Abstract
Rats turned diabetic by alloxan treatment are refractory to systemic anaphylactic shock, in a direct association with reduced intestinal haemorrhage and tissue response to antigen challenge. As diabetic rats show reduction in mast cell numbers in different body compartments, this study was undertaken to investigate the influence of alloxan diabetes on mast cell population as well as the expression of the mast cell growth factor interleukin (IL)-3 in the small intestine of rats. We also analysed the putative involvement of endogenous insulin and glucocorticoid hormones in this phenomenon. There was a significant decrease in the number of mast cells present in the small intestine (ileum segment) of diabetic rats. Likewise, the immunohistochemical analysis revealed that IL-3 labelling was markedly attenuated in diabetic rats, as compared with normal animals, a phenomenon which paralleled with a decreased mRNA expression as attested by Reverse transcriptase-polymerase chain reaction technique. Treatment with insulin and with the steroid receptor antagonist RU 486 restored basal mast cell numbers, normal levels of IL-3 labelling and mRNA expression for IL-3 in the ileum of diabetic rats. In conclusion, our findings show that there is a causative relationship between down-regulation of mast cell numbers and the expression of IL-3 associated with diabetic state. In addition, as both parameters were suppressed by administration of insulin and RU 486, it indicates that an imbalance between the systemic levels of insulin and glucocorticoid hormones seems to be implicated in the reduction in intestinal mast cell population and refractoriness to antigen provocation in alloxan diabetes.
Keywords: Diabetes, glucocorticoids, IL-3, Insulin, mast cells
Mast cells are especially localized near surfaces exposed to the environment, including the skin, airways and gastrointestinal tract, where pathogens, allergens and other environmental agents are frequently found (Galli et al. 2005). In mammals and other vertebrates, mature mast cells are widely distributed throughout vascularized tissues, particularly beneath and, in some cases, within epithelia and in close proximity to the blood vessels, nerves, smooth muscle cells, mucus-producing glands and hair follicles (Kitamura 1989). Mast cells are derivatives of haematopoietic progenitor cells that migrate virtually into all tissues, where they complete their maturation (Metcalfe et al. 1997). In the microenvironment, factors such as interleukin (IL)-3, stem cell factor (SCF) and IL-9 have been identified as the principle cytokines that regulate mast cell growth and differentiation (Godfraind et al. 1990; Crivellato et al. 2004; Galli et al. 2005). IL-3 seems to be important to early mast cell proliferation (Mekori et al. 1993), whereas SCF produced by stromal cells acts to maintain mast cell viability and promote their maturation (Nocka et al. 1990; Irani et al. 1992).
In response to antigen, mast cells can release cytoplasmic granule-associated mediators as vasoactive amines and produce lipid mediators including cysteinyl-leukotrienes and platelet activating factor (Hogan & Schwartz 1997). According to some authors, mast cells also secrete a wide range of cytokines, which may contribute to the initiation and perpetuation of immunoglobulin E-dependent allergic inflammation in different body sites (Galli et al. 2005).
It has been reported that alloxan-treated rats have a selective reduction in the number of pleural mast cells, a phenomenon also observed after treatment with the alternative diabetogenic agent streptozotocin (Diaz et al. 1996). Additionally, we have also demonstrated that diabetic sensitized animals are clearly resistant to local and systemic allergic inflammatory responses (Carvalho et al. 2005). In the case of systemic anaphylaxis, a substantial reduction in the lethality, intestinal haemorrhage and refractoriness of small intestine to antigen stimulation in vitro has been described, suggesting that mast cell depletion in the intestinal tissue might play a role in the refractoriness of diabetic rats to anaphylactic shock (Carvalho et al. 2003). Thus, this study investigated the influence of alloxan diabetes on mast cell population as well as the expression of IL-3 in the small intestine of rats. The involvement of insulin and glucocorticoid hormones in these phenomena was also evaluated.
Materials and methods
Animals and diabetes induction
Male Wistar rats (180–200 g) were obtained from the Oswaldo Cruz Foundation breeding colony and used in accordance with the guidelines of the Committee on Use of Laboratory Animals of the Oswaldo Cruz Foundation (CEUA-FIOCRUZ, license 0085–02). Diabetes was induced by administering a single intravenous (i.v.) injection of alloxan monohydrate (40 mg/kg), diluted with saline (0.9% NaCl), into 12-h-fasted rats supplied with water ad libitum. Animals injected with vehicle and submitted to similar experimental conditions were used as negative controls. Blood glycaemia was determined by means of a glucose monitor (Johnson & Johnson, Milpitas, California, USA) in samples obtained from the tail vein. After alloxan injection, rats with blood glucose levels below 200 mg/dl were excluded from further experiments.
Histology and immunohistochemistry
Animals were killed in CO2 chamber, and the small intestine was washed with phosphate-buffered solution (PBS), fixed in Carnoy's fixative and embedded in paraffin. Small intestine (ileum) sections (4–5 μm) were stained with alcian blue (pH 2.5), safranin (0.1%) and periodic acid–Schiff (PAS). The number of mast cells was counted under a light microscope (Olympus BX50, Center Valley, PA, USA), which was coupled to a video camera (Optronics Engineering, DEI-750, Goleta, CA, USA). The camera output was processed and analysed by image analyser software (Image-Pro Plus 4, Silver Spring, MD, USA). The results were expressed as number of mast cells per mm2.
For immunohistochemical staining, paraffin-embedded ileum sections were deparaffinized by washing with xylene, rehydrated in a graded series of ethanol and washed with Tris-buffered saline (TBS). Endogenous peroxidase activity was blocked by incubation with hydrogen peroxide (3%), and non-specific binding sites were blocked with bovine fetal serum (8%); bovine serum albumin (BSA, 2.5%) and unfatty milk (1%) diluted with TBS. Then primary antibody, polyclonal goat anti-rat IL-3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), was added and incubated on the sections overnight. After rinsing with TBS, the sections were treated with conjugated horseradish peroxidase secondary antibody (polyclonal donkey anti-goat immunoglobulin G (IgG); R&D systems, Minneapolis, MN, USA) for 1 h at room temperature and then incubated with 3-amino-9-ethylcarbazole plus H2O2 as substrate. Sections were counterstained with Harris haematoxylin, rinsed in water and mounted in aqueous medium. In control experiments, no immunostaining was observed when an irrelevant biotinylated goat anti-hamster IgG (Vector Laboratories, Burlingame, CA, USA) was used as primary antibody. The analysis was performed under a light microscope (Olympus BX50) coupled to a video-camera (Optronics Engineering, DEI-750). Images were ultimately analysed using image analyser software (Image-Pro Plus 4). The camera output was processed and analysed by the image analyser software Image-Pro Plus 4. Levels of IL-3 were quantified by densitometric scanning (pixels)/μm2.
Extraction of total RNA and reverse transcription
Total RNA was extracted from intestine tissue of non-diabetic, diabetic and insulin- or RU-486-treated diabetic rats with Trizol (Ivitrogen, Cidade, País) following the manufacturer's instruction. Isolated RNA was diluted with Rnase-free water and quantified by absorbance measurement at 260 nm. Four micrograms of total RNA was incubated with 0.5 μg of random primers for 5 min at 70 °C and allowed to cool down to room temperature. RNA was subsequently reversed transcribed in 1× reverse transcription buffer (75 mm KCl, 3 mm MgCl2, 10 mm dithiothreitol and 50 mm Tris–HCl, pH 8.3) that contained 1 unit/μl Rnasin ribonuclease inhibitor, 1 mm of each dNTP and 10 units/μl Rnase H(−)-Moloney leukaemia virus reverse transcriptase (all reagents from Promega, Madison, WI, USA). The reaction was conducted for 1 h at 37 °C, and the reverse transcriptase was then heat-inactivated at 99 °C for 5 min. The experiments were replicated three times.
PCR of IL-3 and β-actin cDNA
Polymerase chain reactions (PCR) with cDNA obtained were performed in 1× PCR reaction buffer (2 μl of the reaction mix containing FastStart Taq DNA polymerase, dNTP mix and 3 mm MgCl2) (all from Roche Diagnostics, Minneapolis, MN, USA), and 10 ρmol of each primer: IL-3, sense 5′-AAGGGATCTGCAGGAATCGTGTG-3′ and antisense 5′-GCCCAGTGTAGGCTGGAGTCTCCA-3′; β-actin, sense 5′-CCCTGGAGAAG-AGCTACGA-3′ and antisense 5′-TAAAGCCATGCCAATCTCAT-3′ (Invitrogen, Carlsbad, CA, USA), in a 25 μl final volume for 35 cycles. Each cycle consisted of 45-s denaturation at 94 °C, 45-s annealing at 55 °C and 2-min extension at 72 °C. After amplification by PCR with the primers above mentioned, the PCR products were electrophoresed in a 1% agarose gel stained with ethidium bromide. After agarose gel electrophoresis, intensity of bands was measured by densitometric analysis using image Software (Image Pro Plus for Windows) and converted into peaks by the software. The IL-3 mRNA expression was calculated from the area under the peaks.
