Abstract
Humans, apes, and rhesus monkeys demonstrate memory awareness by collecting information when ignorant and acting immediately when informed. In this study, five capuchin monkeys searched for food after either watching the experimenter bait one of four opaque tubes (seen trials), or not watching (unseen trials). Monkeys with memory awareness should look into the tubes before making a selection only on unseen trials because on seen trials they already know the location of the food. In Experiment 1, one of the five capuchins looked significantly more often on unseen trials. In Experiment 2, we ensured that the monkeys attended to the baiting by inter-leaving training and test sessions. Three of the five monkeys looked more often on unseen trials. Because monkeys looked more often than not on both trial types, potentially creating a ceiling effect, we increased the effort required to look in Experiment 3, and predicted a larger difference in the probability of looking between seen and unseen trials. None of the five monkeys looked more often on unseen trials. These findings provide equivocal evidence for memory awareness in capuchin monkeys using tests that have yielded clear evidence in humans, apes, and rhesus monkeys.
Keywords: Metamemory, Metacognition, Explicit memory, Foraging, Visual search
Introduction
Being aware of what we know allows us to behave appropriately according to our knowledge state. For example, imagine that a friend asks if you need directions to their house. If you successfully retrieve a memory of the route, you might answer “no”. In contrast, if your attempt at retrieving the route from memory fails, you will likely answer “yes” and make the adaptive decision to write down directions. This ability to accurately assess the availability or sufficiency of needed information in memory is termed memory awareness (Hampton et al. 2004).
Memory awareness might also be adaptive for nonhuman animals (Griffin 2003). It would allow an individual to decide when it knew enough to act and when it needed to gather more information (Call and Carpenter 2001; Hampton et al. 2004; Kornell et al. 2007). Determining the extent to which nonhuman animals are aware of their knowledge will inform research into when and why memory awareness might have evolved and research into what aspects of cognition we share with other animals.
Memory awareness is one aspect of metacognition, or thinking about thinking. Researchers have gathered evidence of metacognition from both primates and non-primates. A series of studies have used psychophysical discriminations in which an animal can make one of two judgments about the stimulus (e.g., high or low tone, sparse or dense image) or make a third response to “escape” the test. These investigators have hypothesized that if subjects have access to an assessment of their own uncertainty about a particular discrimination they will more often escape difficult discriminations than easy discriminations. Dolphins are more likely to escape difficult auditory discriminations than easy ones (Smith et al. 1995) and rhesus monkeys show the same pattern for visual discriminations (Shields et al. 1997; Smith et al. 2006; Smith et al. 1997). Rats show this pattern of escape responses for duration discriminations (Foote and Crystal 2007), as do pigeons performing visual discriminations (Sole et al. 2003). Most vertebrate species tested thus appear to be able to use an escape response adaptively in psychophysical tasks.
In contrast to psychophysical metacognition tasks, only primates have shown good evidence of memory awareness. One method of testing for memory awareness is to determine the extent to which nonhuman animals, like the human in the opening example, will seek more information if the contents of memory are insufficient for the task at hand, but will act immediately when adequate information is available in memory. This is similar to some metacognitive tests used with humans, which have found a negative correlation between perceived knowledge and information search behavior (Radecki and Jaccard 1995). The less knowledge subjects felt they had about the to-be-tested domain, the more they would study. In the current paradigm, we experimentally controlled the information available in subjects’ memory by either providing or withholding information needed for correct responding on a trial-by-trial basis. The primary dependent measure was whether the monkeys, like human subjects, sought more information when needed, but avoided doing so when more information was not needed.
Call and Carpenter (2001) developed a foraging task based on this principle to assess memory awareness in primates. They presented chimpanzees, orangutans, and 2.5-year-old human children with two or three opaque, horizontal tubes. They then baited one of the tubes either in sight of the subject (“seen” condition) or while the subject’s view of the tubes was blocked by a panel (“unseen” condition). For each trial, the subject could (1) seek more information by looking down the tubes or (2) choose a tube without looking. Either way, the subjects were allowed to select one tube only. In the unseen condition, if no looking occurs, subjects should perform at chance levels of accuracy because they do not know where the food is located. However, subjects could dramatically increase the accuracy of their choices on unseen trials by looking down the tubes to locate the food before choosing. In contrast, on seen trials, looking results in wasted effort and the collection of information redundant with what should already be available in memory. Thus, this foraging paradigm assesses memory awareness by experimentally manipulating the presence of memory for a recent food location and assessing the degree to which animals respond adaptively.
Consistent with memory awareness, chimpanzees, orangutans, and human children looked down the tubes more often on unseen trials than on seen trials (Call and Carpenter 2001). In addition, subjects were significantly more accurate on unseen trials on which they looked compared to unseen trials on which they did not look. When subjects did look, they used an efficient strategy, most often searching the tubes until they saw the food or had searched all tubes. These results show that apes and human children adaptively collect more information when ignorant.
Rhesus macaques, a species of Old World monkey, also adaptively seek new information more often when needed than when not needed. Hampton et al. (2004) used a for-aging task closely modeled after that used by Call and Carpenter (2001). In the study by Hampton et al., nine monkeys chose from among four opaque metal tubes, only one of which contained food. Seven of the nine monkeys looked down the tubes more often on unseen trials than on seen trials. Searching the tubes on unseen trials significantly improved choice accuracy. Reinforcing these findings in rhesus monkeys, Kornell et al. (2007) used a computer-based hint-seeking task in which the monkeys could earn preferred food by touching a set of four images in the proper sequence. Monkeys could touch a button outside the response area to highlight the next image in the sequence, giving them a hint, but reducing their food reward to a less-preferred food. Consistent with memory awareness, monkeys requested fewer hints and were more accurate as they learned the sequence. Monkeys also sought more hints for novel sequences relative to familiar ones. Using a different paradigm, both rhesus monkeys and orangutans have demonstrated memory awareness by declining tests on which their memory is weak. Two rhesus macaques were more accurate on a matching task when they chose to take the test than when they were forced to take it (Hampton 2001). Similarly, one out of five orangutans was significantly more accurate on a foraging task when she chose to take the test than when she was forced to take it (Suda-King 2007). Following a manipulation like that of Call and Carpenter (2001), where the animals either saw or did not see the baiting, the orangutans declined the test significantly more often on unseen trials than on seen trials.
