Abstract
This special issue of Hormones and Behavior, “Androgens in health and disease: new insights into roles and mechanisms of action,” is prompted by a number of relatively recent findings that androgens affect brain morphology and function in ways not previously or widely appreciated. Moreover, recent results also make it clear that androgens utilize a variety of signaling molecules to exert their effects on the brain, which may or may not depend on the classic nuclear androgen receptor (AR). The papers in this issue underscore these two points. This overview is not intended as a comprehensive review of androgen action on the nervous system, since the papers in this issue serve that purpose, but rather to frame the basic issues and themes that tie these papers together. The sum effect of the stories told in this issue encourages us to broaden and refocus our view of androgen action on brain and behavior—to recognize that androgens affect many aspects of brain structure and function throughout the lifespan, from shaping its sexual phenotype to influencing its propensity for disease and repair, and that at least some of these actions are exerted via non-classical modes of action that in many cases were first identified in non-neural tissue or cells.
Setting the stage: lessons from the periphery
Sexual differentiation depends on testicular androgens
Perhaps the first lesson that studies in the periphery taught us is that the same factors driving sexual differentiation of the periphery in mammals also drive sexual differentiation of the brain and behavior. What are these factors? Androgens produced and secreted by the testes. While the story is admittedly a bit more complicated than this, several decades of research support this basic tenet that testicular androgens critically mediate sexual differentiation of both the periphery and the brain and thus, behavior. Among the earlier work that was influential in giving rise to this basic tenet was that of Alfred Jost followed by the work of Phoenix, Goy, Gerall and Young.
In the 1940s and 50s, Jost and colleagues carried out elegant experiments to identify the factors involved in sexual differentiation of the genital tract (Jost, 1953, 1972). By studying the effects of removing the gonads from fetal rabbits and treating castrated fetuses with testosterone (T), Jost concluded that the testes and not the ovaries are responsible for sexual differentiation of the external genitalia, and that the testes exert their influence on genital differentiation by secreting androgens that masculinize genital development. For example, Jost demonstrated that when the testes were removed prenatally in rabbits before any apparent sexual differentiation of their genitalia, feminine genitalia developed. However, if such castrated males were also treated with T during fetal life, then their external genitalia resumed a masculine pattern of development. Importantly, removing testes after birth had no such effect on the sexual phenotype of the genitalia; i.e., they still had a masculine phenotype. On the other hand, when the ovaries were removed from fetal females, their genitalia developed normally, consistent with the genetic sex of the animal, suggesting that the ovaries play little to no role in the sexual differentiation of the external genitalia. Finally, female fetuses treated with T developed masculinized genitalia, showing that the undifferentiated primordium that gives rise to the genitals has the potential to form genitalia of either sex, but the decision of which sex depends on androgens produced by the testes.
The seminal paper published by Phoenix et al in 1959 (Phoenix et al., 1959) reports a series of experimental findings suggesting that this basic principle also holds true for behavior and thus, sex-specific morphogenesis of the brain. Given that Jost had found that prenatal androgen treatment could masculinize the external genitalia of female rabbits, Phoenix et al. tested the role of prenatal androgens on adult sex behavior by treating pregnant guinea pigs with T and examining the behavior of their offspring in adulthood. Their findings clearly showed that early androgen exposure profoundly influences behavior in adulthood, essentially preventing prenatally androgenized females from displaying feminine receptive behavior in adulthood. Paralleling Jost's findings, the effects of early T on the sex behavior of females were permanent, and androgen treatment after birth did not result in the same lasting effects. Based on these results, Phoenix et al. (1959) concluded that “…the prenatal period is a time when fetal morphogenic substances (androgens) have an organizing or “differentiating” action on the neural tissues mediating mating behavior.” They ended this paper by drawing our attention to an important question left unanswered by their studies, that is, whether androgens work in a generalized or localized fashion to influence differentiation of the brain. While evidence gathered since 1959 converges on the idea that hormones act locally to influence specific brain regions and/or circuits, in most cases, the actual cellular targets that receive and transduce gonadal steroid signals remain unidentified. Again, studies of hormone action in the periphery suggest another lesson that is likely to have broad relevance to hormone action throughout the body, including the brain.
Hormones often act indirectly to affect cells
Through another series of technically challenging but elegant experiments conducted by Gerald Cunha and his colleagues (Cunha et al., 2004), it became clear that cellular responses to gonadal hormones in the periphery are often indirect and involve cell-cell interactions. For the last twenty years, Cunha and his group have systematically assessed the role of hormone receptors in epithelial versus mesenchymal cell layers of hormone-dependent sexually differentiated peripheral organs, such as the prostate and the mammary glands. For both of these organs, it is clear that most of the androgen-dependent development of the epithelium is mediated indirectly, via ARs in the mesenchyme, and not via direct action on the epithelial tissue itself. We will discuss briefly their specific findings on the prostate.