Treatments
Diabetic rats were treated with insulin (15 IU/kg) subcutaneously once a day for 18 consecutive days, starting 3 days after diabetes induction. We also tested the effect of the steroid receptor antagonist RU 486 which was dissolved in 0.5% methylcellulose (v/v in water) and administered orally once a day for 18 consecutive days, starting 3 days after diabetes induction at 20 mg/kg. In control animals, the drugs were replaced by their vehicles.
Insulin quantification
Serum levels of insulin were evaluated by radioimmunoassay (de Oliveira Barreto et al. 2003) (ICN, Irvine, CA, USA) following the manufacturer's guidelines.
Drugs
Alloxan monohydrate, RU 486, PBS, BSA, 3-amino-9-ethyl-carbazole, Tris [hydroxymethyl] aminomethane hydrochloride (Tris–HCl) and hydrogen peroxide were purchased from Sigma (St Louis, MO, USA) and insulin NPH from Biobrás (Montes Claros, Brazil). All solutions were freshly prepared immediately before use.
Statistical analysis
The data were reported as mean ± standard error and statistically analysed by anova followed by Newman–Keuls–Student's t-test. Probability values (P) of 0.05 or less were considered significant.
Results
Characteristics of alloxan diabetic rats
Twenty-one days after alloxan (40 mg/kg) intravenous injection into rats, there was an increase in the blood glucose concentration with values of 70.5 ± 14.5 mg/dl for non-diabetic rats and 469.4 ± 36.76 mg/dl for diabetic ones (mean ± SEM, n = 7; P < 0.001). Serum insulin levels were markedly reduced, with values decreasing from 58.79 ± 5.29 μU/ml in non-diabetic rats to 11.99 ± 3.08 μU/ml (mean ± SEM, n = 6; P < 0.001) in alloxan-diabetic rats.
Influence of alloxan-diabetes on mast cell number and IL-3 expression in the small intestine of rats
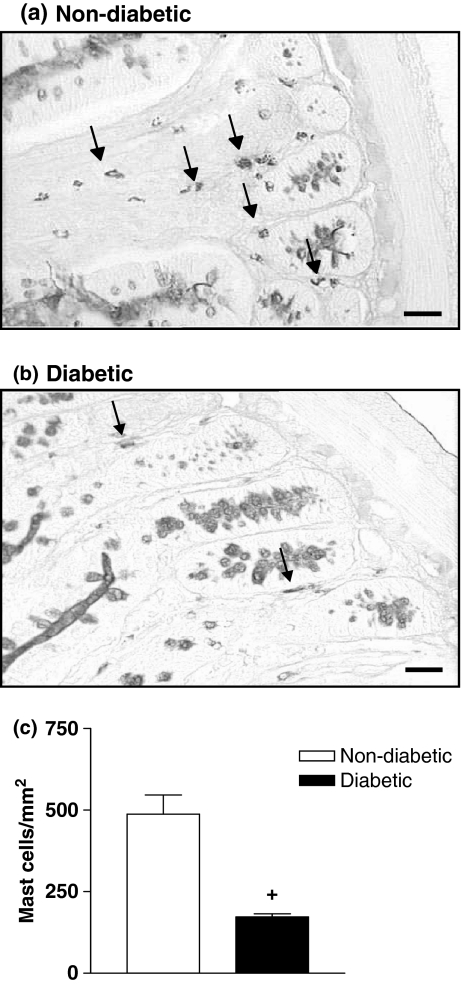
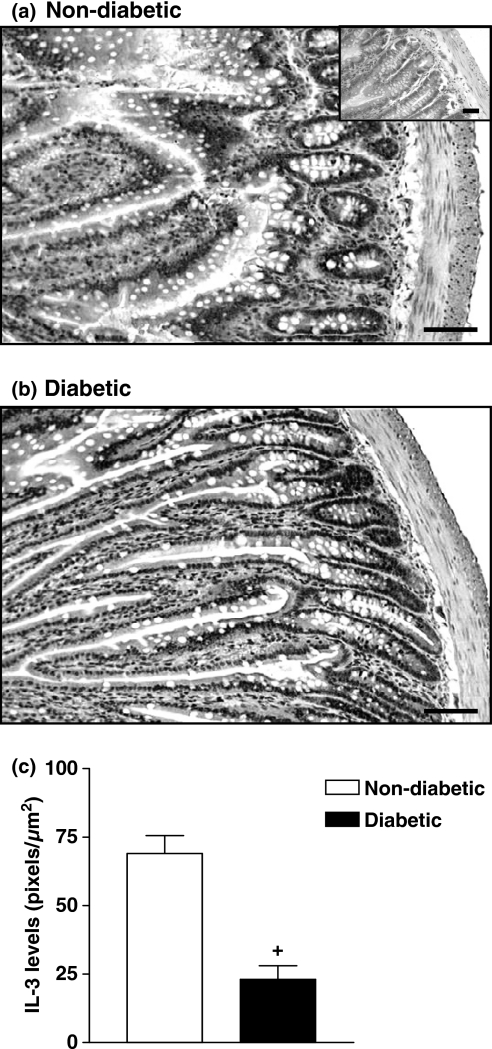