The tendency of apes (Call and Carpenter 2001; Suda-King 2007) and rhesus macaques (Hampton et al. 2004; Kornell et al. 2007) to seek more information when needed suggests that memory awareness may have been present in a common ancestor of humans, apes, and Old World monkeys. By contrast, evidence from non-primates suggests that memory awareness may not be universal among vertebrates. Dogs literally pass by the opportunity to seek a hint when faced with an uncertain choice (Brauer et al. 2004). Pigeons seem unable to adaptively escape memory tests if they have to decide before seeing the test stimuli (Inman and Shettleworth 1999). To determine when memory awareness evolved, it would be useful to study additional primate species, especially New World monkeys. If Old World monkeys show evidence of memory awareness and New World monkeys do not, it would suggest that memory awareness evolved in primates after the split with New World monkeys around 27–36 million years ago (Steiper and Young 2006). However, if New World monkeys do show evidence of memory awareness, it would suggest that our capacity for memory awareness evolved earlier, in a common ancestor of all monkeys and apes.
The current study is the first direct comparison between Old World and New World monkeys’ capacity for memory awareness. One other study has used similar methods to assess capuchins’ knowledge of what they can see (Paukner et al. 2006), although they manipulated the direct visibility of food (perception) rather than the previous visibility of food (memory). We tested tufted capuchins using the same procedures and apparatus used previously to test rhesus macaques (Hampton et al. 2004). Subjects were presented with trials on which they either had or had not observed the baiting (seen and unseen trials, respectively). On each trial, they could either seek more information by searching the four tubes or choose a tube without looking. Based on the successful performance of other primates, we hypothesized that capuchin monkeys would show evidence of memory awareness. We evaluated their behavior with respect to three criteria: (1) whether they looked down the tubes more often on unseen trials than on seen trials, (2) whether looking on unseen trials improved choice accuracy, and (3) whether monkeys appropriately stopped looking down the tubes immediately after finding the food.
Experiment 1
Methods
Subjects
Five adult (aged 8–12 years) male tufted capuchin monkeys (Cebus apella) participated. Two monkeys were housed individually, two were housed together in a socially compatible pair, and one was housed with a juvenile male that did not participate in this study. The housing room was on a 12:12 light–dark cycle with onset at 7:00 a.m. Access to food was not restricted. Monkeys worked for preferred foods (e.g., chocolate candies and grain-based cereal). Water was available ad libitum in the home cage.
Apparatus
We used the same apparatus that was used previously with rhesus monkeys (Hampton et al. 2004). Four tubes (2.5 cm diameter × 16.6 cm length) were affixed to a metal tray 9.5 cm apart (Fig. 1). Each tube attached via a hinge near the front of the tray such that it could be pulled up like a lever, causing any food inside to roll out toward the monkey. Pulling a tube engaged a hidden latching mechanism and locked down the remaining tubes, ensuring that the monkey could choose only one tube per trial. A short metal plate (5.5 cm) served as a visual barrier. When attached to the front of the tray, it was impossible for the monkeys to look down the length of the tubes. However, it did not block monkeys from seeing the tubes from the top, from reaching out and pulling the tubes, or from retrieving any found rewards. Tubes were either transparent (clear acrylic) or opaque (aluminum), depending on the phase of training.
Fig. 1.
The apparatus as seen from experimenter’s side. A The opaque and transparent screens in the down position. B The opaque tubes on the tray. C The tube latching mechanism
Before testing, monkeys were placed in a mobile transport cage, which was then secured to the front of the apparatus. The tray on which the tubes were mounted could be placed at one of five different heights. Height 1 was level with the bottom of the transport cage and height 5 was at the approximate eye level of a sitting capuchin with 8 cm separating each level. Two barriers could be lowered independently between the cage and the tubes apparatus. The first, a transparent screen, allowed visual, but not physical access to the tubes. The second, an opaque screen, prevented both visual and physical access.
A video camera mounted behind the apparatus provided a view down the lengths of the tubes. It was connected to a monitor on the side of the testing set-up so that the experimenter could view and score the monkeys’ looking behavior without staring directly down the tubes and possibly influencing looks or choices.
Behavior scoring
The experimenter sat to the side of the apparatus and watched the closed circuit television monitor. All behaviors were coded in real time. The dependent measures were the number and location of looks down the tubes and choice accuracy (i.e., percent choice of the baited tube). A choice was scored if the monkey lifted the tube enough to engage the latching mechanism. Looks were scored if the monkey’s eye was occluded by the tube, as viewed through the closed circuit television. Sessions were videotaped and reviewed by the first author immediately after each session to ensure accuracy.
Testing procedures
Adaptation Phase 1: selecting and pulling transparent tubes
In Phase 1, we taught monkeys to pull the tube containing the food to retrieve the reward. The apparatus was fitted with clear acrylic tubes through which the monkeys could easily see the location of the food without looking down the tubes. The tray supporting the tubes was placed at height 3 (halfway between eye level and the floor of the transport cage) and the short visual barrier was in place, blocking visual access down the tubes.
We used two trial types, seen and unseen, in order to give monkeys equal experience retrieving food in each condition. All trials began with both clear and opaque screens down, separating the monkey from the tubes. On seen trials the opaque screen was raised prior to baiting, allowing the monkey to observe the baiting procedure through the transparent screen. On unseen trials, the opaque screen was not raised until after the baiting procedure. After either the opaque screen was raised or the tubes were baited, on unseen and seen trials, respectively, the clear screen was kept in place for an additional 2 s, allowing the monkey to observe, but not manipulate the tubes. Next, the experimenter raised the clear screen, which allowed the monkey to choose one tube. If the monkey chose correctly, it could retrieve the reward. Finally, both screens were lowered, thereby terminating the trial.
One session of 24 trials, consisting of 12 seen and 12 unseen trials, was given each day. The sequence of seen and unseen trials and the location of the food reward followed a pseudorandom order, with the constraint that no trial type or location appeared more than four times in a row. Trials were aborted if no choice was made within 60 s. During adaptation, aborted trials were skipped (i.e., not replaced). No correction trials were run after incorrect choices in any of the testing phases. Monkeys were required to select the tube in which the reward was visible 24 out of 24 trials in a single session before advancing to the next phase of adaptation.
Phase 2: transition to opaque tubes
In Phase 2, we taught monkeys to attend the baiting procedure. Phase 2 was conducted similarly to Phase 1 with the exception that opaque metal tubes replaced the transparent tubes and that all trials were seen, as unseen trials would not have allowed the monkeys to learn about the baiting procedure. Because they could no longer see into the tubes (the tubes were opaque and the visual barrier was still in place), the monkeys could reliably choose correctly only if they observed and remembered which tube was baited.