Under ordinary circumstances, only males have a prostate. As clearly demonstrated by Jost, development of the prostate is highly dependent on testicular androgens. If androgens or functional ARs are lacking, the prostate fails to develop. The adult prostate contains sleeves of smooth muscle or stromal cells and fibroblasts (each of mesenchymal origin) that surround the epithelial ducts; adult cells in each layer express ARs. This raises the obvious question of whether androgens are acting via ARs in one or both cell layers to promote prostate development. Using a clever tissue recombinant strategy, Cunha set out to identify which cells in the prostate—epithelial, mechechymal or both—respond directly to androgens to promote its development. To accomplish this, prostate primordia containing both mesenchyme and epithelium were collected from male wildtype (wt) and AR null mice (having the testicular feminization mutant (tfm) allele of the AR gene) and the two cell layers were recombined after enzymatic separation, yielding four different types of recombinants, either wholly wt, wholly tfm, or recombinants of wt and tfm tissue that lacked AR in either the mechenchymal or epithelial cell layers. The tissue recombinants were then grown in hosts to allow for organogenesis and differentiation. The data from these sorts of experiments consistently show that the androgen-dependent formation of the epithelial ducts is mediated by ARs in mesenchymal cells. That is, androgens activate ARs in mesenchymal cells which, via paracrine influences, drive epithelial cell proliferation and other cellular processes involved in duct formation. In short, epithelial ARs are inconsequential for this process. However, not all aspects of prostate development are mediated by mesenchymal AR. One function of epithelial AR is to control the expression of androgen-dependent secretory proteins.
Similar experiments done in other hormone-dependent peripheral organs such as the uterus and the mammary glands lead to similar conclusions that hormone receptors in mesenchymal cells often direct the hormone-dependent development of the adjacent epithelium. Whether indirect action of androgens is also a basic principle that holds for the nervous system has yet to be firmly established. However, findings based on the spinal nucleus of the bulbocavernosus (SNB) system suggest that this may indeed be the case.
The SNB system is a sexually dimorphic neuromuscular system that is present in adult male rodents but essentially absent in adult females. This system is also highly androgen-dependent. Although the SNB system initially develops independent of androgens, survival of the SNB system depends on androgens. As discussed in detail by Sengelaub and Forger in this special issue (Sengelaub and Forger, 2008), both the motoneurons and muscles die perinatally in females due to a lack of androgens. Because both the SNB motoneurons and its target muscles express ARs in adulthood, this too raised the question of where androgens act to keep the system alive. Findings from a number of different experiments led to the conclusion that androgens act via muscle ARs to indirectly keep SNB motoneurons alive, paralleling the indirect mode of androgen action shown in peripheral target organs. The ARs in SNB motoneurons do have some known functions, mediating in adulthood the androgen-regulated size of their cell bodies (Watson et al., 2001) and the level of expression of calcitonin gene-related peptide (Monks et al., 1999). However, androgens regulate the length of adult SNB motoneuronal dendrites via ARs in muscles rather than the AR in the motoneurons themselves (Rand and Breedlove, 1995). Thus, the lesson suggested by these studies is that a cell can be influenced by androgens, either directly, via ARs within that cell itself or indirectly, via ARs in other cells that interact with it.
Androgens can affect cells directly via nonclassical pathways that may or may not depend on nuclear androgen receptors
While it is clear that androgens can influence cells indirectly and that such influences need not involve the cell's own ARs, it is also becoming clear that androgens can and do affect cells directly even in the absence of nuclear ARs. Such responses to androgens tend to be rapid, occurring within seconds to minutes after exposure to androgens, and may involve known receptor systems, such as the GABA receptor or the sex hormone binding globulin receptor, or as yet, unidentified membrane receptors for androgens (Foradori et al., 2007). But it is also clear that androgens have direct and rapid effects on cells that do depend on the classical nuclear AR. The existence of such “nonclassical” or “nongenomic” modes of androgen action were generally first recognized and studied in various cell types from the periphery, but recent data suggest that androgens also influence the brain via many of the same nonclassical modes of action. Since there are several excellent and recent reviews on this topic (Heinlein and Chang, 2002; Losel et al., 2003; Walker, 2003; Foradori et al., 2007; Michels and Hoppe, 2007), we will briefly describe only one example to set the stage for several papers in this issue that suggest or demonstrate such nonclassical modes of androgen action.
Spermatogenesis provides a nice example of a normally occurring biological process that critically depends on androgens activating classical nuclear AR but through nonclassical or nongenomic pathways (Walker, 2003). Moreover, sperm production is also another example of androgens acting directly on one cell type, in this case, Sertoli cells, to promote the development of another, germ cells. This scenario was only recently definitively demonstrated using the cre/lox system to selectively disable AR function in Sertoli cells (De Gendt et al., 2004; Holdcraft and Braun, 2004). Through a series of experiments, Walker and colleagues tell a somewhat surprising story that androgens promote spermatogenesis by acting on Sertoli cells to regulate the expression of CREB-sensitive genes through nonclassical pathways that are dependent on AR but independent of any direct AR-DNA interactions. Activation of the transcription factor CREB by androgens in Sertoli cells is apparently crucial for germ cell survival (Scobey et al., 2001).
The realization that many androgen-regulated genes supporting spermatogenesis lack androgen response elements in their promoters prompted a search for other androgen-regulated pathways in Sertoli cells. While undoubtedly more details are to come, thus far, this nonclassical pathway involves androgens binding to classical nuclear ARs which are then recruited to the plasma membrane to activate CREB rapidly through an androgen-mediated activation of a kinase cascade involving Src and MAPK (Fix et al., 2004; Cheng et al., 2007). As described below, some effects of androgen on the brain also appear involve activation of MAPK via an AR mediated pathway (Pike et al., 2008).
That classic AR is required for these androgen-regulated events in Sertoli cells was shown using the AR antagonist flutamide and AR small inhibitory RNA; each blocked the activation of CREB by androgens. Moreover, while Sertoli cells from tfm rats fail to respond to androgens by activating CREB, this response can be restored by transfecting tfm Sertoli cells with wt AR. Walker (Walker, 2003), among others (Foradori et al., 2007), cautions against using the term “nongenomic” to describe such rapid intracellular responses to androgens since they may ultimately involve a change in gene expression, as demonstrated for Sertoli cells.
Recent evidence suggests a shift in perspective
The papers published in this special issue of Hormones and Behavior are grouped into four main sections that emphasize particular themes.
Role of ARs in sexual differentiation and neural plasticity and what AR “deletion” models can teach us
This first section begins with a discussion of the SNB system (Sengelaub and Forger, 2008), which until recently, was the only widely recognized mammalian neural system in which ARs were known to be critical for sexual differentiation. Discovering that the SNB system is completely feminized in tfm male rats that lack functional ARs provided some of the earliest and strongest evidence that ARs are critical for the masculine development of this system (Breedlove and Arnold, 1981). Sengelaub and Forger provide a cogent discussion of the progress made over the last 25 years in understanding the diverse ways in which androgens influence the SNB system throughout the lifespan and where and how androgens act to exert these influences.
With the emergence of the aromatization hypothesis positing that T is converted to estrogens locally in the brain, and that estrogens are the actual agents masculinizing the mammalian brain and behavior (Naftolin, 1994), little attention was given to the possibility that ARs may also contribute to this process. Indeed, discovering that early estrogen exposure could masculinize not only sexual behavior (Bakker and Baum, 2008) but also the volume of the sexually dimorphic nucleus of the preoptic area (SDN-POA) suggested that there was little reason to consider this possibility (Gorski, 1985). However, the observation that androgens upregulate aromatase (Roselli et al., 1997), the enzyme that converts T to estradiol, and that ARs are abundant in the perinatal brain (McAbee and DonCarlos, 1998) suggested otherwise. Zuloaga and colleagues present a convincing argument in their paper in this issue (Zuloaga et al., 2008) that ARs do indeed play a role in the masculinzation of the brain and/or behavior. For example, even though masculinization of SDN-POA volume depends on estrogen receptors (ERs), masculinization of neuronal soma size within this brain region seems to depend on ARs. On the other hand, ARs seem to be involved in masculinizing both the volume of the posterodorsal nucleus of the medial amygdala (MePD) and the size of neurons in this region. Not surprisingly, recent evidence suggests that AR-mediated effects of androgens also extend to behavior, affecting for example anxiety-related behaviors and the preference for female soiled bedding. For such explorations, the AR-mutant (tfm) rodent models have been enormously helpful in revealing contributions made by AR. Because the AR gene resides on the X chromosome, males having the tfm allele express only the mutant form of the AR. If all else is equal, differences found between tfm and wt males can be attributed to AR. However, given that aromatase expression is regulated by AR in some brain regions, and that AR expression is sometimes regulated by activation of ER in the brain, (Nordeen et al., 1986; McAbee and Doncarlos, 1999; Kim et al., 2004) effects of AR on brain morphology or behavior may in some cases depend on coordinate regulation and activation of both ARs and ERs. Regardless of whether effects of AR on the brain also involve ER, the emerging picture suggests that it is more the rule than the exception that AR plays a role. The particulars of its role, however, may be different depending on the specific brain region of interest.
One significant disadvantage of the tfm model is that ARs are universally disabled throughout the body and throughout the life span of an animal. This means that studies using tfm males provide no definitive data on when AR activity is important, nor which particular cells ARs act through to bring about a change. While manipulating gonadal hormones at different stages in the animal's life yields information about when AR activity is important, figuring out which cells androgens act on to cause a particular change is a much bigger challenge and in many cases, answers have come slowly or not at all. As discussed by Juntti and coauthors (Juntti et al., 2008), the cre/lox system offers a new and potentially powerful inroad for answering previously intractable questions. For example, by crossing transgenic mice that selectively express cre recombinase in only one cell type or region of the brain with other mice that have a “floxed” AR gene, one can selectively disable AR in a particular cell type or region. Such an approach offers great promise for identifying the cascade of events triggered by androgens that underlie sexual differentiation of the brain and behavior. As Juntti et al. point out, such models will also be useful in sorting out which effects of androgens on brain and behavior depend on nuclear AR (acting in particular cells and/or brain regions) and which do not. However, once AR-dependent responses have been identified, other approaches will be required to determine whether such responses are mediated via classical genomic or nonclassical mechanisms.
Substantial evidence indicates that gonadal hormones can also influence the structure of individual neurons not only during development, but also in adulthood, including changing their dendrites and synaptic connections. Hajszan and colleagues in this issue (Hajszan et al., 2008) describe a series of studies showing that synaptic connectivity in the hippocampus and prefrontal cortex of male rats normally depends on androgens. When adult males are castrated, the number of synapses in each region decreases. The story for these two regions then diverges somewhat when specific hormone metabolites are considered. Androgenic but not estrogenic metabolites appear to be involved in maintaining synapses in the hippocampus whereas both androgenic and estrogenic metabolites are implicated in the maintenance of synapses in the prefrontal cortex of adult males. Moreover, evidence from tfm male rats suggests that androgens are working through a nonclassical pathway independent of AR to affect hippocampal synapses, since dihydrotestosterone (DHT), a nonaromatizable androgen, is equally effective in maintaining synapses in both castrated wildtype and tfm males. On the other hand, both nonclassical and classical genomic modes of androgen action appear to be involved in maintaining synapses in the prefrontal cortex. They also present recent evidence that nonhuman primates show similar androgen-dependent changes, and suggest that androgens might be an effective therapeutic for certain neurological dysfunctions such as Alzheimer's disease and schizophrenia, since synapses in these same brain regions are often affected by these diseases. Two other papers (Pike et al., 2008; Raber, 2008) in this special issue examine more directly the role of androgens in Alzheimer's disease and similarly suggest that androgens may have therapeutic potential for treating this disease. Interestingly, androgens may only be beneficial for men and not women.
Role of ARs in social behavior and neuroendocrine function
Papers in the next section examine the role of ARs in the expression of social behaviors, and how androgens themselves appear to serve as reinforcers. The last paper in this section reviews recent data on the deleterious effects of androgens on neuroendocrine function in adult females, data that may help us to understand the mechanisms involved in polycystic ovarian syndrome in humans.
In the review by Sato and coauthors (Sato et al., 2008), there are two main points of discussion: 1) puberty, like perinatal development, is also a time when androgens can have lasting effects on adult behavior, presumably by exerting permanent organizational influences on the brain and 2) androgens are not only anabolic, causing skeletal muscles to grow, but are also rewarding, and this reinforcing action of androgen is mediated via a nonclassical mechanism.
Among the behaviors permanently affected by pubertal androgens is masculine sex behavior. For example, adult male hamsters deprived of pubertal androgens show lasting deficits in sexual performance even when such males are given androgens in adulthood. Another important point raised in this paper is that the pubertal brain appears to respond differently to androgens than the adult brain, and in particular, seems more vulnerable to the harmful effects of high dose androgens that typify androgen abuse in adolescent humans. They find, for example, that treatment with anabolic-androgenic steroids (AAS) induces much more aggression in adolescent male hamsters than does a comparable dose in adults. Notably, the most common behavioral side effect of AAS in humans is increased aggression and irritability.
But it is also apparent from their discussion (Sato et al., 2008) that adolescents and young adults may seek out or continue to use AAS for reasons that have nothing to do with its anabolic effect on muscles, since AAS and T appear to be rewarding in their own right. Recent evidence from their labs suggests that androgens are indeed reinforcing and that the reinforcing action of androgens in the brain appears to be mediated via a nonclassical mechanism involving a rapid action of androgens at the cell membrane that is independent of AR. For example, these investigators find that not only wt male rats but also tfm male rats find androgens rewarding, even though tfm males lack functional ARs. Moreover, androgens appear to be effective reinforcers even when conjugated to bovine serum albumin which renders androgens impermeable to cell membranes. Sato et al (2008) suggest that this rapid signaling pathway may serve the adaptive function of reinforcing behaviors, such as mating and fighting that induce acute increases in androgen secretion, ensuring that such behaviors will happen in the future. On the other hand, that AAS are rewarding and thus susceptible to abuse, particularly in adolescents, poses a great challenge for society.
Bass and Remage-Healey (Bass and Remage-Healey, 2008) discuss their studies on fish vocalization and its regulation by androgens. They too describe a scenario in which some effects of androgens occur with an unusually short delay (within 5 minutes), also implicating a nonclassical mode of androgen action in regulating fish vocalizations. However, in this case, the effects appear to depend on classical nuclear AR, unlike the effects of androgens on hippocampal synapses (Hajszan et al., 2008) and the reward system (Sato et al., 2008). Perhaps the most powerful aspect of the model system in fish is that the neural centers controlling vocalization can be isolated from the other neural inputs and continue to be active in the same rhythmic fashion as before. This offers significant advantages for gaining insight into the neurophysiological mechanisms regulated by androgens that underlie androgen-modulated behaviors.
While aromatized androgens working via ERs have long been implicated in the masculinzation of the neuroendocrine control of gonadal function, recent evidence also implicates AR in this process. Foecking and coauthors (Foecking et al., 2008) review recent work from their lab that examines the impact of androgen excess at different stages of life (prenatal, neonatal and adulthood) on the neuroendocrine function of adult females. They find that while DHT exposure at every stage impedes the ability of acute estrogens to induce a surge of luteinizing hormone (LH) in adult females, prenatal DHT has the most drastic effect, completely blocking the LH surge in response to adult estrogens. Given that the LH surge requires the estrogenic induction of progesterone receptor (PR) expression, they examined whether this response was intact in adult females exposed to early DHT and found that it was not. Thus, one possible mechanism by which prenatal androgens may cause refractoriness to adult estrogens is by permanently modifying chromatin structure (possibly via DNA methylation or histone modification), which prevents estrogens in adulthood from inducing PR expression in critical brain regions. Understanding how androgen excess leads to reproductive incompetence in this animal model has direct clinical relevance, since androgen excess is a core feature of polycystic ovarian syndrome.
Role of ARs in neurodegeneration and recovery of function
The next set of papers examines the role of ARs activated by androgens in neurodegenerative processes such as Alzheimer's disease (AD) and Spinal Bulbar Muscular Atrophy (SBMA), and in recovery of motor function after neural injury.
Two papers examine whether androgens influence the susceptibility to develop AD. The answer is yes, but other factors also matter. Pike and coauthors tackle two related issues: the relationship between androgen levels and AD, and the mechanisms by which androgens act as neuroprotective agents (Pike et al., 2008). Pike et al. review compelling evidence that declining androgen levels in aging men is a significant risk factor for developing AD and/or other neurodegenerative diseases in males. Raber (2008) then discusses evidence from humans and animal studies suggesting that having the ε4 allele of the ApoE gene increases the risk from declining androgen even further. Interestingly, this increased risk introduced by ApoE4 may also involve androgens and/or ARs. For example, Raber discusses recent evidence from his lab that expression of ApoE4 in female mice impairs spatial learning and memory that can be prevented by T or DHT treatment. Moreover, gonadally intact males expressing ApoE4 show less impairment compared to females expressing the gene, presumably because androgen levels are higher in the males than in the females and confer some protection against the toxic effects of ApoE4. That ApoE4 lowers androgen binding to cytosolic AR and may do so by binding directly to AR also suggests that the deleterious effects of ApoE4 on cognitive functioning may depend on an AR-mediated pathway. Whether the increased risk for neurodegenerative processes conferred by ApoE4 is a result of an ApoE4/AR interaction that itself is harmful or is the result of less AR activity is not entirely clear, but Raber discusses evidence that favors the latter possibility over the former, including evidence that elderly men having the ApoE4 allele have higher salivary T levels than non-ε4 carrying men. As Raber suggests, selective AR modulators that are designed to enhance AR activity in the brain may prove useful in treating AD.
Pike et al. (Pike et al., 2008) also discusses recent data from their lab based on animal and cell models suggesting that androgens may influence the risk for AD through both classical and nonclassical pathways. They find, for example, that the accumulation of beta-amyloid (Aβ) protein in the brain, which is thought to trigger and drive AD pathogenesis, is inhibited by androgens. Thus, low androgens may allow Aβ to accumulate to a level that is toxic for neurons, triggering AD. The authors suggest that androgens may limit Aβ accumulation and protect against AD by upregulating the expression of neprilysin, an enzyme that breaks down Aβ. Given that the neprilysin gene has several AR response elements in its promoter, androgens may regulate its expression directly. However, a nonclassical but AR-dependent mode of androgen action also appears to contribute to the susceptibility for AD when androgen levels are low. For example, Pike's group finds that androgens can protect neurons from a toxic insult and that the mechanism of action involves a rapid activation of MAPK that depends on AR. Thus, there is substantial evidence that androgens are important regulators of AD for males but not females, and that the signal transduction mechanisms used by androgens to ward off various toxic threats to neurons include both classical and nonclassical pathways.
The next two papers tackle the role of androgens in neuromuscular function and disease (Fargo et al., 2008; Monks et al., 2008). It is clear from these two papers that androgens can either promote or interfere with neuromuscular function, depending on other factors. Fargo and colleagues (Fargo et al., 2008) discuss perhaps two sides of the same coin—the role of androgens in promoting axonal growth after injury and how the partial loss of AR function in individuals that have SBMA may contribute to the loss of motor function caused by this disease. On the other hand, Monks and colleagues (2008) discuss possible mechanisms underlying SBMA, including the toxic role androgen itself plays in promoting neuromuscular demise in this disease.
Kathryn Jones' group showed a number of years ago that androgen treatment can enhance recovery of motor function after peripheral nerve damage in hamsters and rats by increasing the rate of axonal growth. A central goal since that time has been to determine the molecular mechanisms involved. They describe recent data from the Jones lab and the Poletti lab in both cell culture and in vivo approaches suggesting that androgens directly regulate expression of the neuritin gene to enhance axonal outgrowth (Fargo et al., 2008). For example, androgens can promote both neurite extension and expression of neuritin mRNA in cultured motoneuron-like cells that have been transfected with AR, but not in comparable cells that lack AR. Moreover, the androgen-induced enhancement of neurite extension in culture is blocked by neuritin RNA interference. They further describe supporting evidence from their in vivo model showing that enhanced axonal regeneration by androgens correlates with increased expression of neuritin mRNA, making a compelling argument that neuritin may critically mediate the enhanced recovery of function by androgens.
SBMA is caused by a mutation in the human AR gene that increases the length of the polyglutamine tract in the AR protein. This change alters AR function, causing both a loss of normal function and gain of new functions. Because complete loss of AR function does not appear to trigger SBMA in humans (mutations of the AR gene that disrupt receptor function cause no motor disturbance), it is widely assumed that it is the gain and not the loss of AR function that underlies SBMA pathogenesis. However, recent data suggest that the loss of AR function may also contribute to some of the pathology associated with this disease (Yu et al., 2006). Fargo et al. (Fargo et al., 2008) hypothesize that the loss of AR function may also lead to a depletion of neuritin which could limit the motoneuron's ability to regenerate under pathological conditions. While a direct test of this hypothesis awaits investigation, the possibility that neuritin may be an effective therapeutic for SBMA is intriguing.
In the next paper, Monks et al. review recent work suggesting that ARs trigger SBMA by acting in skeletal muscles rather than in the motoneurons themselves. This idea is contrary to the working assumption in the field that mutant ARs act in motoneurons to cause the disease. They describe a newly developed transgenic mouse model that over-expresses wt AR exclusively in muscle. This model yielded two surprises—that transgenic AR expressed only in muscle triggers a disease that completely mimics SBMA and that the expanded glutamine tract is apparently not necessary for the onset of pathogenesis in this model. Just as in other mouse models of SBMA, only tg males show a loss of motor function, which is triggered by androgens. They discuss possible mechanisms that may cause the loss of motor function, including the formation of protein aggregates, transcriptional dysregulation, aberrant proteolysis of AR, and aberrant protein-protein interactions that involve AR. All have been implicated in SBMA and thus represent potential targets for therapeutics. What this new muscle-specific SBMA model suggests, however, is that all the action of AR is in the muscles and that therapeutics targeted to muscles hold the greatest promise for treating SBMA.
Diverse mechanisms of androgen action
Clearly, an emerging theme shared by several of the papers in this special issue is that androgens affect the brain and behavior through many varied and sometimes unexpected signaling pathways, and that the rules have become considerably more complex than appreciated even a decade ago. The last two papers provide additional evidence for the diverse ways in which androgens can affect nervous system structure and function.
In the simplest terms, androgens have traditionally been thought to regulate gene expression by binding to its receptor which then binds directly to the promoter region of androgen target genes. But as demonstrated in Sertoli cells, androgens can regulate gene expression by regulating the activity of other transcription factors independent of any direct AR-DNA interactions. As discussed by Handa and coauthors, androgen activation of ERβ is yet another example of genomic action via other transcription factors (Handa et al., 2008). In this case, however, the effects of androgens are independent of both AR and direct AR-DNA interactions. Recent evidence suggests that DHT, once converted from T via 5 α-reductase, is further acted on by enzymes to produce either 5α-androstane-3α, 17-βdiol (3α-Diol) or 5α-androstane-3β, 17-βdiol (βα-Diol). Although neither metabolite is very effective in activating AR, they each appear to exert influences on the nervous system through other receptors, the GABA receptor for 3α-Diol and ERβ for 3β-Diol. Because DHT cannot be aromatized, the effects of DHT have traditionally been assumed to reflect activation of ARs and not ERs. As discussed by Handa and colleagues, recent evidence from their lab suggests that this is not a reasonable assumption. Specifically, they find that 3β-Diol derived from DHT represses the hormonal stress response and this action is mediated by ERβ and not AR in the brain. For example, when 3β-Diol is delivered directly to the hypothalamus, corticosterone levels increase less in response to stress. Moreover, ER antagonists but not AR antagonists block the repressive effect of 3β-Diol on the stress hormone response. These authors also describe data based on a hippocampal-derived neuronal cell line suggesting that 3β-Diol derived from DHT can act directly on neurons that lack AR to influence gene expression via ERβ-DNA interactions. While they find that 3β-Diol is able to drive gene expression via classic estrogen response elements (EREs), it appears 3β-Diol also regulates some targets genes, such as arginine vasopression, via promoter elements that are not known EREs. As these researchers emphasize, the fact that metabolites of DHT can act as potent estrogenic agents calls for a re-evaluation of past reports that presumed DHT effects reflect activation of AR.
The last paper in this section and issue (Sarkey et al., 2008) takes a different approach to studying the possibility that classical ARs may have a nonclassical role in neuronal and glial function. These authors review observations from several labs that extranuclear AR immunoreactivity is present in neuronal processes, including axons and axon terminals, as well as glial processes. New work from their lab suggests that, in rats, AR is present in axons emanating from hippocampal CA1 neurons and projecting to the amygdala and limbic cortical regions. These findings are particularly intriguing in light of the rewarding effects of androgens described by Sato et al. (2008) earlier in this issue, since the hippocampus is required for contextual learning as is temporal correspondence of the rewarding stimulus and context; therefore, the AR in axons, if it has a rapid mode of action, is in a position to exert the rapid effects required for associative/contextual learning. Moreover, the hippocampus and its targets are among the brain regions that are most affected by AD, and the extranuclear AR once again is in a position to be involved in the AR-dependent rapid activation of MAPK described by Pike and colleagues (2008) in this issue. Thus, this last contribution demonstrates how recent information from completely distinctive and seemingly disparate types of studies may ultimately converge to form a cohesive picture of how androgens may affect the nervous system in health and disease.
Future questions for the field
In sum, androgens can and do exert a wide variety of effects with far reaching consequences for the organism. It is clear that androgens acting via ARs, in addition to ERs, contribute to sexual differentiation of the brain and behavior. But the role of androgens and ARs in the brain extends well beyond sexual differentiation. Androgens can promote neuronal viability, perhaps related to their ability to maintain synaptic connections and/or to induce neurons to form new connections in the face of trauma. Androgens may serve as neuroprotective agents in males, just as estrogens appear to fulfill this function in females. Under some circumstances, androgens can promote neurodegeneration. Finally, the abuse of androgens by adolescents may cause permanent changes in the brain that interfere with normal function and may ultimately be maladaptive.
The far reaching effects of androgens on brain and behavior are equally matched by the myriad signaling mechanisms that might be involved (Fig. 1). First, androgens can influence neurons indirectly by acting directly on neighboring cells. Even when androgens affect cells directly, that effect may or may not be mediated by the AR. If the direct effect of androgens on a cell is independent of AR, then perhaps other known receptors mediate the effect. Such receptor systems that are known targets for androgens include membrane receptors (such as the GABA receptor) and intracellular receptors (such as ERβ). Other direct effects of androgens that are independent of AR may be mediated by a unique membrane AR that has yet to be identified. When androgen acts directly on cells via AR, there are still two possible pathways: 1) through the well known genomic pathway, resulting in relatively long delay, long-lived responses, or 2) through activation of various kinases and/or other transcription factors that are rapid but either long-lived (altering gene expression) or short lived (not altering gene expression) responses.
Figure 1.
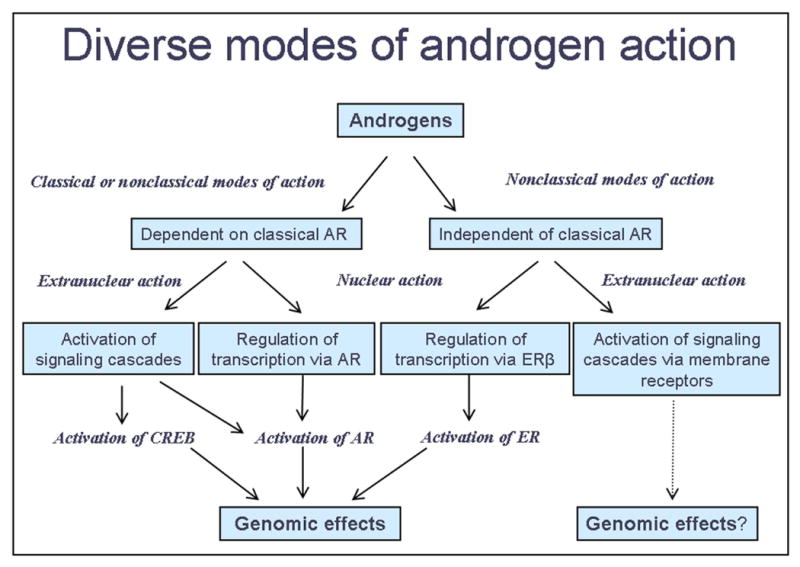
A Multiplicity of Androgen Pathways. In determining the mechanism of androgen action in the CNS, the first question is whether androgens are acting directly upon the neural cells of interest or acting on other cells that affect those cells. Once the cells directly responding to androgens are identified, any of the mechanisms depicted above may be involved, as the reports in this special issue suggest.
So what are some of the important questions remaining? Knowing which cells androgens act directly on in the brain to cause a particular change remains a largely unanswered question in our field. For example, do androgens act directly on AR-containing neurons in the rat MePD to cause them to grow or do androgens act elsewhere to induce this response? We know from the SNB neuromuscular system that androgens can act both directly and indirectly to regulate growth of different aspects of SNB motoneurons. More importantly, what is the relationship between neuronal morphology and behavior? For example, is a change in neuronal morphology in one region of the brain sufficient to trigger a change in behavior? Also we know little to date about the signaling pathways that mediate a particular response, particularly if androgens are interacting with a receptor other than the classical nuclear AR. Given that steroids diffuse passively through cell membranes, many have been reluctant to entertain the possibility that androgens can trigger biologically relevant responses by working on the outer membrane of cells, but a growing number of examples suggest that androgens may indeed regulate brain function through an action at the outer plasma membrane. And if androgens do trigger a response via the outer plasma membrane, what is the receptor mediating this effect? Is it through a known receptor or are there novel ARs yet to be discovered? A lingering question is whether these so-called nonclassical and rapid modes of androgen action that depend on nuclear AR are in fact an early antecedent necessary for the transcriptional capacity of AR as some data suggest (Taneja et al., 2005). Finally, as we get better at deciphering how androgen acts in the brain to influence behavior, we may decide that the terms “nonclassical” and “nongenomic” have little heuristic value, and that these newly recognized modes of androgen action are simply inherent in the brain and not atypical aspects of androgen action at all.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Bakker J, Baum M. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Remage-Healey L. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Cheng J, Watkins SC, Walker WH. Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in sertoli cells. Endocrinology. 2007;148:2066–2074. doi: 10.1210/en.2006-1465. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargo KN, Galbiati M, Foecking EM, Poletti A, Jones KA. Androgen regulation of axon growth and neurite extension in motoneurons. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:10919–10924. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foecking EM, McDevitt MA, Acosta-Martínez M, Horton TH, Levine JE. Neuroendocrine Consequences of Androgen Excess in Female Rodents. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2007 doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA. Sexual dimorphisms of the brain. J Anim Sci. 1985;61 Suppl 3:38–61. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Role of Androgens and the Androgen Receptor in Remodeling of Spine Synapses in Limbic Brain Areas. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5?-androstane-3?,17?-diol. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Prog Horm Res. 1953;8:379–418. doi: 10.1016/b978-1-4831-9825-5.50017-8. [DOI] [PubMed] [Google Scholar]
- Jost A. A new look at the mechanisms controlling sex differentiation in mammals. Johns Hopkins Med J. 1972;130:38–53. [PubMed] [Google Scholar]
- Juntti SA, Coats JK, Shah NM. A genetic approach to dissect sexually dimorphic behaviors. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Perlman WR, Arnold AP. Expression of androgen receptor mRNA in zebra finch song system: developmental regulation by estrogen. J Comp Neurol. 2004;469:535–547. doi: 10.1002/cne.11033. [DOI] [PubMed] [Google Scholar]
- Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, Wehling M. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- McAbee MD, DonCarlos LL. Ontogeny of region-specific sex differences in androgen receptor messenger ribonucleic acid expression in the rat forebrain. Endocrinology. 1998;139:1738–1745. doi: 10.1210/endo.139.4.5940. [DOI] [PubMed] [Google Scholar]
- McAbee MD, Doncarlos LL. Estrogen, but not androgens, regulates androgen receptor messenger ribonucleic acid expression in the developing male rat forebrain. Endocrinology. 1999;140:3674–3681. doi: 10.1210/endo.140.8.6901. [DOI] [PubMed] [Google Scholar]
- Michels G, Hoppe UC. Rapid actions of androgens. Front Neuroendocrinol. 2007 doi: 10.1016/j.yfrne.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Monks DA, Vanston CM, Watson NV. Direct androgenic regulation of calcitonin gene-related peptide expression in motoneurons of rats with mosaic androgen insensitivity. J Neurosci. 1999;19:5597–5601. doi: 10.1523/JNEUROSCI.19-13-05597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks DA, Rao P, Mo K, Johansen JA, Lewis G, Kemp MQ. Androgen Receptor and Kennedy Disease/ Spinal Bulbar Muscular Atrophy. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F. Brain aromatization of androgens. J Reprod Med. 1994;39:257–261. [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ, Arnold AP. Estrogen establishes sex differences in androgen accumulation in zebra finch brain. J Neurosci. 1986;6:734–738. doi: 10.1523/JNEUROSCI.06-03-00734.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Nguyen TV, Ramsden M, Yao M, Murphy MP, Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. 2008 doi: 10.1016/j.yhbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J. AR, apoE, and cognitive function. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM. Androgen alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci. 1995;15:4408–4416. doi: 10.1523/JNEUROSCI.15-06-04408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull. 1997;44:351–357. doi: 10.1016/s0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- Sarkey S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D, DonCarlos LL. Classical androgen receptors in non-classical sites in the brain. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato SM, Schulz KM, Sisk CL, Wood RI. Adolescents and Androgens, Receptors and Rewards. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobey M, Bertera S, Somers J, Watkins S, Zeleznik A, Walker W. Delivery of a cyclic adenosine 3′,5′-monophosphate response element-binding protein (creb) mutant to seminiferous tubules results in impaired spermatogenesis. Endocrinology. 2001;142:948–954. doi: 10.1210/endo.142.2.7948. [DOI] [PubMed] [Google Scholar]
- Sengelaub DR, Forger NG. The spinal nucleus of the bulbocavernosus: Firsts in androgen-dependent neural sex differences. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja SS, Ha S, Swenson NK, Huang HY, Lee P, Melamed J, Shapiro E, Garabedian MJ, Logan SK. Cell-specific regulation of androgen receptor phosphorylation in vivo. J Biol Chem. 2005;280:40916–40924. doi: 10.1074/jbc.M508442200. [DOI] [PubMed] [Google Scholar]
- Walker WH. Nongenomic actions of androgen in Sertoli cells. Curr Top Dev Biol. 2003;56:25–53. doi: 10.1016/s0070-2153(03)01006-8. [DOI] [PubMed] [Google Scholar]
- Watson NV, Freeman LM, Breedlove SM. Neuronal size in the spinal nucleus of the bulbocavernosus: direct modulation by androgen in rats with mosaic androgen insensitivity. J Neurosci. 2001;21:1062–1066. doi: 10.1523/JNEUROSCI.21-03-01062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Dadgar N, Albertelli M, Gruis K, Jordan C, Robins DM, Lieberman AP. Androgen-dependent pathology demonstrates myopathic contribution to the Kennedy disease phenotype in a mouse knock-in model. J Clin Invest. 2006;116:2663–2672. doi: 10.1172/JCI28773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The Role of Androgen Receptors in the Masculinization of Brain and Behavior: What we've learned from the Testicular Feminization Mutation. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


