Abstract
Although beneficial roles of probiotics for inflammatory bowel diseases have been reported, their direct action on immune cells has not been elucidated. In this study, we investigated how three species of Bifidobacterium and Enterococcus faecalis differentially modulate production of cytokines from lipopolysaccharide (LPS)-stimulated macrophages in vitro using RAW264.7 cells. The mRNA levels of proinflammatory cytokines were remarkably increased after exposure to LPS, E. faecalis alone and LPS combined with E. faecalis. In contrast, IL-10 mRNA levels were significantly decreased after exposure to E. faecalis compared with exposure to Bifidobacterium species. When cells were exposed to Bifidobacterium species combined with LPS, mRNA levels of IL12p40 were decreased by co-culture with B. breve and B. longum, IL-1β mRNA levels were decreased by B. breve and B. adorescentis and TNF-α mRNA levels were decreased by B. adolescentis compared with LPS alone. The three species of Bifidobacterium significantly inhibited phosphorylation of IκB-α induced by LPS. The mRNA levels of SOCS1 and SOCS3 were increased by exposure to LPS alone; however, the mRNA levels of SOCS1 or SOCS3 were increased more by exposure to Bifidobacterium species combined with LPS. Conversely, E. faecalis combined with LPS induced significantly lower levels of SOCS mRNA than those induced by Bifidobacterium species combined with LPS. These results indicated that certain species of genus Bifidobacterium could negatively modulate mRNA levels of proinflammatory cytokines produced from LPS-stimulated RAW264.7 cells, which is possibly related to inhibition of IκB-α phosphorylation and stimulation of SOCS signalling.
Keywords: Bifidobacterium, IκB-α, LPS, macrophages, probiotics, proinflammatory cytokines, SOCS
Among several aetiologies of inflammatory bowel diseases (IBDs), including Crohn's disease (CD) and ulcerative colitis, an overly aggressive immune response to commensal intestinal flora has been suggested to be involved in triggering the onset of disease, as observed in several animal models (Dianda et al. 1997; Sellon et al. 1998). Several bacteria in commensal intestinal flora such as Enterococcus faecalis (E. faecalis) and Eschelichia coli have been shown to play a role in exacerbation of IBD both in animal models and human patients (Balish & Warner 2002). On the other hand, certain bacteria such as the genus Bifidobacterium, also residing in intestinal flora, have been reported to be effective for the improvement of IBD. Thus, these living bacteria are defined as probiotics and have been used for the attenuation of inflammation in clinical trials for IBD or in animal models (Ewaschuk & Dieleman 2006). It has been reported that anti-inflammatory mechanisms of probiotics are induced by enhanced barrier functions, production of organic acids, alteration of the intestinal flora and modulation of the mucosal immune system (Ewaschuk & Dieleman 2006), but the direct anti-inflammatory effects of probiotics on immune cells such as macrophages have not been clarified. It has been reported that murine peritoneal macrophages showed differential inflammatory mediator responses to lactobacilli, one kind of probiotics, and to pathogenic intestinal bacteria (Marcinkiewicz et al. 2007), although the intracellular mechanisms of these different responses of cytokines to the two different kinds of bacteria are largely unknown. These findings suggest that the study of macrophages is important for elucidation of the anti-inflammatory mechanisms of probiotics and the proinflammatory mechanisms of pathogenic bacteria in intestinal mucosal immunity.
Lipopolysaccharide (LPS) is a representative molecule of the outer membrane of Gram-negative bacteria. Bacterial endotoxins or LPS have been detected in the plasma of IBD patients, and abnormal microflora and/or an increased permeability of the intestinal mucosa have been suggested to be cofactors responsible for endotoxaemia (Caradonna et al. 2000). Endotoxin can produce severe disturbances of microcirculation in the gut in which activation of leucocytes and platelets and endothelial cell damage are involved, and endotoxin may therefore play a key role in intestinal tissue damage (Miura et al. 1996). LPS can activate macrophages, which release biologically active substances, and in IBD, proinflammatory cytokines and chemokines have been detected in elevated amounts in mucosal tissue and/or in peripheral blood, thus suggesting monocyte/macrophage stimulation by enteric bacteria and/or their constituents (e.g. LPS). Therefore, it is necessary to elucidate how activation of macrophages by LPS can be modified by the presence of probiotics or pathogenic bacteria. The signalling receptor for LPS is toll-like receptor (TLR)-4 (Poltorak et al. 1998), which is well regulated to avoid over-activation of the downstream transcription factor NF-κB. Several regulator molecules that negatively regulate TLR signalling have been recently identified in macrophages (Shibolet & Podolsky 2007). These inhibitory molecules are known to affect the specific site of signal transduction from the TLR receptor complex, thereby inhibiting downstream activation of transcription factors. Among them, we have focused on the suppressor of cytokine signalling (SOCS) family proteins (Baetz et al. 2004), because it has been shown that SOCS1 can directly inhibit the action of LPS on the NF-κB signalling pathway in macrophages (Kinjyo et al. 2002; Mansell et al. 2006), and that SOCS1 plays an important role in the prevention of murine colitis by restricting cytokine signals (Chinen et al. 2006).
The aim of this study was therefore to determine whether three species of the genus Bifidobacterium (B. breve, B. longum and B. adolescentis) and commensal bacteria, E. faecalis, can differentially modulate the function of LPS-stimulated macrophages in vitro using a macrophage cell line, RAW264.7, particularly focusing on the induction of proinflammatory cytokines and molecules that negatively regulate production of these cytokines downstream of LPS receptor, IκB and SOCS1 and 3.
Materials and methods
Bacterial strains, cell line and culture conditions
Three Bifidobacterium species, B. breve (ATCC 15700), B. longum (ATCC 15697) and B. adolescentis (ATCC 15703), and E. faecalis (ATCC19433) were used in this study. The three strains of bifidobacteria were cultured for 16 h at 37 °C in GAM broth (Nissui, Tokyo, Japan) supplemented with 0.1%l-cysteine and 0.1% ascorbic acid under anaerobic conditions. E. faecalis was cultured for 16 h at 37 °C in Enterococcosel broth (BD Japan, Tokyo, Japan) under aerobic conditions.
The mouse macrophage cell line RAW 264.7 was maintained in Dulbecco's modified Eagle's medium (DMEM, Sigma, St Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Sigma) and 4 mM l-glutamine at 37 °C in 5% CO2. For experiments, RAW264.7 cells were seeded at 1x106/ml in a six-well format culture plate and cultured for 24 h, and then the cells were stimulated with LPS (1 μg/ml, serotype B55:O5; E. coli, Sigma) for 16 h. In some experiments, the cells were exposed to each species of living bifidobacteria or E. faecalis with or without LPS for 16 h. All bacteria were resuspended in 0.85% saline, and the concentrations for 3x108 bacterial cells/ml (300 bacteria per RAW264.7 cell) were checked based on the McFarland curve (measured by both turbidity and optical density) before experiments.
Quantitative real-time PCR
Total RNA was extracted from RAW264.7 cells using an RNeasy Mini kit (Qiagen Hidlen, Germany) and used for reverse transcription using a QunatiTect Reverse Transcription kit (Qiagen). Taqman PCR reactions were performed on complementary DNA (cDNA) samples using Taqman Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and an ABI 7900HT Fast Real-Time PCR System. Taqman probes and primers for TNF-α, IL-1β, IL-12p40, IL-10, SOCS1, SOCS3 and 18SrRNA have been developed as TaqMan(R) Gene Expression Assays by Applied Biosystems. Target mRNA was determined using the comparative cycle threshold method of relative quantification. The calibrator sample was isolated from untreated RAW264.7 cells with 18SrRNA used as an internal control. All samples were assayed in duplicate.
Western blot analysis
RAW264.7 cell extracts were applied to a 10% SDS–polyacrylamide gel. Following electrophoresis, the proteins were transferred onto a nitrocellulose membrane. After blocking with 3% bovine serum albumin (Sigma) in Tris-buffered saline (20 mmol/l Tris, pH 7.5, 150 mmol/l NaCl) containing 0.1% Tween 20 (T-TBS) for 1 h, nitrocellulose membranes were incubated with primary antibodies (all diluted 1:1000), anti-phospho-IkB-α (Cell Signaling Technology, Beverly, MA, USA) and anti-actin (Santa Cruz Biotechnology, Inc. Santa Cruz, CA, USA), for 16 h at 4°C and then washed in T-TBS. The secondary antibody, horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG, was incubated with the membranes at a dilution of 1:2000 in T-TBS. After washing, the antibody complexes were detected by using an Amersham ECL plus system (GE Healthcare Bio-Sciences KK, Tokyo, Japan). The detected bands were quantified using LAS-3000 (FUJI Film, Kanagawa, Japan).
Statistical analysis
The parametric data were statistically analysed by Student's t-test, and the non-parametric data were statistically analysed by Mann–Whitney U-test. All results are expressed as mean ± SEM from 10 experiments. A significant difference was defined as P < 0.05.