Because learning to attend to the baiting was by far the hardest phase for the rhesus in the previous study (Hampton et al. 2004), we divided Phase 2 of the current study into four sub-phases. In 2A, both clear and opaque screens were raised together prior to baiting and the monkey was allowed to select a tube immediately after the food was in place. In 2B, the opaque screen was raised first, the tubes were baited in full view of the monkey, then the clear screen was immediately raised (0 s delay), and the monkey was allowed to select a tube. In 2C we lengthened the delay between baiting and raising the clear screen to 1 s. For 2D, we increased the delay to 2 s. In this way, the monkeys were gradually acclimated to attend to the baiting and hold the information in memory for 2 s. In the main experiment, below, this delay provided a period during which the monkeys could collect more information (i.e., look down the tubes to locate the food), but could not yet act on their actual memory (i.e., select a tube).
Criterion for each sub-phase was 21 out of 24 correct in a single complete session. Monkeys moved back to the previous sub-phase if they scored 10 or fewer correct out of 24 trials on both of two consecutive days.
Phase 3: looking
Hampton et al. (2004) found that a pilot rhesus monkey required experience with searching for food by looking down the tubes before they used this behavior reliably under any circumstances. Thus, in Phase 3 we presented the tray at eye level (height 5) without the short visual barrier. All trials were seen trials. This allowed the monkeys to easily detect the location of the food by looking down the tubes.
Sessions were otherwise conducted as in Phase 2. Monkeys were required to look down the tube on 21 or more of 24 trials before advancing to the main task.
Main task
The short visual barrier was removed, giving monkeys the opportunity to visually search the opaque tubes before making a selection. Seen and unseen trials were pseudorandomly intermixed, such that no location and neither trial type occurred more than four times in a row. On seen trials, the monkey observed the baiting of the tube and so did not need to look down the tubes to know the location of the food. On unseen trials, the monkeys could only find the food reliably by conducting a visual search down the lengths of the tubes before selecting a tube. Each daily session consisted of 28 trials: 4 refresher trials, 12 seen trials, and 12 unseen trials. All four refresher trials were conducted at the start of the session and were conducted exactly as in Phase 3.
Because Hampton et al. (2004) found that rhesus showed great individual variation in how much effort they were willing to expend to retrieve the food reward, we manipulated the effort required to look by varying the height of the tray. The tray was raised, making looking easier, if the monkey looked on fewer than 6 of the 24 test trials (seen and unseen trials together) in the previous session. The tray was lowered, increasing the difficulty of looking, if the monkey looked on more than 18 of 24 test trials. We based our titration on the total number of looks, rather than the number of looks in a given trial type, to avoid training the monkeys to show a difference. This titration procedure helped to control for floor and ceiling effects, increasing the probability that putative differences in behavior between seen and unseen trials could be detected.
For the testing phase, aborted trials were noted on the scoring sheet and immediately repeated. Monkeys were tested in this manner until they completed two consecutive sessions in which the tray height had not changed. Only data from these two sessions were analyzed. Our dependent measures were the number and location of looks and choice accuracy (i.e., percent choice of the baited tube). Refresher trials were not included in the analysis. All proportions were arcsine transformed prior to analysis (Howell 1997). There is disagreement about whether to use a Chi-square test or a Fisher’s exact test when expected cell frequencies are low (Aron and Aron 1999, p. 451). Accordingly, we report the more common Chi-square test when both tests agreed on significance and the more conservative Fisher’s exact test when the tests disagreed.
Results and discussion
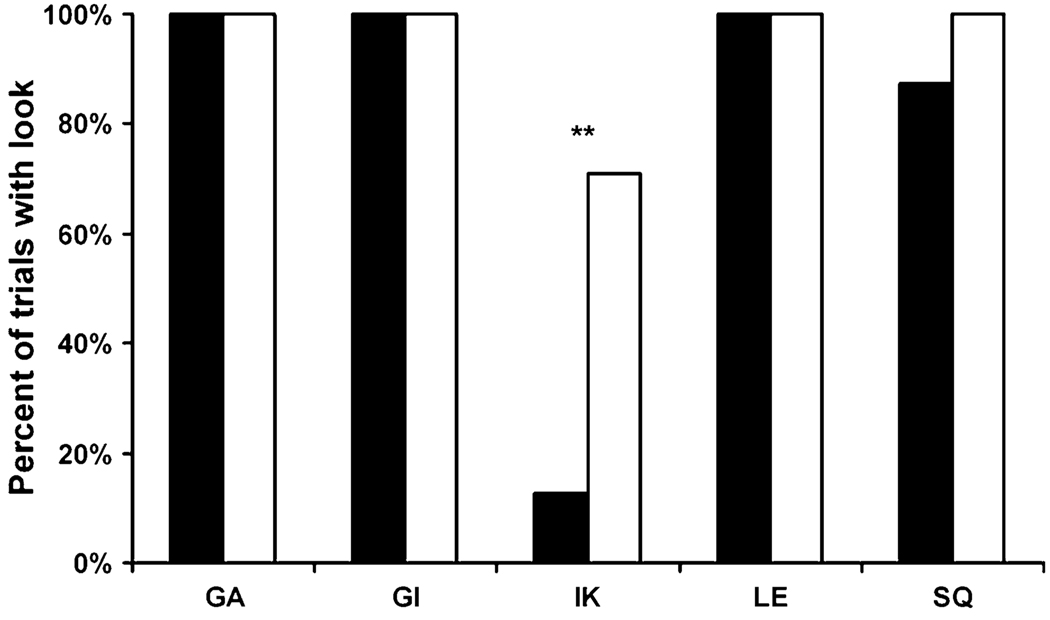
The monkeys required an average of 7.6, 34.2, and 1.4 sessions to reach criteria in adaptation phases 1–3, respectively, indicating that the most difficult aspect of training was learning to attend to the baiting. During the main task, all monkeys looked down the tubes at ceiling levels from the first test session and finished with the tray at floor level (height 1). Review of the videotape showed that one monkey (IK) looked on four trials that were not so scored initially (two seen and two unseen trials). While all trials for all other monkeys were scored correctly as to whether any looks occurred, a small number of additional looks, after the first look on a given trial, were noted (mean = 2.6 trials per monkey).
On the main task, four of the five monkeys failed to show differential looking (Fig. 2; monkeys GA, GI, and LE: , P>0.999; monkey SQ: , P = 0.074). Of these four monkeys, three looked on every trial in both seen and unseen conditions and the forth looked on all but three seen trials. When they searched the tubes, they made their first search to the correct tube significantly more often than expected by chance on seen trials, but not on unseen trials (seen: mean = 0.55, t3 = 3.20, P = 0.049; unseen: mean = 0.32, t3 = 0.98, P = 0.406, chance = 0.25). Although these accurate searches indicate memory of the food’s location, the fact that the monkeys looked does not support the hypothesis that they were aware of their memories. However, their performance is not as high as might be expected if their memories were strong. It is likely that memory of the food’s location was poor, despite our efforts to ensure that they attended to the baiting and were accustomed to the 2 s delay.