Staining of ileum fragments with PAS-alcian blue/safranin revealed a significant reduction in the mast cell numbers in diabetic rats compared with those from non-diabetic animals (Figure 1). Quantitative analyses revealed a 2.7-fold reduction in the number of mast cells in diabetic rats (P < 0.001) (Figure 1). Immunohistochemical staining with a specific antibody against the mast cell growth factors IL-3 was used to determine its tissue expression. As illustrated in Figure 2, ileum tissue from non-diabetic rats showed a marked labelling for IL-3, somehow distributed along the smooth muscle layer and from crypts to villus. Diabetic rats (Figure 2b) exhibited a weaker labelling for IL-3 as compared with non-diabetic animals (Figure 2a). Quantitative data are seen in Figure 2c.
Figure 1.
Decrease in mast cell numbers in the intestine of diabetic rats. Panels (a) and (b) show photomicrographs of PAS-alcian blue/safranin stained mast cells (arrows) from ileum segments of non-diabetic and alloxan-diabetic rats respectively. Bar 0.1 μm. Panel (c) shows that the number of mast cells is significantly reduced in the intestine of diabetic rats compared with controls. Data are expressed as mean ± SEM from three animals. The data are representative of three experiments. +P < 0.05 compared with non-diabetic rats.
Figure 2.
Decrease in IL-3 expression in the intestine of diabetic rats. Panels (a) and (b) show photomicrographs of immunostaining of IL-3 of intestine sections from non-diabetic and alloxan-diabetic rats respectively. Insets represent negative controls when an irrelevant biotinylated goat anti-hamster IgG was used as primary antibody. Sections were counterstained with Harris haematoxylin. Bar 0.1 μm. Quantitative evaluation of IL-3 labelling is seen in panel (c). Data are expressed as mean ± SEM from four animals. The data are representative of two experiments. +P < 0.05 compared with non-diabetic rats.
Influence of hypoinsulinaemia and hypercorticolism on intestinal mast cell and IL-3 depletion in diabetic rats
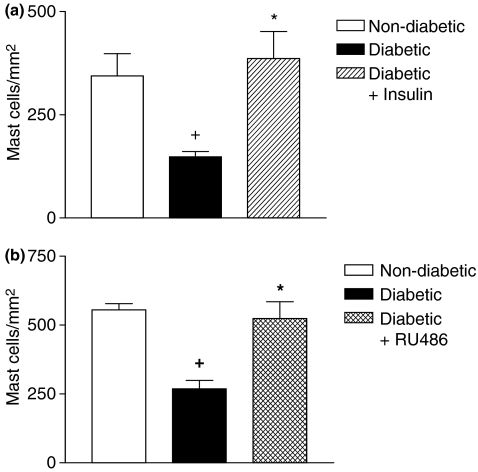
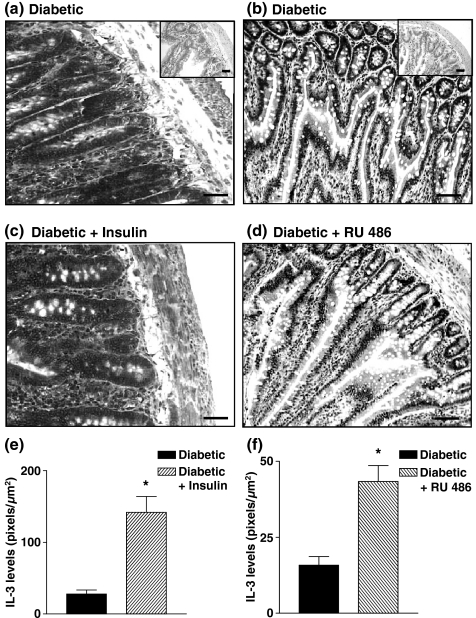
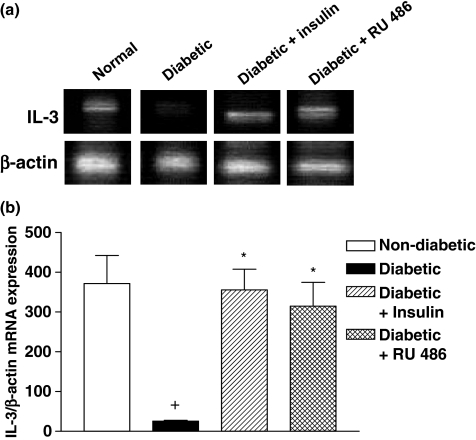
To investigate the contribution of decreased levels of insulin to mast cell depletion in diabetic rats, animals were submitted to a chronic treatment with insulin (15 IU/kg) once a day for 18 consecutive days, starting 3 days after diabetes induction. Insulin had the ability to restore the basal number of mast cells in the ileum tissue of diabetic rats (Figure 3a). In another set of experiments, when diabetic animals were given the steroid receptor antagonist RU 486 (20 mg/kg, p.o.), following the same schedule of drug administration, mast cell numbers returned to basal level (Figure 3b). Furthermore, we noted that administration of insulin (Figure 4c) and RU 486 (Figure 4d) into diabetic rats (Figure 4a,b) restored the normal pattern of IL-3 immunoreactivity in the ileum tissue. Quantitative evaluation of IL-3 expression in diabetic rats treated with insulin and RU 486 is seen in Figure 4e,f respectively.
Figure 3.
Effect of insulin and RU 486 on the intestinal mastocytopenia in diabetic rats. Animals were treated with insulin (3 IU, s.c.) or RU 486 (20 mg/kg, p.o.) once a day for 18 days following diabetes induction. Panel (a) shows that the number of mast cells is significantly reduced in the intestine of diabetic rats compared with non-diabetic rats and that the insulin treatment of diabetic rats is able to restore the number of mast cells. Panel (b) shows that the number of mast cells is significantly reduced in the intestine of diabetic rats compared with control and that RU 486 in diabetic rats is able to restore the number of mast cells. Data are expressed as mean ± SEM from three animals. The data are representative of three experiments. +P < 0.05 compared with non-diabetic rats. *P < 0.05 compared with alloxan-diabetic rats.
Figure 4.
Decrease in IL-3 expression in intestine of diabetic rats and restoring effect by insulin and RU 486. Animals were treated with insulin (3 IU, s.c.) or RU 486 (20 mg/kg, p.o.) once a day for 18 days following diabetes induction. Panels (a) and (b) show photomicrographs of immunostaining of IL-3 of intestine sections from alloxan-diabetic rats. Panels (c) and (d) show photomicrographs of immunostaining of IL-3 of intestine sections from insulin-treated diabetic rats and diabetic rats treated with RU 486 respectively. Insets represent negative controls when an irrelevant biotinylated goat anti-hamster IgG was used as primary antibody. Sections were counterstained with Harris haematoxylin. Bar 0.1 μm. Quantitative evaluations of IL-3 labelling after insulin and RU 486 treatment are seen in panels (e) and (f) respectively. Data are expressed as mean ± SEM from four animals. The data are representative of two experiments. *P < 0.05 compared with alloxan-diabetic rats.
Coherently, both hormones also reversed the drop in the mRNA levels of IL-3 noted in diabetic animals compared with non-diabetic ones (Figure 5a). Quantitative data are seen in the Figure 5b.
Figure 5.
