panel).
Figure 6.
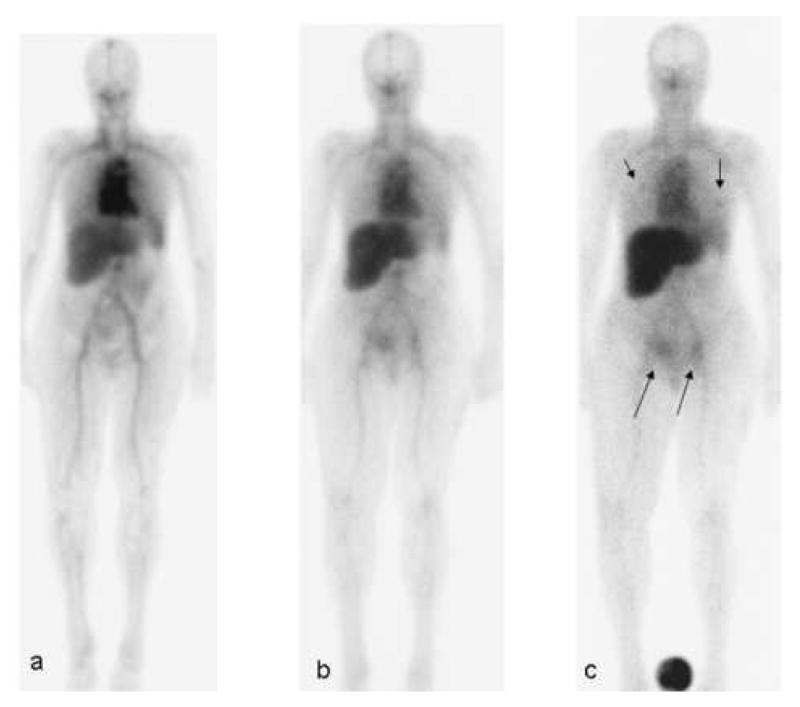
Patient with metastatic ovarian cancer. The patient underwent imaging after intravenous injection of 7.22 mCi of 111In DOTA MORAb-003, co-infused with a total of 677 mg of MORAb-003 (400 mg/m2). Serial 23 minute whole body scans were obtained within 2 hr of injection (a), 2 days (b) and 5 days (c). At 5 days a standard is shown in the field of view between the patient's feet. Arrows indicated areas of metastatic ovarian cancer visualized, 1 in each lung (short arrows) and in the right anterior pelvic wall lesion and a left inguinal node (long arrows).
On average, 111In-DOTA-MORAb-003 plasma levels were 46 ±8 %ID/L at the end of infusion, decreasing to 16 ±2 %ID/L by 5 days post-injection. Plasma clearances were biphasic with an average alpha component of 15 ±4 %ID/L having a biological half-life of 5.4 ±4.5 hours and a beta component of 31 ±7 %ID/L having a biological half-life of 133 ±37 hours. The liver and spleen were clearly visualized with average initial uptakes of 9 ±1% and 0.96 ±0.21 %ID respectively and average uptakes at 5 days post-injection of 13 ±4% and 0.92 ±0.30 %ID respectively. There was gradual accumulation of radioactivity into the liver over the five day observation period and no apparent biological clearance from the spleen.
A total of five soft tissue index lesions were examined in three patients consisting of four pelvic and one lung sites of disease. Maximum lesion uptake was typically observed at 5 days post-administration. The average value of this parameter was estimated as 0.012 ±0.003 %ID/ml (range: 0.008 – 0.017).
Differences in serum pharmacokinetic and planar image-derived parameters between the initial study and the study done during the patients 4th cycle were examined using paired t-tests (Table 2). No significant differences in any parameter were observed.