Fig. 2.
The percent of trials on which monkeys looked down at least one tube on seen (black) and unseen (white) trials in Experiment 1. ** P<0.01, see main text for full statistics
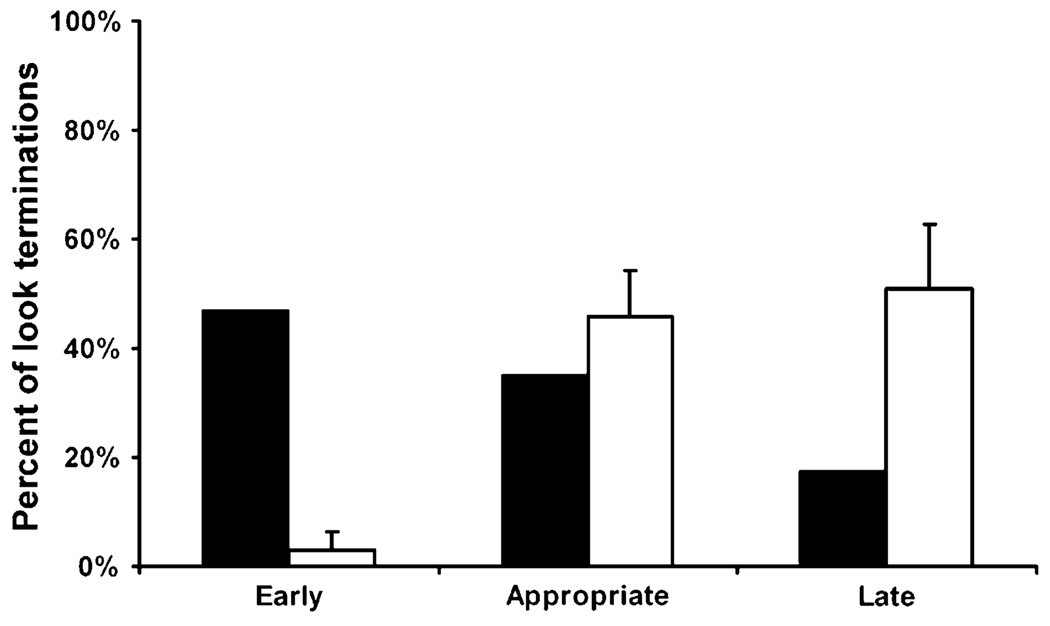
Monkeys could show three patterns of search behavior on trials where they chose to look: appropriate terminations (looking until the food has been located and then choosing a tube), late terminations (continuing to look after having seen the food), or early terminations (choosing a tube before having seen the food). On unseen trials, four monkeys were as likely to appropriately terminate their search as they were to terminate late (Fig. 3, white bars; appropriate: mean = 0.46, late: mean = 0.51, t3 = −0.21, P = 0.845), demonstrating a strong tendency to look even when it should have been unnecessary. On seen trials, all five monkeys terminated the majority of their looks appropriately (mean = 0.88).
Fig. 3.
The percent of unseen trials on which monkeys terminated looking before having seen the food (early), terminated immediately after having seen the food (appropriate), or continued looking after having found the food (late) in Experiment 1. Black bars are the performance of monkey IK. White bars are the mean (+SEM) of the other four monkeys
For unseen trials, we could not directly compare accuracy after looking to accuracy after not looking because monkeys looked on every unseen trial. Consequently, for this and subsequent experiments, we analyzed whether looking on unseen trials resulted in accuracy levels above those expected by chance (0.25). This was the case for the four monkeys that did not show differential looking (mean = 0.93; t3 = 6.33, P = 0.008). Thus, although monkeys did not look strategically, they did successfully use the information gained when they did look.
The fifth monkey (IK) looked significantly more often on unseen trials than he did on seen trials (Fig. 2; , P<0.001). Unlike the other four monkeys, IK showed a strong tendency to look too little, terminating the majority of his searches on unseen trials before having found the food (47% of trials; Fig. 3, black bars). This search strategy resulted in poor performance. Considering both seen and unseen conditions together, monkey IK found the food on only 60% of trials on which he looked. In contrast, the remaining four monkeys found the food 97% of the time. Lastly, monkey IK was the only monkey to abort any trials (n = 2).
In summary, none of the five monkeys met all three of our criteria for memory awareness in Experiment 1. The fact that one monkey (IK) looked significantly more often on unseen than on seen trials would seem to be consistent with memory awareness. However, he most often terminated his searching early, before finding the food, suggesting that his looking behavior was not regulated by his knowledge of the location of the food (although it is possible that he erroneously thought he saw the food when he terminated searching). Probably as a result of the many prematurely terminated searches, looking did not lead to an increase in accuracy, as would be predicted if he were looking down the tubes in order to gather information. This is in contrast to the behavior of apes (Call and Carpenter 2001) and rhesus monkeys (Hampton et al. 2004), both of which showed increased accuracy after looking.
Our prediction that monkeys should look more often on unseen trials than on seen trials was based on two assumptions: (1) the monkeys pay attention to the baiting on seen trials and therefore know the location of the food, and (2) looking is effortful and so monkeys will attempt to minimize unnecessary looks. We attempted to ensure the validity of the first assumption in Experiment 2 and the second assumption in Experiment 3.
Experiment 2
It is possible that the lack of a difference in the probability of looking on seen and unseen trials in Experiment 1 resulted from of the monkeys failing to attend to the baiting. Failing to attend to the baiting effectively turns seen trials into unseen trials. As in the earlier study of rhesus monkeys by Hampton et al. (2004), capuchin monkeys did not spontaneously and reliably attend to the baiting. On average, learning to attend to the baiting took four times longer than any other training phase. In Experiment 1, immediately after learning to attend to the baiting procedure in Phase 2, monkeys experienced three or four sessions where they had the opportunity to look and thus could get all the trials correct without paying attention to the baiting (one or two sessions of Phase 3 and two sessions to titrate the tray height). It is possible that the monkeys had ceased to engage in the already difficult task of paying attention because it was no longer necessary to do so. This interpretation is supported by the surprisingly low proportion of seen trials on which their monkeys looked first in the correct tube in Experiment 1.