Decrease in IL-3 mRNA in the intestine of diabetic rats and restoring effect by insulin and RU 486. Representative samples of RT-PCR performed with mRNA isolated from independent pools of ileum tissue of non-diabetic (lane 1), diabetic (lane 2) and insulin- (lane 3) or RU 486-treated (lane 4) diabetic rats were analysed by a RNA blot with the use of a cDNA probe encoding IL-3 (upper) and β-actin (lower) (a). Results of densitometric analysis of IL-3 and β-actin genes in whole groups are seen in panel (b). Data are expressed as mean ± SEM from four animals. The data are representative of two experiments. +P < 0.05 compared with non-diabetic rats. *P < 0.05 compared with alloxan-diabetic rats.
Discussion
In this study, we investigated the influence of alloxan diabetes on the mast cell population as well as on the growth factors IL-3 in the small intestine of rats. The involvement of insulin and glucocorticoid hormones in these phenomena was also evaluated. There was a significant reduction in the intestinal mast cell population in a good correlation with the decreased expression of IL-3. Treatment with insulin and RU 486 restored mast cell numbers and IL-3 expression in the intestine of diabetic rats. Altogether, our findings indicate that the intestinal mast cell depletion in diabetic animals is associated with reduction in the IL-3 expression, in a clear dependence on the down-regulation of endogenous insulin and up-regulation of endogenous glucocorticoids.
It is noteworthy that mast cells show morphological and functional heterogeneity in different locations in the body (Metcalfe et al. 1997). We showed that the mast cells present in the intestine of non-diabetic rats exhibited the mucosal phenotype, as attested by alcian blue/safranin staining and were significantly reduced in alloxan-diabetic animals. These findings clearly indicated that mucosal mast cell subtype was also affected under conditions of alloxan diabetes.
We further evaluated the influence of the diabetic state upon the intestinal IL-3 expression and noted that a marked decrease in the mRNA and protein of IL-3 in diabetic animals was detected. The survival of mucosal mast cells in tissues is dependent on their direct interaction with IL-3, which is considered to play a crucial role in cell maturation (Kitamura 1989; Metcalfe et al. 1997) as well as proliferation (Lee et al. 1986; Kirshenbaum et al. 1989) and impairing apoptosis (Mekori et al. 1993). We also observed the expression of SCF, a cytokine with multiple activities which acts on several cell types including mast cells. No significant labelling was detected in the ileum sections from normal animals (data not shown), indicating that IL-3 seems to play a much more important role in the regulation of the number of mucosal mast cells. Mast cell numbers and reactivity were previously shown to be down-regulated under diabetic conditions, in a clear association with the percentage of cells developing apoptosis (Barreto et al. 2006). Thus, one possible explanation is that changes in the IL-3 content might contribute to mast cell depletion noted in diabetic rats. Decreased IL-3 in alloxan-diabetic rats might be associated with severe T-cell reduction in circulating blood (Graber et al. 1999; Otton et al. 2004), in mesenteric lymph nodes (Otton et al. 2004) and in thymus (Barreto et al. 2005). We showed that insulin treatment restored baseline levels of intestinal mast cells in diabetic rats, a phenomenon correlated with restoration of basal expression of IL-3 mRNA and protein expression. These findings were clearly supported by previous data showing that the decrease in insulin levels appeared to play a pivotal role in the reduction in pleural and peritoneal mast cell number and reactivity in diabetic rats (Diaz et al. 1996). As insulin was shown to induce cell proliferation and activation in different biological systems (Straus 1984), one can speculate that insulin may be acting in the control of proliferation and/or survival of mast cells (Lessmann et al. 2006) by means of IL-3 control.
It was previously described that low levels of serum insulin were clearly associated with hypertrophy of the adrenal glands (Nunes & Curi 1989). Some authors reported a rise in the serum corticosterone levels in parallel with an increase in adrenal-gland/body weight rate following diabetes caused by alloxan treatment in rats (Wexler & Lutmer 1975; Diaz et al. 1997). As insulin and glucocorticoids were shown to exhibit antagonistic effects, it was plausible to speculate that the balance between the systemic levels of these hormones may be important to the control of mast cell numbers and reactivity in rats. We showed that the drop in intestinal mast cell numbers noted during alloxan-diabetes was inhibited in animals treated with the steroid receptor antagonist RU 486 (Diaz et al. 2001). This was an indicative that endogenous glucocorticoids seemed to play a role in the depletion of intestinal mast cell population associated with diabetes. These findings were consistent with the previous reports that demonstrated the effect of glucocorticoids on inducing depletion of mast cells in different sites including intestine (Pipkorn et al. 1989; Goldsmith et al. 1990; Finotto et al. 1997) and also provided evidence similar to what was noted in the case of pleural mast cells (Diaz et al. 1997); there was a close-relationship between mast cell depletion and increase in the levels of endogenous glucocorticoids. It is noteworthy that the lower expression of IL-3 mRNA and protein in the intestine of diabetic rats was sensitive to RU treatment, indicating that this response was at least partially dependent on the increase in glucocorticoid hormones associated with diabetic state. These findings are supported by previous observations indicative that glucocorticoids inhibit IL-3 production by competent cells (Culpepper & Lee 1985; Powell et al. 2001).
We conclude that alloxan-diabetes induces reduction in intestinal mast cell numbers and reactivity, in clear association with depletion of local mast cell growth factor IL-3. In addition, both phenomena seem to be directly correlated with decreased levels of insulin and augmented endogenous glucocorticoids associated with diabetes.
Acknowledgments
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and PAPES 3/FIOCRUZ Scientific Programme, Brazil.
References
- Barreto EO, Riederer I, Arantes AC, et al. Thymus involution in alloxan diabetes: analysis of mast cells. Mem. Inst. Oswaldo Cruz. 2005;100(Suppl. 1):127–130. doi: 10.1590/s0074-02762005000900022. [DOI] [PubMed] [Google Scholar]
- Barreto EO, Carvalho VF, Oliveira MS, et al. High levels of filamentous actin and apoptosis correlate with mast cell refractoriness under alloxan-evoked diabetes. Life Sci. 2006;79:1194–1202. doi: 10.1016/j.lfs.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Carvalho VF, Barreto EO, Diaz BL, et al. Systemic anaphylaxis is prevented in alloxan-diabetic rats by a mechanism dependent on glucocorticoids. Eur. J. Pharmacol. 2003;472:221–227. doi: 10.1016/s0014-2999(03)01934-4. [DOI] [PubMed] [Google Scholar]
- Carvalho VF, Barreto EO, Cordeiro RS, et al. Mast cell changes in experimental diabetes: focus on attenuation of allergic events. Mem. Inst. Oswaldo Cruz. 2005;100(Suppl. 1):121–125. doi: 10.1590/s0074-02762005000900021. [DOI] [PubMed] [Google Scholar]
- Crivellato E, Beltrami CA, Mallardi F, et al. The mast cell: an active participant or an innocent bystander? Histol. Histopathol. 2004;19:259–270. doi: 10.14670/HH-19.259. [DOI] [PubMed] [Google Scholar]
- Culpepper JA, Lee F. Regulation of IL 3 expression by glucocorticoids in cloned murine T lymphocytes. J. Immunol. 1985;135:3191–3197. [PubMed] [Google Scholar]
- Diaz BL, Serra MF, Alves AC, et al. Alloxan diabetes reduces pleural mast cell numbers and the subsequent eosinophil influx induced by allergen in sensitized rats. Int. Arch. Allergy Immunol. 1996;111:36–43. doi: 10.1159/000237343. [DOI] [PubMed] [Google Scholar]
- Diaz BL, Serra MF, Barreto EO, et al. Antigen-induced pleural eosinophilia is suppressed in diabetic rats: role of corticosteroid hormones. Mem. Inst. Oswaldo Cruz. 1997;92(Suppl. 2):219–222. doi: 10.1590/s0074-02761997000800031. [DOI] [PubMed] [Google Scholar]
- Diaz B, Barreto E, Cordeiro R, et al. Enhanced serum glucocorticoid levels mediate the reduction of serosal mast cell numbers in diabetic rats. Life Sci. 2001;68:2925–2932. doi: 10.1016/s0024-3205(01)01087-6. [DOI] [PubMed] [Google Scholar]