Table 2.
| Dx1 | Dx2 | pValue | |
|---|---|---|---|
| Administered activity (mCi) | 7.29 ± 0.39 | 7.22± 0.18 | 0.83 |
| Co (%ID/L) | 47 ± 11 | 45 ± 8 | 0.58 |
| Vd (L) | 2.21± 0.48 | 2.26 ± 0.35 | 0.76 |
| Terminal T1/2 (h) | 142 ± 46 | 125 ± 35 | 0.69 |
| AUC (%ID*h/L) | 6500 ± 1600 | 6200 ± 1300 | 0.85 |
| Initial Liver uptake (%ID) | 10.1 ± 1.8 | 8.7 ± 0.2 | 0.31 |
| Final Liver uptake (%ID) | 12.2 ± 4.3 | 13.3 ± 4.0 | 0.28 |
| Initial Spleen uptake (%ID) | 1.1 ± 0.2 | 0.8 ± 0.2 | 0.31 |
| Final Spleen uptake (%ID) | 0.95 ± 0.3 | 0.9 ± 0.4 | 0.28 |
| Maximum lesion uptake (%ID/ml) | 0.013 ± 0.003 | 0.011 ± 0.003 | 0.35 |
Co=concentration at T=zero
Vd=Volume of Distribution
Follow up in these patients showed stabilization of disease in two patients and progression in third patient at 6-8 weeks.
Discussion
In order to understand the characteristics of, and select the best imaging/therapeutic agent, we studied binding characteristics and retention rates of 131I and 111In labeled forms of MORAb-003 using IGROV1 cells which express the folate receptor alpha.
The initial labeling of the mAb with 131I, up to a specific activity of 350 MBq/mg, resulted in little or no apparent loss of immunoreactivity. Similarly the conjugation of up to an average of 5.5 DOTA chelates per mAb enabled specific activities of up to 350 MBq 111In/mg DOTA-MORAb-003 to be obtained with no apparent loss of immunoreactivity. Site specific modification of the antibody is sometimes required when this type of random DOTA coupling results in loss of immunoreactivity due to presence of a lysine residue in the antigen binding domain. High specific activities are often required for accurate mAb characterization and particularly when large amounts of the radiotherapeutic agent are administered to a patient. This level of DOTA conjugation allows us to label at specific activities of 350 MBq/mg with excellent incorporation and is sufficient for in vitro binding studies and in vivo studies.
As our previous studies have demonstrated, internalizing antibodies are best labeled with radiometals to enhance the retention of radioactivity [10]. Early approaches to labeling mAbs with radiometals utilized diethylenetriaminepentaacetic acid (DTPA), which in its dicyclic anhydride form could be conveniently coupled to mAbs [13]. Unfortunately this simple coupling chemistry produced a more labile chelate than bifunctional forms of the same unconjugated DTPA chelator [14]. Macrocyclic chelators have shown even higher kinetic stability [15], but truly bifunctional forms are even more time consuming to chemically synthesize [16] than bifunctional DTPA. The monofunctional 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA) can be coupled directly to mAbs using simple chemistry and commercially available materials [10].
DTPA binds 111In more rapidly than DOTA, but an incubation period of 5 minutes can give high labeling yields for the DOTA-mAb conjugate. The DOTA chelator was immensely superior to DTPA in its ability to tightly chelate 111In in the presence of an excess of competing ligand. This is in agreement with other studies [16-17] and underlies the importance of using stable chelates with monoclonal antibodies, which can stay in circulation for prolonged periods of time in the presence of competing ligands (e.g. transferrin). The higher stability of the 111In-DOTA complex relative to the 111In-DTPA complex also applies for the 90Y complex [16-17] and is an important prerequisite for radiolabeled mAbs used for either diagnosis or therapy since optimal tumor/non-tumor ratios are often achieved after several days. Since 111In-DOTA-MORAb-003 is stable to DTPA competition, it enables non-specifically bound 111In to be removed by challenging with DTPA and a simple column separation to yield a highly pure radiopharmaceutical.
Effective systemic targeting of tumors has been achieved with both iodinated and radiometal chelated antibodies. A significant factor in selecting between the two approaches is in whether the antibody is internalized or not. In the case of an internalizing antibody, directly iodinated antibodies are metabolized within the cell, and the main metabolites of iodide and iodotyrosine are then freely released from the cell. Conversely the metal chelate labeled antibody is metabolized to leave a chelate-amino acid fragment which is typically not released from the cell and is trapped in for further degradation. This effect can produce vastly different residence times for two differently labeled forms of the same antibody [18]. FRA is known to be an internalizing receptor system [19], these studies clearly support the notion that radiometal labeled mAbs are superior for cells expressing internalizable FRA and that metabolites from the DOTA-mAb conjugate are not appreciably released from IGROV1 cells whereas 131I is released.
FRA levels and internalization rates have been determined by various groups [20-21]. Miotti et al [20] found around 106 and 105 FRAs per cell for IGROV1 and SKOV3 cells using 125I-folic acid and Paulos et al [21] found around 10 × 106 sites using 3H-folic acid and 111In-DTPA-folate. Rates of internalization of 1 to 3 × 105 molecules per hour were reported for cells line such as HeLa, KB, IGROV1, M109 and HS597T. In our studies we observed around 8.3 × 105 FRAs sites per IGROV1 cell using 111In-MORAb-003 and around 1.5 × 105 molecules internalized during the first hour of incubation.
Comparison of the blood clearance of 111In-DOTA-MORAb-003 with 125/131I labeled murine MOv18 [5] showed a longer single half-life of around 110 hours compared to shorter 3.6 hour alpha and 80-90 hour beta phases. The 90Y labeled MOv-18 demonstrated a beta phase blood clearance of 51±7 hours non tumor bearing animals [8]. 111In-DOTA-MORAb-003 showed a lower initial accumulation than 90Y-MOv18 in normal organs such as liver (5.36 vs 11.15), spleen (4.36 vs 6.16) and kidneys (4.03 vs 6.16) at one day post injection as well as at 6 days post injection (3.39, 4.71 and 2.4 vs 7.43, 6.30 and 4.27 respectively).
The images of the clinical study subject demonstrated good tumor uptake and retention of the 111In-DOTA-MORAb-003. For the patients studied with radiolabeled MORAb-003, blood clearances were biphasic with an average beta biological half-life of 133 ±37 hours. This was comparable to that observed for mice (112 ±23 hours). The kinetics of clearance of 111In-DOTA-MORAb-003 from liver and spleen were also comparable between humans and mice in that there was little clearance over the 4 day human and 8 day murine study periods.
In conclusion,111In-DOTA-MORAb-003 is able to associate more radioactivity with IGROV1 cells than the comparable iodinated forms. The 111In labeled DOTA conjugate is stable against loss of 111In in vitro and in vivo. In mice 111In-DOTA-MORAb-003 has a slow monophasic blood clearance and a high 32±5 %ID/g tumor uptake at 4 days p.i..
Given the distribution of FRA, the specificity of binding of 111In-DOTA-MORAb-003 to this receptor, the in vitro stability of the chelated radiopharmaceutical, the fully humanized status of this reagent and the excellent targeting in tumor xenografts, these findings make DOTA-MORAb-003 an attractive candidate for further evaluation as either diagnostic or radiotherapeutic agents. Phase 2 imaging and immunotherapy clinical trails are ongoing in patients with ovarian cancer
Acknowledgments
The authors are grateful for the support of this research through grants from the Ludwig Center for Cancer Immunotherapy and the NIH Immunology Program Project Grant 5PO1 CA33049.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rettig WJ, Cordon-Cardo C, Koulos JP, Lewis JL, Jr, Oettgen HF, Old LJ. Cell surface antigens of human trophoblast and choriocarcinoma defined by monoclonal antibodies. Int J Cancer. 1985;35(4):469–75. doi: 10.1002/ijc.2910350409. [DOI] [PubMed] [Google Scholar]
- 2.Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int J Cancer. 2006;119(2):243–50. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]
- 2.Lu Y, Low PS. Immunotherapy of folate receptor-expressing tumors: review of recent advances and future prospects. J Control Release. 2003;91(12):17–29. doi: 10.1016/s0168-3659(03)00215-3. [DOI] [PubMed] [Google Scholar]
- 3.van Zanten-Przybysz I, Molthoff C, Gebbinck JK, et al. Cellular and humoral responses after multiple injections of unconjugated chimeric monoclonal antibody MOv18 in ovarian cancer patients: a pilot study. J Cancer Res Clin Oncol. 2002;128(9):484–92. doi: 10.1007/s00432-002-0348-z. [DOI] [PubMed] [Google Scholar]
- 4.Molthoff CF, Buist MR, Kenemans P, Pinedo HM, Boven E. Experimental and clinical analysis of the characteristics of a chimeric monoclonal antibody, MOv18, reactive with an ovarian cancer-associated antigen. J Nucl Med. 1992;33(11):2000–5. [PubMed] [Google Scholar]
- 5.Gadina M, Canevari S, Ripamonti M, Mariani M, Colnaghi MI. Preclinical pharmacokinetics and localization studies of the radioiodinated anti-ovarian carcinoma MAb MOv18. Int J Rad Appl Instrum B. 1991;18(4):403–8. doi: 10.1016/0883-2897(91)90067-u. [DOI] [PubMed] [Google Scholar]
- 6.Andersson H, Lindegren S, Back T, Jacobsson L, Leser G, Horvath G. Biokinetics of the monoclonal antibodies MOv 18, OV 185 and OV 197 labelled with 125I according to the m-MeATE method or the Iodogen method in nude mice with ovarian cancer xenografts. Acta Oncol. 1999;38(3):323–8. doi: 10.1080/028418699431393. [DOI] [PubMed] [Google Scholar]
- 7.Andersson H, Elgqvist J, Horvath G, et al. Astatine-211-labeled antibodies for treatment of disseminated ovarian cancer: an overview of results in an ovarian tumor model. Clin Cancer Res. 2003;9(10 Pt 2):3914S–21S. [PubMed] [Google Scholar]
- 8.Coliva A, Zacchetti A, Luison E, et al. 90Y Labeling of monoclonal antibody MOv18 and preclinical validation for radioimmunotherapy of human ovarian carcinomas. Cancer Immunol Immunother. 2005;54(12):1200–13. doi: 10.1007/s00262-005-0693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebel W, Routhier EL, Foley B, et al. Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-alpha. Cancer Immun. 2007;7:6–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Markwell MA, Fox CF. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978;17(22):4807–17. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- 11.Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, et al. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res. 2000;60(18):5237–43. [PubMed] [Google Scholar]
- 12.Lindmo T, Bunn PA., Jr Determination of the true immunoreactive fraction of monoclonal antibodies after radiolabeling. Methods Enzymol. 1986;121:678–91. doi: 10.1016/0076-6879(86)21067-8. [DOI] [PubMed] [Google Scholar]
- 13.Roselli M, Schlom J, Gansow OA, et al. Comparative biodistributions of yttrium- and indium-labeled monoclonal antibody B72.3 in athymic mice bearing human colon carcinoma xenografts. J Nucl Med. 1989;30(5):672–82. [PubMed] [Google Scholar]
- 14.Meares CF, Moi MK, Diril H, et al. Macrocyclic chelates of radiometals for diagnosis and therapy. Br J Cancer Suppl. 1990;10:21–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Moi MK, DeNardo SJ, Meares CF. Stable bifunctional chelates of metals used in radiotherapy. Cancer Res. 1990;50(3 Suppl):789s–793s. [PubMed] [Google Scholar]
- 16.Camera L, Kinuya S, Garmestani K, et al. Evaluation of the serum stability and in vivo biodistribution of CHX-DTPA and other ligands for yttrium labeling of monoclonal antibodies. J Nucl Med. 1994;35(5):882–9. [PubMed] [Google Scholar]
- 17.Lewis MR, Raubitschek A, Shively JE. A facile, water-soluble method for modification of proteins with DOTA. Use of elevated temperature and optimized pH to achieve high specific activity and high chelate stability in radiolabeled immunoconjugates. Bioconjug Chem. 1994;5(6):565–76. doi: 10.1021/bc00030a012. [DOI] [PubMed] [Google Scholar]
- 18.Stein R, Chen S, Haim S, Goldenberg DM. Advantage of yttrium-90-labeled over iodine-131-labeled monoclonal antibodies in the treatment of a human lung carcinoma xenograft. Cancer. 1997;80(12 Suppl):2636–41. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2636::aid-cncr39>3.3.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Lee RJ, Low PS. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J Biol Chem. 1994;269(5):3198–204. [PubMed] [Google Scholar]
- 20.Miotti S, Facheris P, Tomassetti A, et al. Growth of ovarian-carcinoma cell lines at physiological folate concentration: effect on folate-binding protein expression in vitro and in vivo. Int J Cancer. 1995;63(3):395–401. doi: 10.1002/ijc.2910630316. [DOI] [PubMed] [Google Scholar]
- 21.Paulos CM, Reddy JA, Leamon CP, Turk MJ, Low PS. Ligand binding and kinetics of folate receptor recycling in vivo: impact on receptor-mediated drug delivery. Mol Pharmacol. 2004;66(6):1406–14. doi: 10.1124/mol.104.003723. [DOI] [PubMed] [Google Scholar]