In Experiment 2, we encouraged the monkeys to attend to the baiting by interleaving sessions in which the short visual barrier was in place and the monkey had to attend to succeed, with the test sessions on which they could choose whether to look down the tube. Monkeys were required to reach criterion on each of three consecutive sessions with the visual barrier before each test session. Our hypothesis was that interleaving sessions with the visual barrier with test sessions would increase the monkeys’ attention to the baiting on test trials. On seen trials, the increased attention should decrease overall searches down the tubes and increase the proportion of first-looks to the correct tube when the monkeys do look.
Methods
Subjects and apparatus
All subjects and apparatus are the same as those used in Experiment 1.
Procedure
Refresher training
Sessions with the visual barrier in place ensured that the monkeys were reliably attending to the baiting. Trials were conducted as those in sub-phase 2D of Experiment 1. The delay between baiting and access was decreased by 1 s if the monkeys scored 10 or fewer correct out of 24 on each of two consecutive days. Meeting criterion on this reduced delay returned the monkeys to the full 2-s delay. Monkeys moved on to the main task if they performed at criterion level (21 out of 24 correct) with the 2-s delay for each of three consecutive days.
Main task
The main task was conducted as in Experiment 1 with the exception that the tray was already at the lowest height. After one complete test session, monkeys were required to complete three more sessions of criterion-level performance with the visual barrier in place. The second session of the main task was then conducted. In this way, the two test sessions were interleaved with refresher sessions on which monkeys were required to attend to the baiting.
Results and discussion
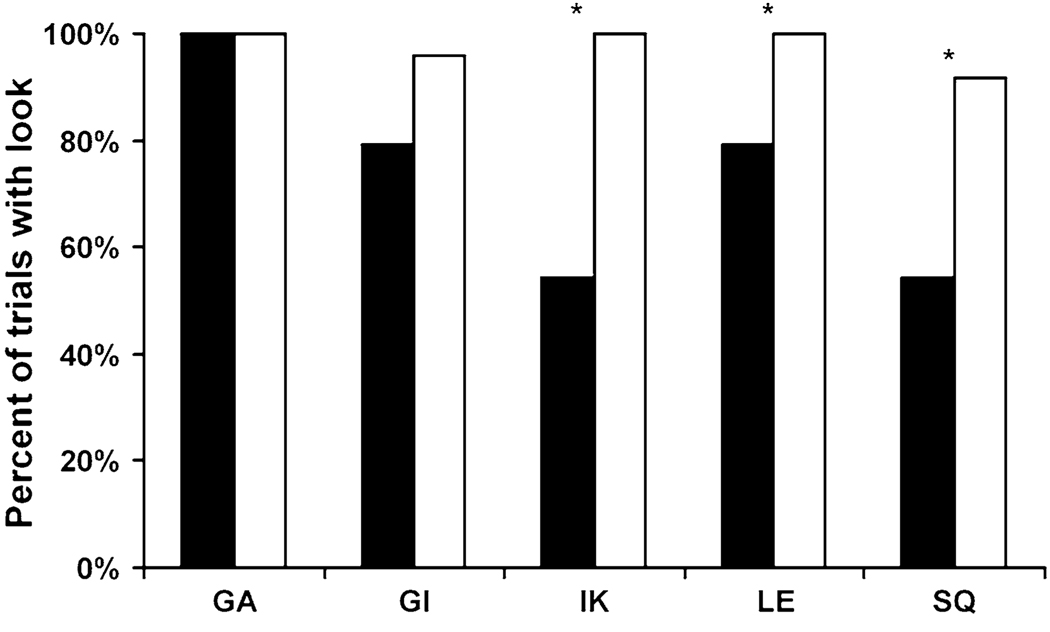
Monkeys required nearly as many sessions to relearn to attend to the baiting as they did to learn initially in Phase 2 of Experiment 1, suggesting that they were indeed not attending to the baiting by the end of Experiment 1. A paired t test revealed that the number of sessions to reach criteria did not differ between the two phases (Experiment 1: mean = 34.2, Experiment 2: mean = 24.4; t4 = 1.04, P = 0.359). After reviewing the videotape, no monkeys required recoding of the number of trials on which they looked. Three monkeys (GA, GI, and IK) required recoding of search pattern data for at least one trial (mean = 2 trials). Additionally, monkeys demonstrated that they knew the location of the food on seen trials, making their first search to the correct tube significantly more often than chance (mean = 0.77, t4 = 6.74, P = 0.003; chance = 0.25).
In the main task, three of the five monkeys (IK, LE, and SQ) met all three of our criteria for memory awareness. First, like the other species of primates tested, they searched the tubes more often when they needed information than when they did not, looking down the tubes significantly more often on unseen trials than on seen trials (Fig. 4; GA: , P>0.999; GI: , P = 0.081; IK: , P<0.001; LE: , P = 0.018; SQ: , P = 0.003). Second, when they did look, their behavior was consistent with control by knowledge of the location of the food. Group analysis of all five monkeys showed that appropriate look terminations occurred significantly more often than late terminations in both conditions (seen: appropriate mean = 0.74, late mean = 0.25, t4 = 3.30, P = 0.030; unseen: appropriate mean = 0.70, late mean = 0.29, t4 = 4.24, P = 0.013, Fig. 5), indicating that monkeys did not look more often than needed to determine the location of the food. This search pattern is strikingly similar to that observed in chimpanzees and human children, who also efficiently searched until they found the food and then appropriately terminated looking (Call and Carpenter 2001, Experiments 2 and 3, respectively). On seen trials, the monkeys also terminated the majority of their trials appropriately (mean = 0.74). Third, the monkeys’ group accuracy after looking on unseen trials was higher than expected by chance (mean = 0.99; t4 = 23.33, P<0.001; chance = 0.25). Thus, the monkeys successfully used the information gained by looking. Monkey IK aborted one trial.
Fig. 4.
The percent of trials on which monkeys looked down at least one tube on seen (black) and unseen (white) trials in Experiment 2. *P < 0.05, see main text for full statistics
Fig. 5.
The percent of unseen trials (+SEM) on which monkeys terminated looking before having seen the food (early), terminated immediately after having seen the food (appropriate), or continued looking after having found the food (late) in Experiment 2
In summary, the monkeys based their decision to look down the tubes on whether they had seen the food, based their search pattern on their knowledge of the food’s location, and thus accurately found the food on almost all unseen trials. Their behavior is highly similar to that of apes, children (Call and Carpenter 2001), and Old World monkeys (Hampton et al. 2004), but dissimilar to that of dogs (Brauer et al. 2004), who showed knowledge of the food when they had seen it, but could not adaptively seek more information when they had not. This suggests that capuchin monkeys can adaptively seek more information when they do not know the answer.
Experiment 3
Looking takes time and effort, and it should only improve accuracy when the monkey is ignorant of the food location. Thus, the monkeys should base their decision to either look down the tubes or immediately attempt to retrieve the food on the costs and benefits of each action. In Experiments 1 and 2, the tubes were at the lowest level (height 1). Because this height required monkeys to crouch to look down the tubes, the act of looking was presumably effortful. In addition, looking behavior delayed the retrieval of food, thereby incurring a temporal cost as well. Nevertheless, the monkeys still made many apparently unnecessary looks down the tubes in both experiments (though less so in Experiment 2). The three monkeys that showed differential looking in Experiment 2 still searched the tubes on an average of 62.5% of the seen trials. They also continued to search the tubes after having looked in the one containing the food on an average of 30.1% of seen trials and 25.5% of unseen trials on which they did look. We hypothesized that further increasing the cost of looking would decrease the number of unnecessary looks and thereby increase the difference in looks on seen and unseen trials.
In Experiment 3, we increased the cost of looking by attaching hinged metal flaps to the front of the tubes (Fig. 6). Monkeys had to lift the flap, hold it open, and crouch to look down a tube. The monkeys could not just move their head across the tube openings and quickly scan all tubes, as they could in Experiments 1 and 2. The addition of the flaps increased both the effort required to look and the delay to reinforcement.
Fig. 6.
Top view of a monkey holding up one of the flaps to look down the tube (left) and side view of a monkey selecting a tube by pulling up on it (right) in Experiment 3
Methods
Subjects and apparatus
The same subjects used previously were used again. The apparatus was the same except for the addition of the four hinged, metal flaps (Fig. 6). The flaps were attached to the front of each tube (i.e., the end nearest the monkey). In the down position, the flaps prevented the monkey from looking down the tubes. Monkeys could independently raise each flap to gain visual access to the tube. A short metal bolt on the top of each tube prevented the flaps from staying in the up position, requiring the monkeys to physically hold it up for the duration of their look down a particular tube. When a tube was pulled up, the flap would fall open, letting the food roll out toward the monkey.
Procedure
Adaptation
In adaptation for Experiment 1, all monkeys immediately began lifting the tubes without any previous experience. Accordingly, we expected the monkeys to use the flaps upon first exposure. A single unrewarded pilot trial with monkey GA confirmed this expectation. Thus, we did not give monkeys any full sessions to familiarize them with the flaps. Prior to the first trial of the main task, monkeys were shown the tubes apparatus with the flaps attached, the experimenter demonstrated how to lift the flaps, and then the monkeys were allowed time (<60 s) to lift the flaps themselves in the absence of food reward.
Refresher training
Refresher sessions were similar to that in Experiment 2, with the exception that the tray was positioned at the middle level (height 3). The lowest level was not used because we were unsure how effortful the monkeys would find lifting the flaps and did not want to risk extinguishing the response. As in Experiment 2, we required the monkeys to meet criterion (21 out of 24 correct) for three consecutive refresher sessions before receiving 1 session of the main task.
Main task
The main task was similar to that of Experiment 2 except that the tray was positioned at the middle level and the hinged flaps were attached to the end of the tubes.
Results and discussion
Increasing the cost of searching did have the expected effect of decreasing unnecessary looking, but also decreased the number of necessary searches. Four of the five monkeys showed a significant decrease in the total number of trials (collapsed across both conditions) on which they looked when compared to Experiment 2 (GA: P = 0.012; GA: P = 0.111; IK: P<0.001; SQ: P<0.001; LE: P<0.001; two-sided Fisher’s exact test). This decrease was not the result of the monkeys’ unfamiliarity with using the flaps, as all monkeys immediately lifted the flaps during Adaptation and continued to do so sporadically throughout the sessions. Monkeys IK and SQ aborted six and four trials, respectively. As in Experiment 1, the aborted trials were immediately re-administered. After reviewing the videotape, no monkeys required recoding of the number of trials on which they looked or their pattern of searches.
Despite the increased cost of looking, which we had predicted would increase the difference observed in Experiment 2, none of the five monkeys searched the tubes significantly more often on unseen trials than on seen trials (Fig. 7; GA: , P = 0.683; GI: , P = 0.312; IK: , P = 0.064; LE: , P = 0.248; SQ: , P = 0.221). The performance of the three monkeys that showed differential looking in Experiment 2 remained in the predicted direction, although the differences were no longer significant. This lack of differential looking is not due to a lack of knowledge, as evidenced by looking behavior on seen trials. When the monkeys looked, they first searched the correct tube significantly more often than chance on seen trials, but not on unseen trials (seen: mean = 0.88, t4 = 6.55, P = 0.03; unseen: mean = 0.20, t4 = −0.92, P = 0.412; chance = 0.25). After looking on unseen trials, monkeys showed a nonsignificant trend toward being more accurate than expected by chance in selecting the correct tube (mean = 0.65; t4 = 2.68, P = 0.055; chance = 0.25). However, when accuracy was broken down into trials on which the monkeys looked in the correct tube and trials on which the monkeys terminated looking before finding the correct tube, the accuracy rates were 0.94 and 0.19, respectively. This suggests that the monkeys used the information they gained from looking, but did not always correctly identify the baited tube.
Fig. 7.
The percent of trials on which monkeys looked down at least one tube on seen (black) and unseen (white) trials in Experiment 3
When the monkeys did look down the tubes, their search pattern was consistently different from that observed in Experiment 2. Rather than appropriately terminating their searches after having found the food on unseen trials, they were equally likely to terminate their looking appropriately (on the first look to the baited tube) as they were to terminate looking early (prior to finding the food; appropriate: mean = 0.52, early: mean = 0.44, t4 = 0.02, P = 0.982). The monkeys made very few late terminations (mean = 0.04). On seen trials, the monkeys made the majority of their terminations appropriately (mean = 0.92). After early terminations, there was no evidence that subjects were inferring the location of the food. The monkeys’ choice accuracy was low (mean = 0.21, chance = 0.25) and they did not choose an unsearched tube more often than expected by chance, given the average number of unsearched tubes remaining (expected: 0.66; observed: 0.58; P = 0.554, two-tailed binomial test). The monkeys almost never continued looking after having found the food. Their accuracy after seeing the food on unseen trials was high (mean = 93.75%), indicating that they did use information appropriately when available.
The addition of the flaps on the ends of the tubes may have increased the cost of looking beyond the optimal level for detecting metamemory. Adding the flaps increased the time and physical effort required to look down the tubes. Under these conditions, the monkeys initiated fewer searches of the tubes and when they did search, they often gave up before finding the food (proportion of early search terminations: Experiment 2 = 0.00; Experiment 3 = 0.44). As a result, the monkeys’ choice accuracy after looking on unseen trials decreased (Experiment 2: 99.09%; Experiment 3: 65.13%). The monkeys also aborted more trials in Experiment 3 than in Experiment 2 (total aborted trials: Experiment 2 = 1; Experiment 3 = 10). Collectively, these behaviors suggest that the cost of looking now out-weighed the benefit of increased choice accuracy that would result from a thorough search of the tubes. The contrast in the results of Experiments 2 and 3 highlight the importance of the balance between the costs and benefits of searching the tubes before making a selection.
General discussion
With respect to our three criteria, Experiment 2 demonstrated that capuchin monkeys (1) looked more often on unseen trials than on seen trials, (2) chose tubes more accurately when they did look than expected by chance, and (3) most often appropriately terminated searching immediately after finding the food. Collectively, these three behaviors suggest that capuchins have memory awareness.
Experiment 3 demonstrates the importance of balancing the costs and benefits in memory tasks. In previous work, rhesus monkeys showed a wide range of variation in how much effort they were willing to expend to look down the tubes (Hampton et al. 2004). Seven of the nine rhesus monkeys showed floor or ceiling effects, requiring that the overall difficulty be altered (Table 1: monkeys K, C, G, F, B, H, and L), and two of those monkeys continued to show floor or ceiling effects even at the extreme edges of difficulty (Fig. 2: monkeys G and F). Similarly, we had to adjust the difficulty of looking for all five of our capuchin monkeys. However, unlike the high individual variability of the rhesus, all of the capuchins looked too often, leading us to increase the difficulty of looking. This may reflect the extremely explorative nature of capuchins (Fragaszy et al. 2004). Brauer et al. (2004) experienced the opposite problem with dogs in a similar experiment. The dogs refused to search for food and continued to do so even when looking was made significantly easier. Future studies of memory awareness might benefit from manipulating the benefit of the food rather than the cost of looking, either through the number or quality of rewards or the hunger of the subject. Increasing the benefit of obtaining the food might avoid the apparent frustration effect we found in Experiment 3.
The general tendency of capuchins to look more than necessary (initiate looking on seen trials and continue looking after they had already seen the food) is consistent with their natural foraging behavior. Capuchins are exhaustive foragers, tending to over-search one food cluster before moving to the another (DeLillo et al. 1997). Similarly, Paukner et al. (2006) found that capuchins reliably searched for food down transparent tubes (when they were already able to see it from above) and down bent tubes (where it was impossible to see the food). Demonstrating memory awareness may therefore require capuchins to inhibit their natural foraging behavior. If capuchins have memory awareness, they may only employ it in restricted circumstances. Future studies may benefit from taking this into account by designing more ecologically valid foraging tasks, perhaps with multiple bait locations.
These unnecessary looks may also represent a “passport effect” (Call and Carpenter 2001). This can occur when the costs and benefits of seeking more information are unbalanced. For example, the small cost of double-checking your passport is far outweighed by the large cost of forgetting to bring it to the airport. In this case, you will likely make an unnecessary search for your passport even if you are aware of a strong memory for packing it. In the current experiment, the monkeys may have searched unnecessarily because the small cost of double-checking the correct location was outweighed by the cost of not obtaining the food. Thus, positive evidence for memory awareness (some of which was reported here) should not be discounted due to the presence of unnecessary searches on some trials or under some conditions.
Non-metacognitive accounts
The inference of memory awareness is valid only if capuchins base their search strategy on judgments about the contents of their own memory. Here we briefly explore two possible alternative explanations for the current data: conditioned responding and response competition.
Conditioned responding
Call and Carpenter (2001), Brauer et al. (2004), and Hampton et al. (2004) all suggest the possibility that during training the animals were reinforced for looking down the tubes on unseen trials and for choosing a tube without looking on seen trials. In the current study, the visibility of baiting on seen trials could have served as a cue that choosing a tube immediately would result in a high probability of success. However, the reinforcement history during training is unlikely to provide sufficient differential reinforcement to support the observed behavior. Prior to their successful performance in Experiment 2, monkeys received an average of 58.6 sessions during training where they were reinforced for immediately choosing a tube and only an average of 5.6 sessions during which they could have learned a differential rule about looking. Accordingly, we would expect capuchins to have an overall tendency to choose without looking. This was not the case. Conditioning predicts insufficient looking, whereas the capuchins looked excessively. Conditioning alone is an unlikely explanation for the current results.
Response competition
Hampton et al. (2004) suggest that successful behavior in the tubes task might come about through competition between the natural responses of the animal. Searching for available food may be the monkeys’ default behavior in any foraging situation. The sight of the food may release a competing reaching behavior. On seen trials, the drive to reach overpowers the drive to look, whereas the opposite is true on unseen trials. Kornell et al. (2007) further developed this argument, suggesting that the tubes task may reflect a generalization of this natural foraging behavior and that it may not require awareness of one’s knowledge.
It is difficult to entirely rule out the response competition account. It is the case that rather than immediately reaching for the food after seeing it, as response competition would predict, the monkeys sometimes continued to search the remaining tubes. While this could suggest a search for more information (e.g., an exhaustive search), it could also suggest that, in capuchins, the reaching response is weak compared to the searching response under these testing conditions. It is unclear how we can best determine whether the decision to look or reach is controlled by competing impulses or by the search for additional information. Both accounts predict that the monkey should attempt to retrieve visible food. Future studies might manipulate the amount of information by baiting multiple tubes or manipulate memory strength by including a delay condition, similar to what was done with apes (Call and Carpenter 2001).
Comparison with rhesus monkeys
Because we tested capuchins using procedures and apparatus closely modeled after those used previously with rhesus monkeys (Hampton et al. 2004), we can make fairly direct comparisons between the two species. First, the capuchins were initially more explorative and less willing to attend to the baiting. Whereas the base rate of looking varied among the rhesus, all capuchins showed high looking rates from the first session of testing. When the capuchins did show more looking on unseen than on seen trials in Experiment 2, they looked at a higher overall rate on seen trials than did the rhesus. The capuchins were also more likely to continue looking after having found the food. Second, despite the fact that the capuchins looked more often than the rhesus overall, the pattern of the capuchins’ performance in Experiment 2 was generally similar to that of the rhesus. Looking on unseen trials resulted in high choice accuracy, first-looks on seen trials were most often to the correct tube, and the monkeys most often immediately stopped looking after seeing the food. Overall, the capuchins showed evidence for memory awareness under a narrower set of circumstances than did the rhesus.
The lack of evidence of memory awareness from Experiments 1 and 3 suggests that we should interpret the positive findings from Experiment 2 with caution. The arguments made here that the monkeys were not attending to the baiting in Experiment 1 and that we exceeded the ideal amount of effort required to look in Experiment 3 have yet to be systematically explored. Future studies are needed to unequivocally determine whether New World monkeys have memory awareness.
In summary, we found evidence of memory awareness in Experiment 2. Three of the five capuchins tested met all three of our criteria. First, they sought information more often when they needed it than when they did not. Second, searching led to highly successful performance at retrieving the food. Third, their searches were appropriate, continuing until the food was found and then stopping. However, these positive findings should be interpreted conservatively because the capuchins did not continue to show this pattern when we increased the difficulty of searching in Experiment 3. Nonetheless, the positive results from Experiment 2 are consistent with the existence of memory awareness in capuchins. Combined with the results from similar experiments with humans, apes, and rhesus monkeys (Call and Carpenter 2001; Hampton et al. 2004; Suda-King 2007), the present results may indicate that memory awareness is widespread among primates.
Acknowledgments
We thank David Ide of Research Services Branch, NIMH for designing and fabricating the apparatus. We also thank Ruth Woodward of Research Animal Management Branch, NICHD for excellent veterinary care. This research was supported by the NIMH Intramural Research Program. Additional support for preparation of this manuscript was provided by Yerkes Center base grant No. RR-00165 awarded by the Animal Resources Program of the National Institutes of Health, and by the Center for Behavioral Neuroscience under the STC Program of the National Science Foundation under Agreement No. IBN-9876754. These experiments comply with US law.
Contributor Information
Benjamin M. Basile, Email: bbasile@emory.edu, National Institute of Mental Health, Bethesda, MD, USA.
Robert R. Hampton, National Institute of Mental Health, Bethesda, MD, USA
Stephen J. Suomi, National Institute of Child Health and Human Development, Bethesda, MD, USA
Elisabeth A. Murray, National Institute of Mental Health, Bethesda, MD, USA
References
- Aron A, Aron E. Statistics for psychology. Upper Saddle River: Prentice Hall; 1999. [Google Scholar]
- Brauer J, Call J, Tomasello M. Visual perspective taking in dogs (Canis familiaris) in the presence of barriers. Appl Anim Behav Sci. 2004;88(3–4):299–317. [Google Scholar]
- Call J, Carpenter M. Do apes and children know what they have seen. Anim Cogn. 2001;4:207–220. [Google Scholar]
- DeLillo C, Visalberghi E, Aversano M. The organization of exhaustive searches in a patchy space by capuchin monkeys (Cebus apella) J Comp Psychol. 1997;111(1):82–90. [Google Scholar]
- Foote AL, Crystal JD. Metacognition in the rat. Curr Biol. 2007;17(6):551–555. doi: 10.1016/j.cub.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragaszy DM, Visalberghi E, Fedigan LM. The complete capuchin : the biology of the genus Cebus. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- Griffin DR. Significant uncertainty is common in nature. Behav Brain Sci. 2003;26(3):346. doi: 10.1017/S0140525X03290087. [DOI] [PubMed] [Google Scholar]
- Hampton RR. Rhesus monkeys know when they remember. Proc Natl Acad Sci U S A. 2001;98:5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR, Zivin A, Murray EA. Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Anim Cogn. 2004;7:239–254. doi: 10.1007/s10071-004-0215-1. [DOI] [PubMed] [Google Scholar]
- Howell DC. Statistical methods for psychology. 4th edn. Belmont: Duxbury Press; 1997. [Google Scholar]
- Inman A, Shettleworth SJ. Detecting metamemory in nonverbal subjects: A test with pigeons. J Exp Psychol Anim Behav Process. 1999;25(3):389–395. [Google Scholar]
- Kornell N, Son LK, Terrace HS. Transfer of metacognitive skills and hint seeking in monkeys. Psychol Sci. 2007;18(1):64–71. doi: 10.1111/j.1467-9280.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Paukner A, Anderson J, Fujita K. Redundant food searches by capuchin monkeys (Cebus apella): a failure of metacognition. Anim Cogn. 2006;9(2):110–117. doi: 10.1007/s10071-005-0007-2. [DOI] [PubMed] [Google Scholar]
- Radecki CM, Jaccard J. Perceptions of knowledge, actual knowledge, and information search behavior. J Exp Soc Psychol. 1995;31(2):107–138. [Google Scholar]
- Shields WE, Smith JD, Washburn DA. Uncertain responses by humans and rhesus monkeys (Macaca mulatta) in a psycho-physical same-different task. J Exp Psychol Gen. 1997;126(2):147–164. doi: 10.1037//0096-3445.126.2.147. [DOI] [PubMed] [Google Scholar]
- Smith JD, Schull J, Strote J, McGee K, Egnor R, Erb L. The uncertain response in the bottlenosed dolphin (Tursiops truncatus) J Exp Psychol Gen. 1995;124(4):391–408. doi: 10.1037//0096-3445.124.4.391. [DOI] [PubMed] [Google Scholar]
- Smith JD, Shields WE, Schull J, Washburn DA. The uncertain response in humans and animals. Cognition. 1997;62(1):75–97. doi: 10.1016/s0010-0277(96)00726-3. [DOI] [PubMed] [Google Scholar]
- Smith JD, Redford JS, Beran MJ, Washbum DA. Dissociating uncertainty responses and reinforcement signals in the comparative study of uncertainty monitoring. J Exp Psychol Gen. 2006;135(2):282–297. doi: 10.1037/0096-3445.135.2.282. [DOI] [PubMed] [Google Scholar]
- Sole LM, Shettleworth SJ, Bennett PJ. Uncertainty in pigeons. Psychon Bull Rev. 2003;10(3):738–745. doi: 10.3758/bf03196540. [DOI] [PubMed] [Google Scholar]
- Steiper ME, Young NM. Primate molecular divergence dates. Mol Phylogenet Evol. 2006;41(2):384–394. doi: 10.1016/j.ympev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Suda-King C. Do orangutans (Pongo pygmaeus) know when they do not remember. Anim Cogn. 2007;11(1):21–42. doi: 10.1007/s10071-007-0082-7. [DOI] [PubMed] [Google Scholar]