- Finotto S, Mekori YA, Metcalfe DD. Glucocorticoids decrease tissue mast cell number by reducing the production of the c-kit ligand, stem cell factor, by resident cells: in vitro and in vivo evidence in murine systems. J. Clin. Invest. 1997;99:1721–1728. doi: 10.1172/JCI119336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Godfraind C, Louahed J, Faulkner H, et al. Intraepithelial infiltration by mast cells with both connective tissue type and mucosal-type characteristics in gut, trachea, and kidneys of IL-9 transgenic mice. J. Immunol. 1990;160:3989–3996. [PubMed] [Google Scholar]
- Goldsmith P, McGarity B, Walls AF, et al. Corticosteroid treatment reduces mast cell numbers in inflammatory bowel disease. Dig. Dis. Sci. 1990;35:1409–1413. doi: 10.1007/BF01536749. [DOI] [PubMed] [Google Scholar]
- Graber R, Farine JC, Fumagalli I, et al. Apoptosis and oxidative status in peripheral blood mononuclear cells of diabetic patients. Apoptosis. 1999;4:263–270. doi: 10.1023/a:1026408409916. [DOI] [PubMed] [Google Scholar]
- Hogan AD, Schwartz LB. Markers of mast cell degranulation. Methods. 1997;13:43–52. doi: 10.1006/meth.1997.0494. [DOI] [PubMed] [Google Scholar]
- Irani AM, Nilsson G, Miettinen U, et al. Recombinant human stem cell factor stimulates differentiation of mast cells from dispersed human fetal liver cells. Blood. 1992;80:3009–3021. [PubMed] [Google Scholar]
- Kirshenbaum AS, Goff JP, Dreskin SC, et al. IL-3-dependent growth of basophil-like cells and mastlike cells from human bone marrow. J. Immunol. 1989;142:2424–2429. [PubMed] [Google Scholar]
- Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu. Rev. Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- Lee F, Yokota T, Otsuka T, et al. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc. Natl Acad. Sci. U. S. A. 1986;83:2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann E, Grochowy G, Weingarten L, et al. Insulin and insulin-like growth factor-1 promote mast cell survival via activation of the phosphatidylinositol-3-kinase pathway. Exp. Hematol. 2006;34:1532–1541. doi: 10.1016/j.exphem.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Mekori YA, Oh CK, Metcalfe DD. IL-3-dependent murine mast cells undergo apoptosis on removal of IL-3. Prevention of apoptosis by c-kit ligand. J. Immunol. 1993;151:3775–3784. [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol. Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Nocka K, Buck J, Levi E, et al. Candidate ligand for the c-kit transmembrane kinase receptor: KL, a fibroblast derived growth factor stimulates mast cells and erythroid progenitors. EMBO J. 1990;9:3287–3294. doi: 10.1002/j.1460-2075.1990.tb07528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes MT, Curi R. Hyperthyroid-like metabolic changes induced by chronic administration of compound BW 48/80. Braz. J. Med. Biol. Res. 1989;22:417–420. [PubMed] [Google Scholar]
- de Oliveira Barreto E, de Frias Carvalho V, Diaz BL, et al. Adoptive transfer of mast cells abolishes the inflammatory refractoriness to allergen in diabetic rats. Int. Arch. Allergy Immunol. 2003;131:212–220. doi: 10.1159/000071489. [DOI] [PubMed] [Google Scholar]
- Otton R, Soriano FG, Verlengia R, et al. Diabetes induces apoptosis in lymphocytes. J. Endocrinol. 2004;182:145–156. doi: 10.1677/joe.0.1820145. [DOI] [PubMed] [Google Scholar]
- Pipkorn U, Hammarlund A, Enerback L. Prolonged treatment with topical glucocorticoids results in an inhibition of the allergen-induced weal-and-flare response and a reduction in skin mast cell numbers and histamine content. Clin. Exp. Allergy. 1989;19:19–25. doi: 10.1111/j.1365-2222.1989.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Powell N, Till SJ, Kay AB, et al. The topical glucocorticoids beclomethasone dipropionate and fluticasone propionate inhibit human T-cell allergen-induced production of IL-5, IL-3 and GM-CSF mRNA and protein. Clin. Exp. Allergy. 2001;31:69–76. [PubMed] [Google Scholar]
- Straus DS. Growth-stimulatory actions of insulin in vitro and in vivo. Endocr. Rev. 1984;5:356–369. doi: 10.1210/edrv-5-2-356. [DOI] [PubMed] [Google Scholar]
- Wexler BC, Lutmer RF. Adrenal glandular lipids and circulating corticosterone in severely diabetic rats. Br. J. Exp. Pathol. 1975;56:299–306. [PMC free article] [PubMed] [Google Scholar]