Results
mRNA expression of proinflammatory and anti-inflammatory cytokines in RAW264.7 cells
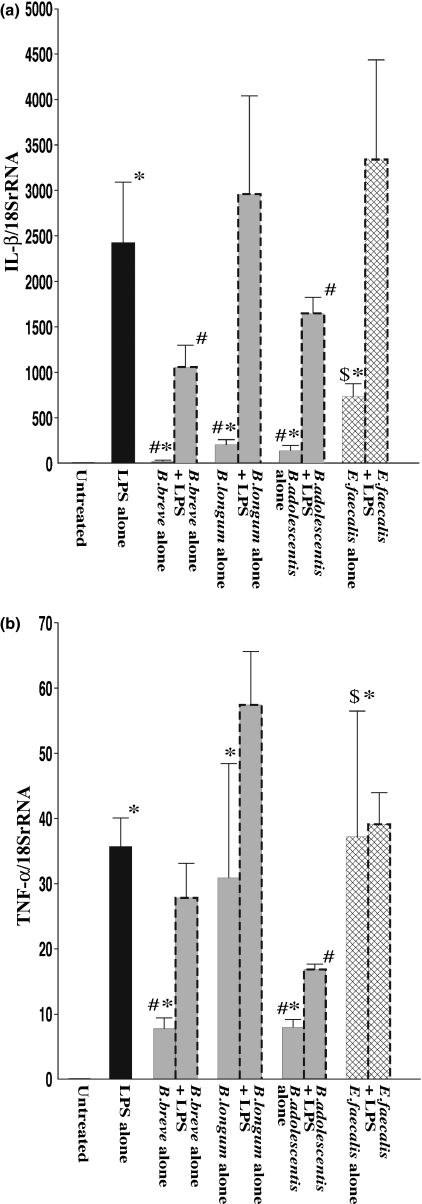
The effects of three Bifidobacterium species (B. breve, B. longum and B. adolescentis) and E. faecalis on mRNA expression of various proinflammatory cytokines in RAW264.7 cells were determined by quantitative real-time PCR under unstimulated and LPS-stimulated conditions. As shown in Figure 1a, mRNA level of IL-1β was significantly increased in LPS-treated RAW264.7 cells (LPS alone) compared with the level in untreated controls. In the case of exposure to each species of bifidobacteria alone (without LPS), IL-1β mRNA was also induced, but the degree of induction was much smaller than that in the case of exposure to LPS alone. Exposure to E. faecalis also increased IL-1βmRNA to a level significantly higher than the levels in the case of exposure to each species of bifidobacteria alone. When macrophages were exposed to each of the Bifidobacterium species combined with LPS, these species except for B. longum significantly attenuated the increase in mRNA level of IL-1β compared with that in the case of exposure to LPS alone. However, in case of exposure to E. faecalis combined with LPS, there was an increase in IL-1β expression level compared with that in the case of exposure to LPS alone, but the difference was not statistically significant.
Figure 1.
mRNA expression of inflammatory cytokines in RAW264.7 cells after exposure to LPS and effects of Bifidobacterium species and Enterococcus faecalis. RAW264.7 cells were exposed to each species of living bacterial cells in condition medium with or without LPS (1 μg/ml) for 16 h. IL-1β (a) and TNF-α (b) mRNA levels were determined by quantitative real-time PCR. The columns represent the average ratio of cytokines and 18SrRNA from 10 experiments. Results are expressed as mean ± SEM. *P < 0.05 vs. negative control (untreated RAW264.7 cells); #P < 0.05 vs. LPS alone; $P < 0.05 vs. exposure to each of the bifidobacteria alone.
There was also a significant increase in the TNF-α mRNA level in RAW264.7 cells after LPS treatment (LPS alone) compared with the level in untreated controls (Figure 1b). Examination of the effects of exposure to each of the Bifidobacterium species and E. faecalis showed that all these bacteria increased TNF-α mRNA levels in RAW264.7 cells by direct contact. However, the responses were different: in the case of exposure to B. breve and B. adolescentis, mRNA levels of TNF-α were significantly lower than that in the case of exposure to LPS alone, while in case of exposure to B. longum and E. faecalis, the levels were similar to the level in the case of exposure to LPS alone. When macrophages were exposed to Bifidobacterium species combined with LPS, B. adolescentis significantly attenuated the increase in TNF-α mRNA level compared with that in the case of exposure to LPS alone. However, the mRNA level of TNF-α was not significantly affected by exposure to B. breve, B. longum or E. faecalis combined with LPS compared with the level in the case of exposure to LPS alone.
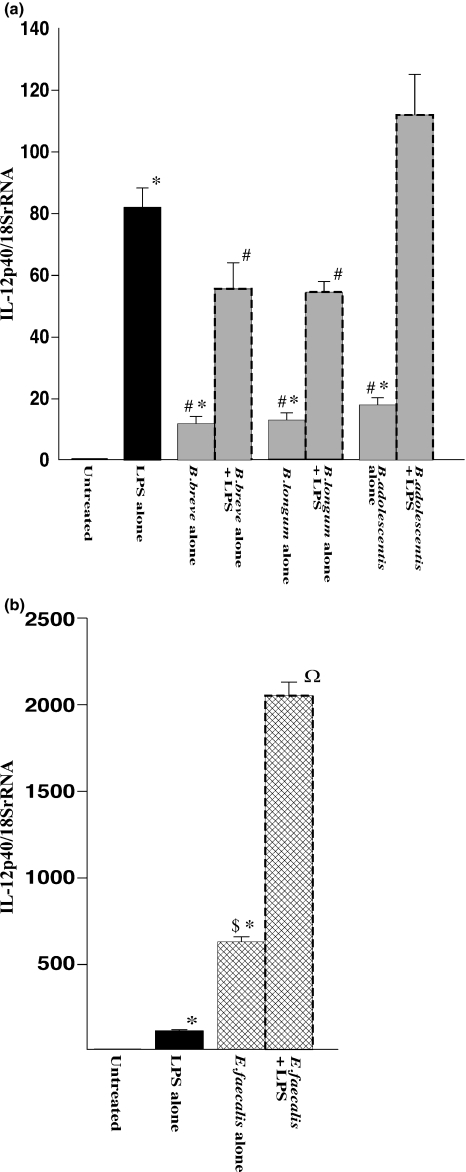
Figure 2 shows mRNA expression levels of IL-12p40 in RAW264.7 cells determined by real-time PCR. IL-12p40 levels were significantly increased in LPS-treated macrophages compared with the levels in untreated controls. Small but significant increases were also observed in macrophages exposed to each of the Bifidobacterium species alone, although the expression levels were significantly lower than the level in macrophages exposed to LPS alone (Figure 2a). In groups of macrophages exposed to each of the three species of bifidobacteria combined with LPS, the LPS-induced increase in mRNA level of IL12p40 was significantly decreased by B. breve and B. longum, but not by B. adolescentis, compared with the level in macrophages exposed to LPS alone. On the other hand, it should be noted that exposure to E. faecalis alone increased the mRNA level of IL-12p40 by more than 25-fold compared with the levels in macrophages exposed to each of the three species of bifidobacteria (Figure 2b). Moreover, exposure to E. faecalis in the presence of LPS increased the mRNA level of IL-12p40 by 15- to 30-fold compared with the levels in macrophages exposed to each of the three species of bifidobacteria under the same LPS-stimulated conditions. These observations indicated that there was a drastic up-regulation of IL-12p40 mRNA levels in RAW264.7 cells after exposure to either E. faecalis alone or E. faecalis combined with LPS, in clear contrast to the exposure to the species of Bifidobacterium, which resulted in reduction in IL-12p40 mRNA levels.
Figure 2.
mRNA expression of IL-12p40 in RAW264.7 cells after exposure to LPS and effects of Bifidobacterium species (a) and Enterococcus faecalis (b). RAW264.7 cells were exposed to each species of living bacterial cells in condition medium with or without LPS (1 μg/ml) for 16 h. The mRNA levels were determined by quantitative real-time PCR. The columns represent the average ratio of cytokines and 18SrRNA from 10 experiments. Results are expressed as mean ± SEM. *P < 0.05 vs. negative control (untreated RAW264.7 cells); #P < 0.05 vs. LPS alone; $P < 0.05 vs. exposure to each of the bifidobacteria alone; ΩP < 0.05 vs. exposure to each of the bifidobacteria combined with LPS.
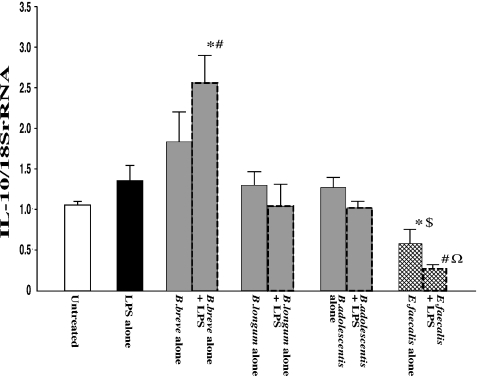
The effects of the three Bifidobacterium species (B. breve, B. longum and B. adolescentis) and E. faecalis on mRNA expression of IL-10, a major anti-inflammatory cytokine, in RAW 264.7 cells were determined under unstimulated and LPS-stimulated conditions (Figure 3). IL-10 mRNA levels were slightly increased in the case of exposure to each of the bifidobacteria species alone and exposure to LPS alone, but the degrees of increase were not significantly different to that in controls. When macrophages were exposed to each of the Bifidobacterium species combined with LPS, B. breve increased IL-10 mRNA to a significantly higher level than that in untreated controls or that in the case of exposure to LPS alone. However, mRNA level of IL-10 was not significantly affected by exposure to either B. longum or B. adolescentis combined with LPS. Interestingly, in the case of exposure to E. faecalis, IL-10 mRNA level was significantly lower than that in untreated controls or that in the case of exposure to each of the Bifidobacterium species alone. Moreover, in the case of exposure to E. faecalis combined with LPS, IL-10 mRNA level was significantly decreased compared with that in the case of exposure to LPS alone or each of the Bifidobacterium species combined with LPS.
Figure 3.
mRNA expression of IL-10 in RAW264.7 cells after exposure to LPS and effects of Bifidobacterium species and Enterococcus faecalis. RAW264.7 cells were exposed to each species of living bacterial cells in condition medium with or without LPS (1 μg/ml) for 16 h. mRNA levels were determined by quantitative real-time PCR. The columns represent the average ratio of cytokines and 18SrRNA from 10 experiments. Results are expressed as mean ± SEM. *P < 0.05 vs. negative control (untreated RAW264.7 cells); #P < 0.05 vs. LPS alone; $P < 0.05 vs. exposure to each of the bifidobacteria alone; ΩP < 0.05 vs. exposure to each of the bifidobacteria combined with LPS.
Changes in phosphorylation of IκB-α in RAW264.7 cells
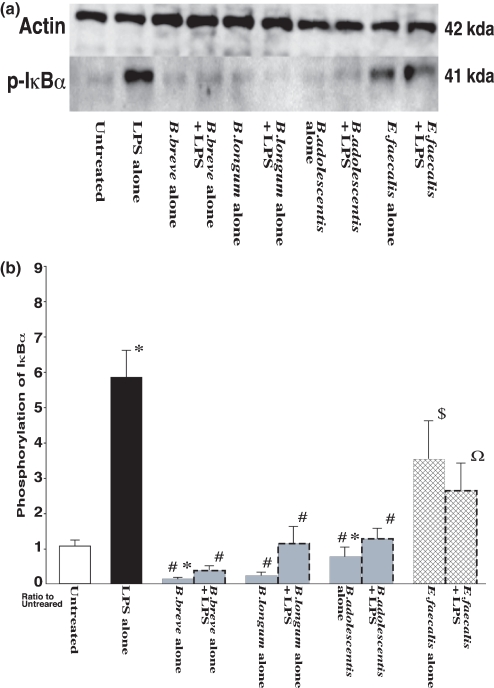
The effects of exposure to the three species of Bifidobacterium (B. breve, B. longum and B. adolescentis) and E. faecalis on phosphorylation of IκB-α in RAW264.7 cells are shown in Figure 4. The phosphorylation level of IκB-α was significantly increased by exposure to LPS compared with that in the negative controls. In contrast, in the case of exposure to B. breve and B. longum alone, phosphorylation of IκB-α was significantly suppressed compared with that in untreated controls. Moreover, exposure to each of the three species of bifidobacteria in combination with LPS significantly suppressed IκB-α phosphorylation compared with that in the case of exposure to LPS alone. On the other hand, the phosphorylation level of IκB-α induced by exposure to E. faecalis alone was significantly higher than that in the negative controls or that in the case of exposure to bifidobacteria alone, and E. faecalis combined with LPS induced a significantly stronger response than that in the case of exposure to bifidobacteria with LPS.
Figure 4.
(a) Phosphorylation of IκB-α in RAW264.7 cells after exposure to LPS and effects of Bifidobacterium species and Enterococcus faecalis. RAW264.7 cells were exposed to each species of living bacterial cells in condition medium with or without LPS (1 μg/ml) for 16 h. Proteins (50 μg/lane) of total cell lysates were separated by SDS–PAGE, transferred to a nitrocellulose membrane, and then incubated with specific antibodies against actin as confirmation of equal loading of crude protein levels (upper blot) and phospho-IκBa (lower blot). (b) The detected bands were quantified using LAS-3000. The IκB-α phosphorylation level in the negative control (untreated RAW264.7 cells) was assigned as 1.0, and results are expressed as mean ± SEM (n = 10). *P < 0.05 vs. negative control (untreated RAW264.7 cells); #P < 0.05 vs. LPS alone; $P < 0.05 vs. exposure to each of the bifidobacteria alone; ΩP < 0.05 vs. exposure to each of the bifidobacteria combined with LPS.
Expression of SOCS family mRNA in RAW264.7 cells
Figure 5 shows changes in mRNA levels of SOCS1 and SOCS3 in RAW264.7 cells after exposure to the three species of Bifidobacterium (B. breve, B. longum and B. adolescentis) and E. faecalis. Treatment with LPS significantly increased mRNA levels of SOCS1 and SOCS3 compared with those in negative controls. Exposure to each of the three species of bifidobacteria alone also induced SOCS1 and SOCS3 mRNA expression, but the induction levels were lower than that in the case of exposure to LPS alone. However, in the case of exposure to B. longum and B. adolescentis combined with LPS, induction of mRNA was significantly stronger than that in the case of exposure to LPS alone. Again, a clear difference was found between the effects of Bifidobacterium species and E. faecalis. Interestingly, exposure to E. faecalis alone resulted in increases in SOCS1 mRNA and SOCS3 mRNA to levels equivalent to those in the case of exposure to each of the three species of bifidobacteria alone, but in the case of E. faecalis combined with LPS, the increases in mRNA levels of SOCS1 and SOCS3 were remarkably inhibited compared with those in the case of exposure to LPS alone or exposure to each of the three species of bifidobacteria combined with LPS.
Figure 5.
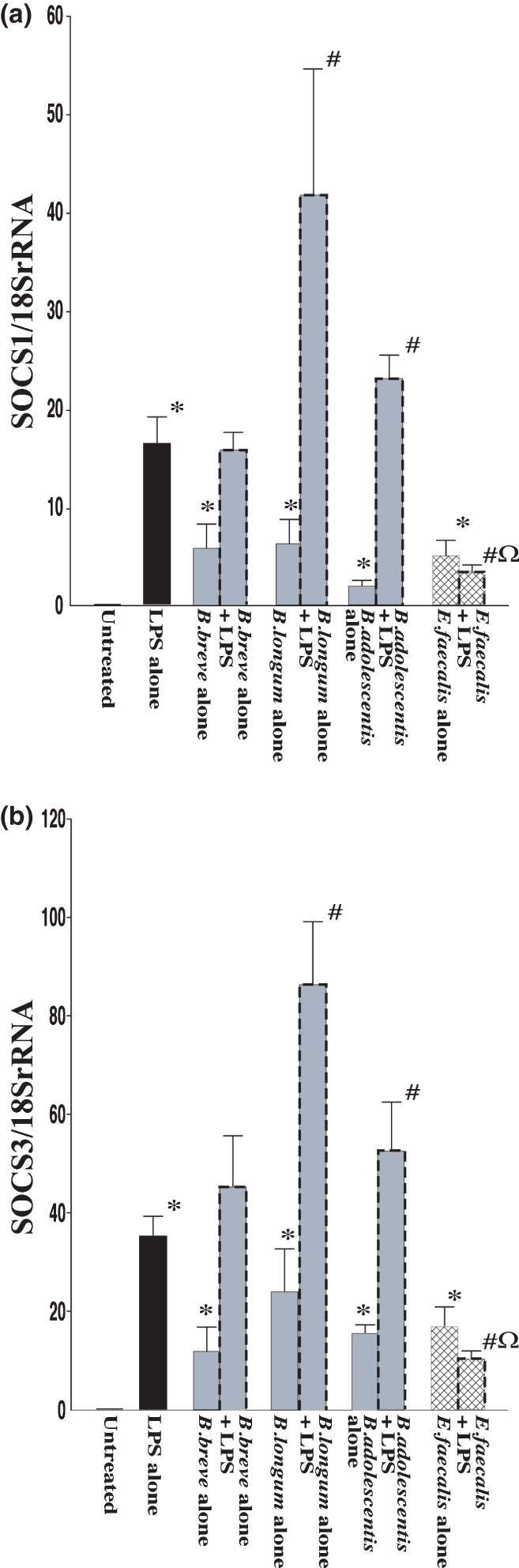
mRNA expression of SOCS family in RAW264.7 cells after exposure to LPS and effects of Bifidobacterium species and Enterococcus faecalis. RAW264.7 cells were exposed to each species of living bacterial cells in condition medium with or without LPS (1 μg/ml) for 16 h. SOCS1 (a) and SOCS3 (b) mRNA levels were determined by quantitative real-time PCR. The columns represent the average ratio of cytokines and 18SrRNA from 10 experiments. Results are expressed as mean ± SEM. *P < 0.05 vs. negative control (untreated RAW264.7 cells); #P < 0.05 vs. LPS alone; $P < 0.05 vs. exposure to each of the bifidobacteria alone; ΩP < 0.05 vs. exposure to each of the bifidobacteria combined with LPS.
Discussion
Macrophages, a main player in innate immunity not only interact with intestinal bacteria but also have the ability to produce several proinflammatory cytokines, which lead to Th1 polarization and tissue damage in intestinal inflammation of IBD (Parronchi et al. 1997). Among them, IL-1β and TNF-α are up-regulated in inflamed intestinal tissue of IBD, and macrophages are their main producing cells (Murch et al. 1993). Therefore, we initially quantified the effect of direct contact of bacteria with macrophages on mRNA expression of IL-1β and TNF-α (Figure 1). Our findings indicate that each of the three species of bifidobacteria, and E. faecalis have the ability to directly influence macrophage function and consequently increase mRNA levels of IL-1β and TNF-α. These findings are in accordance with the results of a study by Marcinkiewicz et al. showing a differential pattern of TNF-α induction from murine macrophages in the case of exposure to killed lactobacillus strains and in the case of exposure to E. faecalis (Marcinkiewicz et al. 2007). These findings also suggest that although bacteria defined as ‘probiotics’, such as species of the genus Bifidobacterium and the genus Lactobacillus, can weakly activate macrophages under physiological conditions, potentially pathogenic bacteria such as E. faecalis have a stronger ability to enhance proinflammatory cytokine production than that of ‘probiotics’. Next, we investigated whether the genus Bifidobacterium has an anti-inflammatory effect on macrophages under the condition of inflammation. To mimic inflammatory conditions, we further determined the effects of the three species of bifidobacteria on the production of cytokines from RAW264.7 cells in the presence of LPS. Our results suggested that some Bifidobacterium species could negatively modulate production of IL-1β or TNF-α induced by activated macrophages under inflammatory conditions but that potentially pathogenic bacteria such as E. faecalis could exacerbate the inflammation via augmentation of the production of these proinflammatory cytokines.
It has been reported that the expression level of IL-12 was increased in inflamed intestinal tissue (Monteleone et al. 1997; Parronchi et al. 1997) and that anti-IL-12 treatment effectively ameliorated intestinal inflammation in several animal models of colitis (Davidson et al. 1998). Thus, we investigated mRNA expression levels of IL-12p40 in RAW264.7 cells under unstimulated and LPS-stimulated conditions. Interestingly, significant different responses in IL-12p40 mRNA expression were observed after exposure to the three Bifidobacterium species and E. faecalis. That is, the increase in IL-12p40 expression induced by LPS was significantly inhibited by exposure to bifidobacteria, whereas IL-12p40 expression was drastically further up-regulated after exposure to E. faecalis in both unstimulated and LPS-stimulated conditions. It is well known that IL-12 may be a key player in sustaining chronic inflammatory disorders of CD characterized by favouring the differentiation of IFN-γ-secreting CD4+ Th1 cells (Trinchieri 2003). In addition, the number of infiltrating CD4+ lymphocytes in inflamed tissue has been shown to be strongly correlated with histological damage score in experimental colitis models (Kato et al. 2000). We speculate that the mechanism underlying the anti-inflammatory effects of bifidobacteria in vivo is not only the down-regulation of IL-1β and TNF-α released from macrophages but also possibly the reduction in differentiation and infiltration of Th1 cells because of the decreased release of IL-12 from activated macrophages. Furthermore, although there was no significant additive effect on induction of mRNA expression of IL-1β and TNF-α by E. faecalis when combined with LPS, mRNA expression level of IL-12p40 induced by E. faecalis with LPS was more than 25-fold higher than that in the case of exposure to LPS alone (Figure 2b). This drastic enhancement suggests that the production of IL-12 from macrophages stimulated by potentially pathogenic bacteria, including E. faecalis, plays a central role in exacerbation of intestinal inflammation in IBD.
Moreover, we quantified the effect of direct contact of bacteria with macrophages on mRNA expression of IL-10 (Figure 3). It is well known that IL-10 is an important regulatory cytokine for suppressing immune responses by blocking the action of many proinflammatory cytokines, including IL-1β, TNF-α and IL-12, produced by monocytes and macrophages (Bogdan et al. 1991; de Waal Malefyt et al. 1991). In addition, certain Bifidobacterium species enhance IL-10 production in peripheral blood mononuclear cells or a macrophage-like cell line, thus suggesting that the genus Bifidobacterium has anti-inflammatory effects against IBD (He et al. 2002; Imaoka et al. 2008). Our results indicated that each of the three species of bifidobacteria has the ability to increase the mRNA level of IL-10 compared with the level in untreated controls, while IL-10 mRNA was significantly down-regulated by exposure to E. faecalis in both unstimulated and LPS-stimulated conditions. These findings suggest that the intestinal regulatory function is maintained physiologically by IL-10 secreted by bacteria defined as ‘probiotics’ including Bifidobacterium species. In conditions mimicking inflammation, among the three Bifidobacterium species, only B. breve combined with LPS significantly increased IL-10 mRNA level compared with that in the case of exposure to LPS alone. Hoarau et al. reported that B. breve induced prolonged survival of dendritic cells (DCs) with high IL-10 and low IL-12 production levels compared with the effects of LPS on DCs (Hoarau et al. 2006). These findings indicate the possibility that B. breve has a specific anti-inflammatory effect by directly influencing antigen-presenting cells (APCs) such as macrophages or DCs and consequently increasing mRNA levels of IL-10.
We also analysed upstream signal transduction of cytokine production. IκB-α phosphorylation levels were determined using Western blot analysis, because gene expression of proinflammatory cytokines, including IL-1β, TNF-α and IL-12, is regulated by release of NF-κB dimers from the cytoplasmic NF-κB-IκB complex caused by the phosphorylation of IκB-α (Ghosh & Karin 2002). As shown in Figure 4, phosphorylation level of IκB-α was significantly increased by exposure to LPS. In contrast, exposure to each of the three species of bifidobacteria alone or in combination with LPS significantly suppressed IκB-α phosphorylation. On the other hand, the phosphorylation level of IκB-α induced by exposure to E. faecalis was significantly higher than that induced by exposure to each species of bifidobacteria, in either the unstimulated or LPS-stimulated condition. These results suggest that components of the genus Bifidobacterium directly influence macrophages, thus attenuating NF-κB-dependent induction of proinflammatory cytokine production by preventing phosphorylation of the NF-κB inhibitory subunit IκB-α.
We also investigated whether the genus Bifidobacterium modifies the expression of negative regulators for proinflammarory cytokine production. Although it is commonly accepted that many of the negative regulators of toll-like receptor-mediated immune responses are induced by components of bacteria in a negative feedback manner (Liew et al. 2005), we focused on changes in SOCS family expression, as SOCS1 mRNA and SOCS3 mRNA have been shown to be induced by LPS or CpG-DNA stimulation in macrophages (Stoiber et al. 1999; Crespo et al. 2000; Dalpke et al. 2001). SOCS proteins constitute a class of negative regulators for Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathways (Baetz et al. 2004). It has been reported that there were abnormally high levels of the proinflammatory cytokines IFN-γ, TNF-α and IL-12 and activated circulating T cells in mice with SOCS1 deficiency (Chong et al. 2005). Recently, Chinen et al. also demonstrated that much more severe colitis developed in SOCS1/T-cell receptor alpha (TCRα) double knockout mice (DKO) than in TCRα-KO mice and that signal transducer and activator of transcription 1, NF-κB and their target genes were hyperactivated in infiltrated mononuclear cells and colonic epithelial cells in DKO mice, indicating that SOCS1 plays an important role in prevention of murine colitis by restricting the cytokine signals (Chinen et al. 2006). Our results showed that treatment with LPS can induce mRNA of SOCS1 and SOCS3 in macrophages as a kind of protective mechanism. Exposure to bifidobacteria, especially B. longum and B. adolescentis, resulted in further induction of these mRNAs when combined with LPS. In the case of exposure to E. faecalis combined with LPS, however, expression of these mRNAs was remarkably inhibited compared with that in the case of exposure to LPS alone or exposure to each of the three species of bifidobacteria combined with LPS. These findings suggest that under an inflammatory condition in the gut such as the active inflammatory stage of IBD, exposure to bifidobacteria leads to increases in SOCS1 and SOCS3 expression levels at the same time in a negative feedback manner and consequently to suppression of secretion of proinflammatory cytokines from macrophages. On the other hand, indigenous but potentially pathogenic bacteria, including E. faecalis, might enhance the production of proinflammatory cytokines under an inflammatory condition because of the lower expression levels of SOCS1 and SOCS3. Furthermore, as Ding et al. showed that SOCS1 and SOCS3 are induced by IL-10 (Ding et al. 2003), and the lower level of IL-10 mRNA expression in the case of exposure to E. faecalis may further impair the induction of SOCS1 and SOCS3. However, our results indicated that IL-10 mRNA expression pattern in the case of exposure to B. longum and B. adolescentis combined with LPS did not fully correlate to the patterns of SOCS1 and SOCS3 mRNA expression (Figures 3 and 5). These findings suggest that SOCS1 and SOCS3 mRNA expression in the case of B. longum and B. adolescentis combined with LPS is not only regulated by IL-10 alone but also induced by other feedback mechanisms stimulated by bifidobacterial components.
Both the genus Bifidobacterium and E. faecalis are Gram-positive bacteria, and components of these bacteria are therefore similar (Beveridge 1981). From the results of this study, we cannot determine the exact reason why RAW264.7 cells are able to recognize Bifidobacterium species as ‘probiotics’ and respond differently from their response to potentially pathogenic bacteria. Our results also indicated that although the three species of bifidobacteria (B. breve, B. longum and B. adolescentis) may have similar cellular components, there was a significant heterogeneity of response in the induction of mRNA of IL-1β, TNF-α, IL-12p40, IL-10 and SOCS1&3 in RAW264.7 cells among these species (Figures 1, 2, 3 and 5). In this regard, Iwabuchi et al. obtained similar results showing that although most of the bifidobacteria strains induced only low levels of IL-12p70 in murine splenic cells compared with levels induced by lactobacilli and streprococci, three species of bifidobacteria (B. breve, B. longum and B. adolescentis) induced different quantities of IL-12p70 (Iwabuchi et al. 2007). Hence, further study is necessary to elucidate which molecular mechanisms are responsible for the modification of production of proinflammatory cytokines and signal transduction in macrophages when macrophages encounter various kinds of pathogenic and non-pathogenic bacteria. In summary, the genus Bifidobacterium could negatively modulate production of mRNA of proinflammatory cytokines in LPS-stimulated RAW264.7 cells. In this condition, increased mRNA levels of SOCS1 and SOCS3 and decreased phosphorylation of IκB protein were observed, and these might be related to the suppression of production of inflammatory cytokines such as IL-12. These characteristic changes induced in macrophages by the genus Bifidobacterium are significantly different from those induced by E. faecalis, and these functional changes may greatly contribute to the anti-inflammatory effect of the genus Bifidobacterium, which can play a beneficial role as probiotics and may be a possible therapeutic tool in intestinal inflammation.
Acknowledgments
This research was supported by grants from the National Defense Medical College and Food Science Institute Foundation, Japan.
Conflict of interest
None declared.
References
- Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J. Biol. Chem. 2004;279:54708–54715. doi: 10.1074/jbc.M410992200. [DOI] [PubMed] [Google Scholar]
- Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am. J. Pathol. 2002;160:2253–2257. doi: 10.1016/S0002-9440(10)61172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ. Ultrastructure, chemistry, and function of the bacterial wall. Int. Rev. Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J. Exp. Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caradonna L, Amati L, Magrone T, Pellegrino NM, Jirillo E, Caccavo D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J. Endotoxin Res. 2000;6:205–214. [PubMed] [Google Scholar]
- Chinen T, Kobayashi T, Ogata H, et al. Suppressor of cytokine signaling-1 regulates inflammatory bowel disease in which both IFNgamma and IL-4 are involved. Gastroenterology. 2006;130:373–388. doi: 10.1053/j.gastro.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Chong MM, Metcalf D, Jamieson E, Alexander WS, Kay TW. Suppressor of cytokine signaling-1 in T cells and macrophages is critical for preventing lethal inflammation. Blood. 2005;106:1668–1675. doi: 10.1182/blood-2004-08-3049. [DOI] [PubMed] [Google Scholar]
- Crespo A, Filla MB, Russell SW, Murphy WJ. Indirect induction of suppressor of cytokine signalling-1 in macrophages stimulated with bacterial lipopolysaccharide: partial role of autocrine/paracrine interferon-alpha/beta. Biochem. J. 2000;349:99–104. doi: 10.1042/0264-6021:3490099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpke AH, Opper S, Zimmermann S, Heeg K. Suppressors of cytokine signaling (SOCS)-1 and SOCS-3 are induced by CpG-DNA and modulate cytokine responses in APCs. J. