Abstract
Injectable, biodegradable scaffolds are important biomaterials for tissue engineering and drug delivery. Hydrogels derived from natural polysaccharides are ideal scaffolds as they resemble the extracellular matrices of tissues comprised of various glycosaminoglycans (GAG). Here, we report a new class of biocompatible and biodegradable composite hydrogels derived from water-soluble chitosan and oxidized hyaluronic acid upon mixing, without the addition of a chemical crosslinking agent. The gelation is attributed to the Schiff-base reaction between amino and aldehyde groups of polysaccharide derivatives. In the current work, N-succinyl-chitosan (S-CS) and aldehyde hyaluronic acid (A-HA) were synthesized for preparation of the composite hydrogels. The polysaccharide derivatives and composite hydrogels were characterized by FTIR spectroscopy. The effect of the ratio of S-CS and A-HA on the gelation time, microstructure, surface morphology, equilibrium swelling, compressive modulus, and in vitro degradation of composite hydrogels was examined. The potential of the composite hydrogel as an injectable scaffold was demonstrated by encapsulation of bovine articular chondrocytes within the composite hydrogel matrix in vitro. The results demonstrated that the composite hydrogel supported cell survival and the cells retained chondrocytic morphology. These characteristics provide a potential opportunity to use the injectable, composite hydrogels in tissue engineering applications.
Introduction
Various hydrogels and microspheres have been employed as injectable scaffolds for a variety of biomedical applications [1–5]. Injectable, biodegradable hydrogels could be utilized as delivery systems, cell carriers, and scaffolds for tissue engineering [6–8], which allow easy and homogenous drug or cell distribution within any defect size or shape. Recently, many methods have been employed for the preparation of injectable in situ forming hydrogels, including photopolymerization of their custom-made monomers [9–10] and chemical crosslinking with agents such as carbodiimide, glutaraldehyde, genipin, and adipic dihydrazide [11–15]. However, photopolymerization often requires a photosensitizer and prolonged irradiation, thus limiting their applications. The chemical crosslinking agents are the major obstacle in the use of injectable in situ forming polymer scaffolds, due to their toxicity to cells [16–17].
Presently, several polysaccharides such as dextran [18], gum arabic [19], chondroitin sulfate [20] and hyaluronic acid [21–22] are partially oxidized and employed for possible medical applications such as drug release and peritoneal adhesion prevention. However, little has been reported on the use of oxidized polysaccharides for the preparation of hydrogels as cell carriers for tissue engineering applications. Hererin, we describe a new injectable, in situ forming biodegradable hydrogel by self-crosslinking of water-soluble chitosan and oxidized hyaluronic acid, without employing any extraneous chemical crosslinking agents.
Chitosan, a partially deacetylated derivative from chitin composed of glucosamine and N-acetylglucosamine, is structurally similar to glycosaminoglycan (GAG) and its analogs. Chitosan has been widely applied in drug delivery, gene therapy and tissue engineering because of its biocompatibility and biodegradability [23–25]. However, chitosan has poor solubility in physiological solvents due to its strong intermolecular hydrogen bonding, thereby greatly limiting further biomedical applications, particularly as an injectable scaffold. N-Succinyl-chitosan (S-CS), a water soluble chitosan derivative, was synthesized via introduction of succinyl groups at the N-position of the glucosamine units of chitosan. It is attractive as a drug carrier as it shows biocompatibility and long-term retention in vivo [26–28]. By incorporating with other polysaccharides such as hyaluronic acid, S-CS can create a more biomimetic microenvironment with improved biocompatibility and biodegradation for tissue regeneration.
Hyaluronic acid is a linear high-molecular-weight polysaccharide, composed of repeating disaccharide units of N-acetyl-D-glucosamine and D-glucuronic acid [29–30]. In ECM, hyaluronic acid is the backbone of GAG superstructure complexes, mostly associated with other polysaccharides such as chondroitin sulfate [31]. Due to its good biocompatibility, biodegradability, as well as excellent gel-forming properties, hyaluronic acid shows promise in biomedically-relevant hydrogel systems. Hyaluronic acid can be oxidized, and the carbon-carbon bonds of the cisdiol groups in molecular chain are cleaved and generate reactive aldehyde functions (aldehyde hyaluronic acid, A-HA), which can develop chemical crosslinking action with amino functions via Schiff-base linkage [21–22].
The aim of this work was to prepare a non-toxic in situ forming biodegradable S-CS/A-HA composite hydrogel, and to study the effects of varying the ratio of S-CS and A-HA on gelation time, microstructure, morphology, equilibrium swelling, compressive modulus and degradation in vitro. Bovine articular chondrocytes were encapsulated within the hydrogels in vitro to assess their biological performance and applicability as cell carriers.
MATERIALS AND METHODS
Materials
Chitosan (deacetylation degree: 85%, Mη: 4×105), hyaluronic acid sodium, succinic anhydride, sodium periodate, ethylene glycol, t-butyl carbazate, ninhydrin and L-lactic acid were purchased from Sigma-Aldrich, USA. CyQuant Cell Proliferation Assay Kit was purchased from Invitrogen, Eugene, Oregon, USA. All chemicals and reagents were used as received.
Synthesis of S-CS
S-CS was synthesized according to an already reported procedure slightly modified [27]. 0.5g of chitosan was dissolved in 40mL 5%(v/v) lactic acid solution and then 160mL methanol was added to dilute the solution. 1.5g of succinic anhydride was added to this solution with stirring at room temperature. After 24h, the succinyl modified chitosan was precipitated by adjusting the solution pH to 6~7. The precipitate was filtered, re-dissolved in H2O, and dialyzed for 3 days. The purified product was freeze-dried and stored at 4°C. The substitution degree of S-CS was determined by the ninhydrin assay [32].
Synthesis of A-HA
A-HA was synthesized according to an already reported procedure slightly modified [21–22]. 1.0g HA (~2.5mmol) was dissolved in 100mL nanopure H2O at a concentration of 10mg/ml. An aqueous solution of sodium periodate (0.5M, 5ml) was added dropwise, and the reaction was stirred for 2h at room temperature in the dark. 1mL Ethylene glycol was then added to inactivate any unreacted periodate. The reaction was stirred for 1h at ambient temperature and the solution was purified by exhaustive dialysis against H2O for 3 days, and the dry product was obtained by freeze-drying. The percentage oxidation of A-HA was quantified by measuring the number of aldehydes in the polymer using t-butyl carbazate [33].
Fabrication of composite hydrogels
S-CS and A-HA were dissolved in PBS separately at a concentration of 20mg/ml. The crosslinked composite hydrogels were formed by mixing S-CS and A-HA solutions at various volume ratios of 1/9, 3/7, 5/5, 7/3 and 9/1 at room temperature. The gelation time of composite hydrogels was monitored.
Characterization of composite hydrogels
Morphologies
Morphologies of composite hydrogels were characterized by utilizing scanning electron microscopy (SEM) after gelation. The hydrogels were freeze-dried and then gold-coated using a Cressington 108 Auto (Cressington, Watford UK). The surface and cross-sectional morphologies were viewed using a JSM-6330F SEM (JEOL, Peabody, MA) operated at 10kV accelerating.
Infrared (IR) spectroscopic measurement
Fourier transformed infrared (FTIR) spectra of polysaccharides and hydrogel membranes were measured to confirm the expected pendant functionalities. Various samples were recorded with FTIR spectrometer (Nicolet Avatar 360, USA) against a blank KBr pellet background.
Equilibrium swelling
The known weights of freeze-dried hydrogels were immersed in DMEM/F12/10%FBS and PBS, respectively, and kept at 37°C for 2h until equilibrium of swelling had been reached. The swollen hydrogels were removed and immediately weighed with a microbalance after the excess of water lying on the surfaces was absorbed with a filter paper. The equilibrium swelling ratio (ESR) was calculated using the following equation:
where Ws and Wd are the weights of the hydrogels at the equilibrium swelling state and at the dry state, respectively.
Compressive modulus
Mixtures of solutions described above were injected into a 12-well culture plate for 15 min to obtain columned hydrogels (22mm diameter, 6mm height). Compressive modulus of elasticity was measured in the elastic region of hydrogel using a dynamic mechanical analyzer (ELF3200, Endura TEC) in unconfined compression at a constant stress rate of 40 mN min−1 up to 20% strain at room temperature.
Degradation in vitro
Degradation of composite hydrogels was also examined with respect to weight loss. Weight loss of initially weighed hydrogels (W0) was monitored as a function of incubation time in PBS at 37°C. At specified time intervals, hydrogels were removed from the PBS and weighed (Wt). The weight loss ratio was defined as 100%×(W0−Wt)/W0. The weight remaining ratio was defined as 1–100%×(W0−Wt)/W0.
Chondrocyte culture
Bovine articular chondrocytes were isolated from bovine knees under the institutional guidelines [34–35]. Chondrocytes were isolated through enzymatic digestion and the pellet was resuspended in DMEM/F12 with 10% FBS and 1% penicillin/streptomycin. The cells were then seeded in plastic tissue culture flasks and were incubated in a humidified atmosphere of 95% air and 5% CO2 at 37oC. Adherent chondrocytes were expanded for a period of 5~8 days at 37°C, and the medium was changed every other day until the cells achieved 80% confluence.
Chondrocyte adhesion to the composite hydrogels was assessed. S-CS and A-HA were sterilized under UV irradiation for 1h and then dissolved in sterilized PBS to obtain 20mg/mL solution, respectively. A total of 300μL of the S-CS and A-HA solutions at various volume ratios were injected into the 24-well culture plate, mixed, and incubated at 37°C for 10min to form composite hydrogels. 1mL DMEM/10% FBS solution containing 50,000 cells was added into each well. After 24h, the number of chondrocytes attached to the composite hydrogels was quantified using a CyQuant Cell Proliferation assay [36].
Encapsulation of chondrocytes within the composite hydrogel was also evaluated. 2mL S-CS solution was added into a centrifugal tube containing 2,000×104 chondrocytes. After sufficient mixing, the cell containing S-CS solutions were injected into 24-well culture plate to crosslink with the A-HA solutions. The mixture with cells was then incubated at 37°C to form a composite cells/hydrogel matrix. The cell density was 500×104/mL hydrogel. After 10min, DMEM/F12/10%FBS solution was added into each well.
Chondrocytes were observed by confocal laser scanning microscopy (CLSM, Olympus IX 81). The live cells were dyed with Cell Tracker Orange CMRA (Red) and all cell nuclei were dyed with Hoechst 33342 (Blue). The cell/hydrogel matrices were further observed by SEM after fixation, sequential dehydration, critical point drying and gold coating.
Statistical analysis
The experimental data from all the studies were analyzed using Analysis of Variance (ANOVA). Statistical significance was set to p value ≤ 0.05. Results are presented as mean ± standard deviation.
RESULTS
Structure of polysaccharide derivatives
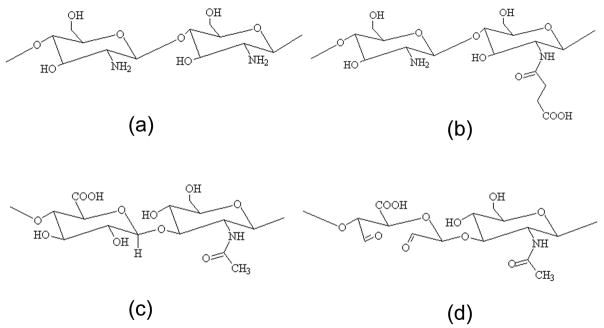
S-CS was obtained by introduction of succinyl groups into chitosan N-terminal of the glucosamine units (Figure 1a). The succinylation reaction consists of a condensation reaction between the polysaccharide amine group and the electrophilic carbonyl group of the anhydride, with the formation of an amidic bond with opening of the anhydride ring. The chemical structure of S-CS is shown in Figure 1b, which displays good water soluble property at various pHs. The determined substitution degree of S-CS was 38.4%. Aldehyde groups were introduced to hyaluronic acid (Figure 1c) by reaction with sodium periodate, which oxidizes the vicinal hydroxyl groups to dialdehydes, thereby opening the sugar ring to form dialdehyde derivatives (Figure 1d). Determination of the actual aldehyde content of A-HA revealed an extent of oxidation of 46.6%.
Figure 1.
Chemical structures of chitosan (CS) (a), N-succinyl-chitosan (S-CS) (b), hyaluronic acid (HA) (c) and aldehyde hyaluronic acid (A-HA) (d).
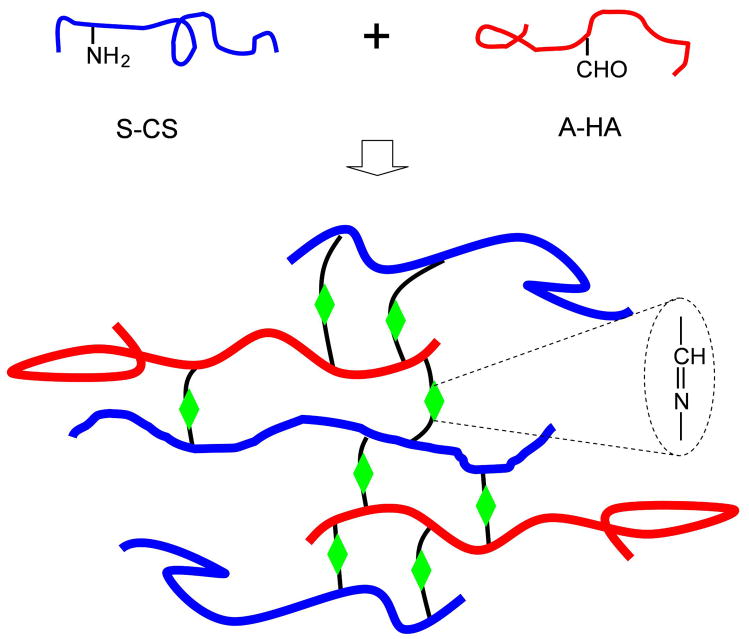
The mechanism of gelation is attributed to the Schiff-base reaction between amino and aldehyde groups of polysaccharide derivatives (Figure 2). Polysaccharide derivatives and composite hydrogels were characterized by FTIR (Figure 3). By comparing with CS, the spectrum of S-CS shows a new absorption peak around 1733 cm−1, which corresponds to the carboxylic group [27]. The spectra of HA and A-HA were very similar and it is hard to detect any signal corresponding to the aldehyde functionalities, and this may be due to the formation of hemiacetals. In the spectrum of the composite hydrogel, the characteristic peak of the hemiacetal structure at 886 cm−1 suggests that the coupling reaction was followed between –CHO groups of A-HA and –NH2 of S-CS [28].
Figure 2.
The scheme of S-CS and A-HA composite hydrogel via Schiff’s base cross-linking reaction.
Figure 3.
FTIR spectra of CS (a), S-CS (b), HA (c), A-HA (d), and 5/5 S-CS/A-HA composite hydrogel (e).
Gelation time of hydrogels
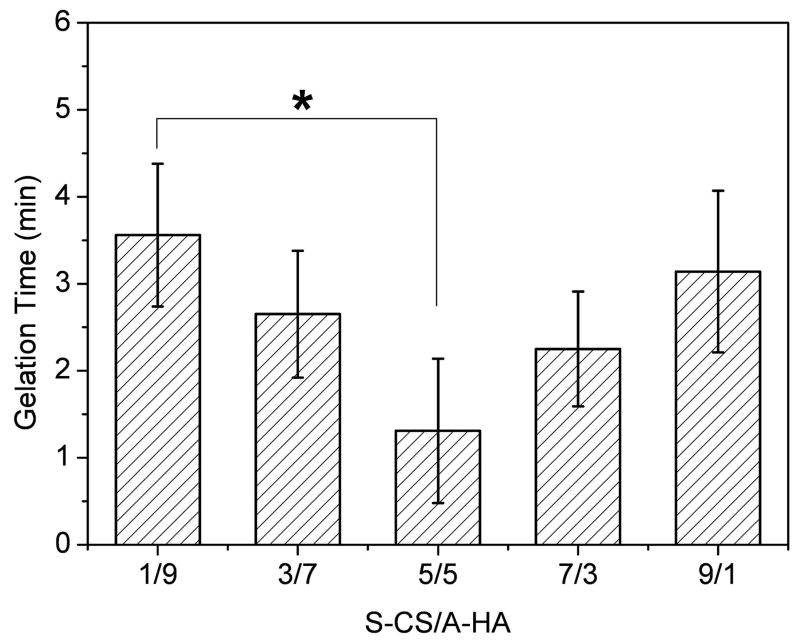
The gelation rate of composite hydrogels was monitored at room temperature. When 20mg/mL S-CS and A-HA were mixed with five different volume ratios, gelation occurred within 1~4 min (Figure 4). With increasing ratios of S-CS in the composite hydrogels, the gelation time first decreased, then increased. The gelation rate of 5/5 composite hydrogel was the fastest, which showed a significantly faster gelation rate than 1/9 hydrogels (p<0.05).
Figure 4.
Gelation time of S-CS/A-HA composite hydrogels as a function of volume ratio at room temperature. The total polymer concentration was 20mg/mL. Values reported are an average n=3, ± standard deviation.
Morphologies of hydrogels
SEM images were obtained to characterize the microstructure morphologies of three freeze-dried S-CS/A-HA composite hydrogels (Figure 5). The surface images of the hydrogels are presented in Figure 5a-c. A thin polymer layer can be observed, which is likely related to the collapse of surface pores by the freeze-drying process. According to cross-section SEM images (Figure 5d-f), the hydrogels displayed a continuous and porous structure by virtue of the freeze-drying step, with the pores being the result of ice crystal formation, resembling other natural macromolecular hydrogel system structures, with pore diameters in the range of 10~100μm. The internal morphology was dependent on the content ratio of polysaccharide. Higher ratio of S-CS resulted in smaller pore sizes.
Figure 5.
SEM photographs of freeze-dried S-CS/A-HA composite hydrogels. (a)–(c) Surface morphologies of hydrogels with the volume ratio of 3/7 (a), 5/5 (b) and 7/3 (c). (d)–(f) Cross-sectional morphologies of hydrogels with the volume ratio of 3/7 (d), 5/5 (e) and 7/3 (f).
Equilibrium swelling of hydrogels
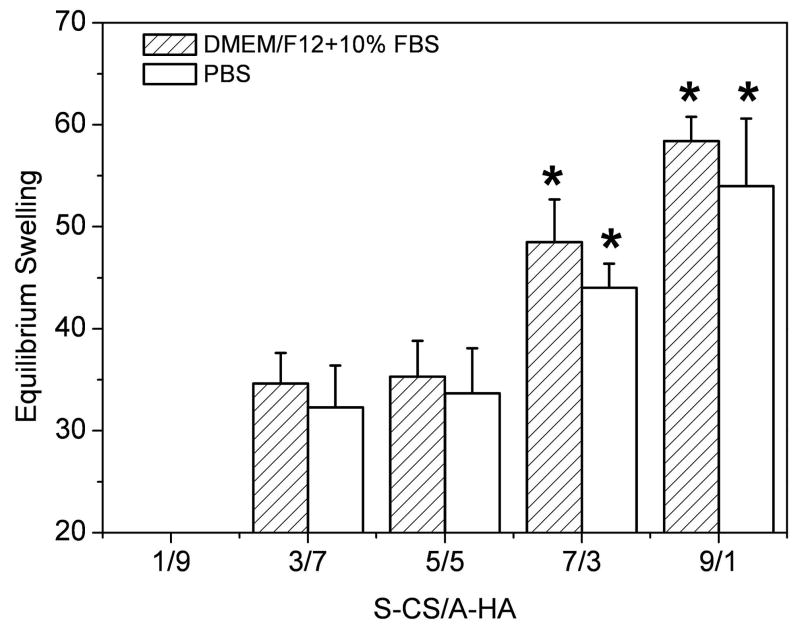
Figure 6 indicates the equilibrium swelling ratio of freeze-dried composite hydrogels determined in DMEM/F12/10%FBS and PBS. The swelling ratio was increased along with increase of S-CS content, except for the dried 1/9 hydrogel, which became water-soluble after a 2h incubation due to poor crosslinking. The swelling ratio in DMEM/F12/10%FBS was slightly higher than those in PBS, whereas no difference was found between them. The swelling value rapidly increased with the increase in the S-CS content from 5/5 to 7/3 (p<0.05). In the case of DMEM/F12/10%FBS medium, the swelling value was 35.3 for 5/5 hydrogels, while it was 48.5 for 7/3 hydrogels (Figure 6).
Figure 6.
Equilibrium swelling ratio of S-CS/A-HA composite hydrogels as a function of volume ratio incubated in DMEM/F12/10%FBS and PBS at 37°C. Values reported are an average n=5, ± standard deviation.
Compressive modulus of hydrogels
Compressive modulus of the hydrogels was studied by a dynamic mechanical analysis method (Table 1). With increasing ratios of S-CS content from 30% to 70% (v/v), the compressive modulus of the composite hydrogels were improved correspondingly. The 5/5 and 7/3 hydrogels had significantly larger compressive modulus than the 3/7 hydrogel (p<0.05), which were 25 and 28 kPa, respectively, whereas no difference was found between them.
Table 1.
Compressive modulus of S-CS/A-HA composite hydrogels at room temperature. Values reported are an average n=3, ± standard deviation.
| %(v/v) S-CS | %(v/v) A-HA | Compressive modulus (kPa) |
|---|---|---|
| 30 | 70 | 12±4 |
| 50 | 50 | 25 ± 3 |
| 70 | 30 | 28 ± 6 |
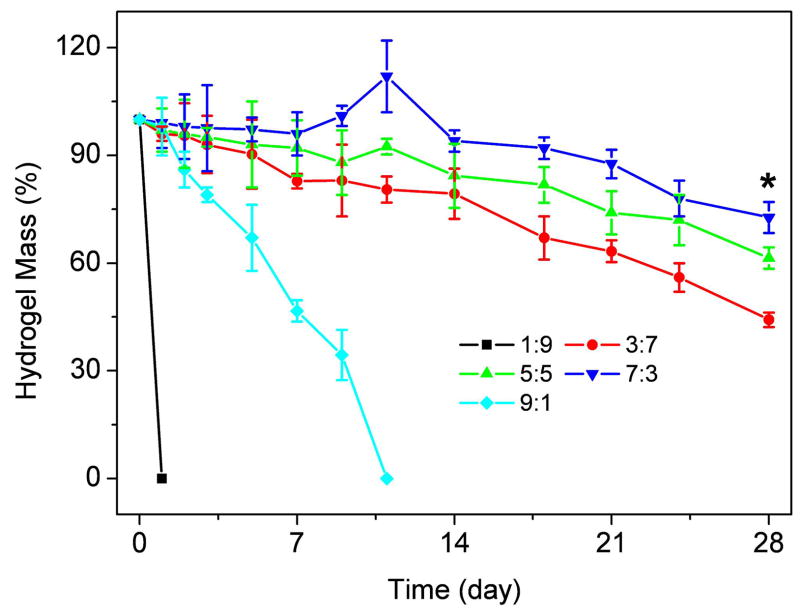
Degradation of composite hydrogels
Degradation of composite hydrogels was monitored as a function of incubation time in PBS at 37°C, as shown in Figure 7. The ratio of S-CS/A-HA had a significant influence on weight loss. The hydrogels with a ratio of 1/9 and 9/1 showed a significantly faster weight losing rate due to less crosslinking, and dissolved in 1 day and 11 days, respectively. The other hydrogels lost their weight steadily up to 28 days (Figure 7). The hydrogels with more S-CS showed a slower weight loss rate than hydrogels with less S-CS composition. At day 28, the weight remaining ratio of 3/7, 5/5, and 7/3 hydrogel were 44.2%, 61.4% and 72.7%, respectively.
Figure 7.
Degradation of S-CS/A-HA composite hydrogel in PBS at 37°C with respect to weight loss. Values reported are an average n=5, ± standard deviation.
Cell Biocompatibility of composite hydrogels
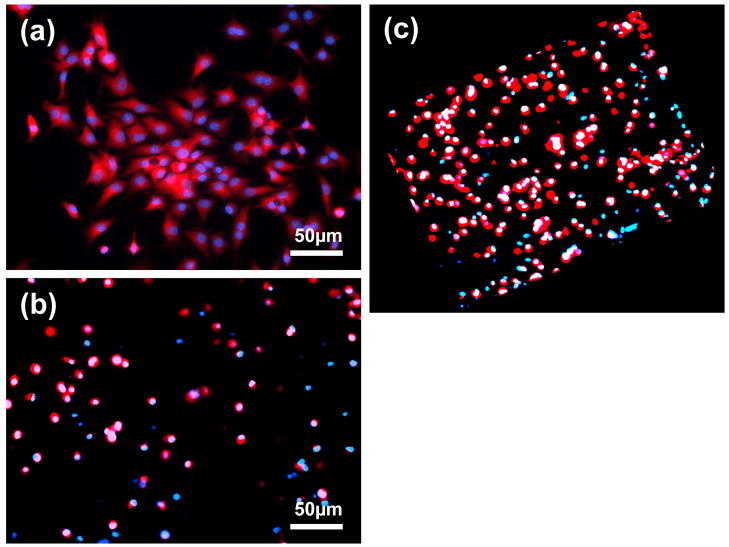
The adhesion of bovine chondrocytes to the top surface of composite hydrogels after culture for 24h was characterized (Figure 8). All hydrogel samples showed less attached cells than the control TCP. The number of cells on the surface of 5/5 and 7/3 hydrogels was significantly greater than that of 3/7 and 9/1 hydrogels (p<0.05). The attached chondrocytes on the surface of the 5/5 hydrogel were present in the superficial area of the hydrogel and maintained their polygonal morphology (Figure 8a). The chondrocytes encapsulated in the hydrogels for 3D culture were also observed, and were found to remainviable after 24h of culture (Figure 8b). Elliptical or round shaped chondrocytes were uniformly distributed in the hydrogel. The 3D calculation results demonstrated that more than 93% of the encapsulated chondrocytes survived (Figure 8c).
Figure 8.
Number of bovine chondrocytes adhered to surface of S-CS/A-HA composite hydrogels versus control wells. Values reported are an average n=5, ± standard deviation.
SEM images of the 5/5 composite hydrogel encapsulated with chondrocytes are presented in Figure 8. The existence of the cobble-stone like appearance on the top surface of the hydrogel reflects the encapsulation of the cells, and one can estimate the cell size as ~10μm. The morphology of the chondrocytes residing in the hydrogel was further observed (Figure 8b). The cells were encapsulated within the composite hydrogel and possessed normal spherical morphology.
DISCUSSION
Hydrogels can be utilized for cell delivery, as well as growth factor or drug delivery. An injectable hydrogel is clinically desired as this system could result in minimally invasive surgeries. Hydrogels derived from naturally occurring polysaccharides mimic many features of extracellular matrix (ECM) and thus have the potential to direct the migration, growth and organization of encapsulated and transplanted cells during tissue regeneration. In this study, a new in situ forming biodegradable hydrogel was prepared, which may be used as an injectable scaffold for tissue repair. The hydrogel was obtained by the crosslinking of S-CS with A-HA at a relatively stable physiological pH, with H2O as the only by product. Five different volume ratios of S-CS/A-HA composite hydrogels were prepared by Schiff’s base crosslinking reaction (−C=N−) between –NH2 of S-CS and –CHO of A-HA (Figure 2).
In our experiment, ratios of S-CS/A-HA did not significantly influence the gelation time, i.e. the gelation time for all five groups were within 1~4min (Figure 4). However, the gelation time was shorter when the ratios of S-CS/A-HA were 3/7~7/3. The cross-sectional SEM images of the freeze-dried 3/7 and 7/3 composite hydrogels demonstrated that a higher content of S-CS results in the formation of smaller pore diameters and tighter network structure in composite hydrogels, which is likely due to the comparatively sufficient crosslinking (Figure 5).
Swelling properties of the as-prepared hydrogels are crucial for substance exchange when they are used as injectable scaffolds for biomedical applications. Both S-CS and A-HA have an abundant number of hydrophilic groups, such as hydroxyl, amino and carboxyl groups, which can easily produce hydration with water. The amount of S-CS and A-HA used in the hydrogel synthesis significantly affects the hydrogels’ swelling properties. The 1/9 and 9/1 hydrogels revealed less crosslinking, consequently increasing the exposure of polymer chains to water molecules, and significantly leading to water absorption enhancement (Figure 6) and faster weight loss (Figure 7). Crosslinking density influences many of the macroscopic properties of hydrogels. In general, an increase in the crosslinking density results in a decrease in the water content and mass weight loss. The 3/7, 5/5 and 7/3 hydrogels showed a slower degradation rate than the 1/9 and 9/1 hydrogels in PBS at 37°C (Figure 7), which is possibly due to the sufficient crosslinking and subsequent microstructure. Although some reports have indicated that Schiff’s base crosslinking structure of hyaluronic acid is instable [21–22], interestingly, we found the S-CS/A-HA hydrogels were stable in PBS during the four week period, which indicated that the hydrolysis rate of S-CS/A-HA Schiff’s base is slow under physiological conditions. Compressive modulus is particularly important for cartilage tissue engineering. The compressive modulus of composite hydrogels were improved with increasing ratios of S-CS content from 30% to 70% (v/v) (Table 1). However, measures must be taken in the future to further improve the mechanical strength of the present systems if they are used as the injectable scaffold for cartilage restoration, although many types of hydrogels with similar strength have been diversely used for the same purpose.
Another important issue for injectable hydrogels is the encapsulation efficiency for the target cells. Since the microstructure and high water content are very similar to that of the extracellular matrix of natural cartilage, hydrogels may preserve the phenotype of chondrocytes. The S-CS/A-HA composite hydrogels were then similarly assessed in terms of cell biocompatability. The bovine chondrocyte attachment study indicated that the hydrogels support cell adhesion (Figure 8). A significantly higher number of attached chondrocytes were observed on the 5/5 and 7/3 composite hydrogels than the 3/7 and 9/1 hydrogels. The cells which were encapsulated within the 5/5 composite hydrogel possessed normal spherical morphology (Figure 9 and Figure 10), as per that in normal cartilage, predicting a potential application of the hydrogel as an injectable scaffold in cartilage tissue engineering.
Figure 9.
(a) CLSM image showing bovine chondrocytes upon the surface of a 5/5 S-CS/A-HA composite hydrogel after 24h culture. Cell seeding density: 50,000/well (24-well cell culture plate). (b) CLSM image showing bovine chondrocytes encapsulated in a 5/5 S-CS/A-HA composite hydrogel after 24h culture. Cell seeding density: 5×106/mL. (c) The 3D image of (b). The live cells were dyed with Cell Tracker Orange CMRA (Red) and all cell nuclei were dyed by Hoechst 33342 (Blue).
Figure 10.
(a) SEM image depicting surface morphology of the 5/5 composite hydrogel encapsulated with chondrocytes after 24h culture. (b) SEM image depicting the morphology of encapsulated chondrocytes after 24h culture. Cell seeding density: 5×106/mL.
The evaluation of these properties contributes to a further understanding of the formation mechanism of hydrogels and biological applicability. As this process of the CS-HA hydrogel formation is simple, feasible, and usually performed under mild conditions without employing any extraneous toxic crosslinking agents, such as glyoxal, glutaraldehyde, carbodiimide and diepoxy compounds, we believe that such a composite matrix will have potential applications in wound management, drug delivery, tissue engineering, and other related biomedical fields. However, further optimization of the system is required to promote cell proliferation and ECM production in addition to maintenance of their phenotype.
CONCLUSIONS
The in situ forming S-CS and A-HA composite hydrogels were prepared via Schiff’s base cross-linking reaction. The gelation time, structure, equilibrium swelling, compressive modulus and degradation in vitro were dependent upon crosslinking and structure of composite hydrogels. The composite hydrogels with a higher S-CS composition showed a slower degradation rate than hydrogels with a lower S-CS composition. The compressive modulus of composite hydrogels were improved with increasing ratios of S-CS content from 30% to 70% (v/v). Bovine articular chondrocytes cultured within the gels indicated that the S-CS/A-HA composite hydrogel is able to support cell adhesion. Encapsulation of chondrocytes demonstrated that the composite hydrogel promoted cell survival and the cells retained regular, chondrocytic spherical morphology. These preliminary studies indicate that the composite hydrogel supports chondrocyte adhesion and encapsulation, and may have potential uses in cartilage tissue engineering applications.
Acknowledgments
We thankfully acknowledge the Center for Biologic Imaging for SEM, Andrew Feola and Steve Abramowich for assistance with the mechanical testing, and NIH R01051963 (CRC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tememoff JS, Mikos AG. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials. 2000;21:2405–2412. doi: 10.1016/s0142-9612(00)00108-3. [DOI] [PubMed] [Google Scholar]
- 2.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 3.Hou QP, De Bank PA, Shakesheff KM. Injectable scaffolds for tissue regeneration. J Mater Chem. 2004;14:1915–1923. [Google Scholar]
- 4.Pratt AB, Weber FE, Schmoekel HG, Müller R, Hubbell JA. Synthetic extracellular matrices for in situ tissue engineering. Biotechnol Bioeng. 2004;86(1):27–36. doi: 10.1002/bit.10897. [DOI] [PubMed] [Google Scholar]
- 5.Lao L, Tan H, Wang Y, Gao C. Chitosan modified poly(l-lactide) microspheres as cell microcarriers for cartilage tissue engineering. Colloids and Surfaces B: Biointerfaces. 2008;66:218–225. doi: 10.1016/j.colsurfb.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 6.McGlohorn JB, Grimes LW, Webster SS, Burg KJL. Characterization of cellular carriers for use in injectable tissue-engineering composites. J Biomed Mater Res. 2003;66A:441–449. doi: 10.1002/jbm.a.10546. [DOI] [PubMed] [Google Scholar]
- 7.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51(2):164–171. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.DeFail AJ, Chu CR, Izzo N, Marra KG. Controlled release of bioactive TGF-β1 from microspheres embedded within biodegradable hydrogels. Biomaterials. 2006;27:1579–1585. doi: 10.1016/j.biomaterials.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Anseth KS, Metters AT, Bryant SJ, Martens PJ, Elisseeff JH, Bowman CN. In situ forming degradable networks and their application in tissue engineering and drug delivery. J Control Rel. 2002;78(1–3):199–209. doi: 10.1016/s0168-3659(01)00500-4. [DOI] [PubMed] [Google Scholar]
- 10.Leach JB, Bivens KA, Collins CN, Schmidt CE. Development of photocrosslinkable hyaluronic acid polyethylene glycol-peptide composite hydrogels for soft tissue engineering. J Biomed Mater Res. 2004;70A:74–82. doi: 10.1002/jbm.a.30063. [DOI] [PubMed] [Google Scholar]
- 11.Balakrishnan B, Jayakrishnan A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials. 2005;26:3941–3951. doi: 10.1016/j.biomaterials.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Holland TA, Tessmar JK, Tabata Y, Mikos AG. Transforming growth factor-beta1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Rel. 2004;94(1):101–114. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Wieland JA, Houchin-Ray TL, Shea LD. Non-viral vector delivery from PEG-hyaluronic acid hydrogels. Journal of Controlled Release. 2007;120:233–241. doi: 10.1016/j.jconrel.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moffat KM, Marra KG. Biodegradable Poly(ethylene glycol) Hydrogels Crosslinked with Genipin for Tissue Engineering Applications. J Biomed Mater Res Part B: Appl Biomater. 2004;71B:181–187. doi: 10.1002/jbm.b.30070. [DOI] [PubMed] [Google Scholar]
- 15.Bouhadir KH, Kruger GM, Lee KY, Mooney DJ. Sustained and Controlled Release of Daunomycin from Cross-Linked Poly(aldehyde guluronate) Hydrogels. Journal of Pharmaceutical Sciences. 2000;89:910–919. doi: 10.1002/1520-6017(200007)89:7<910::AID-JPS8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Sung HW, Huang RN, Huang LLH, Tsai CC, Chiu CT. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J Biomed Mater Res. 1998;42:560–567. doi: 10.1002/(sici)1097-4636(19981215)42:4<560::aid-jbm12>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Ferretti M, Marra KG, Kobayashi K, Defail AJ, Chu CR. Controlled in vivo degradation of genipin crosslinked polyethylene glycol hydrogels within osteochondral defects. Tissue Engineering. 2006;12(9):2657–2663. doi: 10.1089/ten.2006.12.2657. [DOI] [PubMed] [Google Scholar]
- 18.Maia J, Ferreira L, Carvalho R, Ramos MA, Gil MH. Synthesis and characterization of new injectable and degradable dextran-based hydrogels. Polymer. 2005;46:9604–9614. [Google Scholar]
- 19.Nishi KK, Jayakrishnan A. Preparation and in Vitro Evaluation of Primaquine-Conjugated Gum Arabic Microspheres. Biomacromolecules. 2004;5:1489–1495. doi: 10.1021/bm0499435. [DOI] [PubMed] [Google Scholar]
- 20.Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, et al. Multifunctional chondroitin sulphate for cartilage tissue–biomaterial integration. Nat Mater. 2007;6(5):385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruhela D, Riviere K, Szoka FC. Efficient Synthesis of an Aldehyde Functionalized Hyaluronic Acid and Its Application in the Preparation of Hyaluronan-Lipid Conjugates. Bioconjugate Chem. 2006;17:1360–1363. doi: 10.1021/bc0600721. [DOI] [PubMed] [Google Scholar]
- 22.Ito T, Yeo Y, Highley CB, Bellas E, Benitez CA, Kohane DS. The Prevention of Peritoneal Adhesions by in situ Cross-linking Hydrogels of Hyaluronic Acid and Cellulose Derivatives. Biomaterials. 2007;28:975–983. doi: 10.1016/j.biomaterials.2006.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Martino A, Sittinger M, Risbud MV. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Kim SE, Park JH, Cho YW, Chung H. Porous chitosan scaffold containing microspheres loaded with transforming growth factor: Implication for cartilage tissue engineering. J Controlled Release. 2003;91:365–374. doi: 10.1016/s0168-3659(03)00274-8. [DOI] [PubMed] [Google Scholar]
- 25.Tan H, Gong Y, Gao C. Gelatin/chitosan/hyaluronan ternary complex scaffold containing basic fibroblast growth factor for cartilage tissue engineering. Journal of Materials Science: Materials in Medicine. 2007;18:1961–1968. doi: 10.1007/s10856-007-3095-5. [DOI] [PubMed] [Google Scholar]
- 26.Kato Y, Onishi H, Machida Y. Biological characteristics of lactosaminated N-succinyl-chitosan as a liver-specific drug carrier in mice. J Controlled Release. 2001;70:295–307. doi: 10.1016/s0168-3659(00)00356-4. [DOI] [PubMed] [Google Scholar]
- 27.Sun S, Wang A. Adsorption properties of N-succinyl-chitosan and cross-linked N-succinyl-chitosan resin with Pb(II) as template ions. Separation and Purification Technology. 2006;51:409–415. [Google Scholar]
- 28.Kato Y, Onishi H, Machida Y. Lactosaminated and intact N-succinyl-chitosans as drug carriers in liver metastasis. International Journal of Pharmaceutics. 2001;226:93–106. doi: 10.1016/s0378-5173(01)00777-3. [DOI] [PubMed] [Google Scholar]
- 29.Ameer GA, Mahmood TA, Langer R. A biodegradable composite scaffold for cell transplantation. J Orthop Res. 2002;20:16–19. doi: 10.1016/S0736-0266(01)00074-2. [DOI] [PubMed] [Google Scholar]
- 30.Fan HB, Hu YY, Qin L, Li XS, Wu H, Lv R. Porous gelatin-chondroitin-hyaluronate tri-copolymer scaffold containing microspheres loaded with TGF-β1 induces differentiation of mesenchymal stem cells in vivo for enhancing cartilage repair. J Biomed Mater Res. 2006;77A:785–794. doi: 10.1002/jbm.a.30647. [DOI] [PubMed] [Google Scholar]
- 31.Bulpitt P, Aeschlimann D. New Strategy for Chemical Modification of Hyaluronic Acid: Preparation of Functionalized Derivatives and Their Use in the Formation of Novel Biocompatible Hydrogels. J Biomed Mater Res. 1999;47:152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Curotto E, Aros F. Quantitative determination of chitosan and the percentage of free amino groups. Analytical Biochemistry. 1993;211:240–241. doi: 10.1006/abio.1993.1263. [DOI] [PubMed] [Google Scholar]
- 33.Bouhadir KH, Hausman DS, Mooney DJ. Synthesis of cross-linked poly(aldehyde guluronate) hydrogels. Polymer. 1999;40:3575–3584. [Google Scholar]
- 34.Karpie J, Chu CR. Lidocaine Exhibits Dose- and Time-Dependent Cytotoxic Effects on Bovine Articular Chondrocytes In Vitro. The American Journal of Sports Medicine. 2007;35:1621–1627. doi: 10.1177/0363546507304719. [DOI] [PubMed] [Google Scholar]
- 35.Chu CR, Izzo N, Papas NE, Fu FH. In Vitro Exposure to 0.5% Bupivacaine Is Cytotoxic to Bovine Articular Chondrocytes Arthroscopy. The Journal of Arthroscopic and Related Surgery. 2006;22:693–699. doi: 10.1016/j.arthro.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Marra KG, DeFail AJ, Clavijo-Alvarez JA, Badylak SF, Taieb A, Schipper B, et al. FGF-2 enhances vascularization for adipose tissue engineering. Plastic and Reconstructive Surgery. 2008;121(4):1153–1164. doi: 10.1097/01.prs.0000305517.93747.72. [DOI] [PubMed] [Google Scholar]
- 37.Yu H, Lu J, Xiao C. Preparation and Properties of Novel Hydrogels from Oxidized Konjac Glucomannan Cross-Linked Chitosan for in vitro Drug Delivery. Macromol Biosci. 2007;7:1100–1111. doi: 10.1002/mabi.200700035. [DOI] [PubMed] [Google Scholar]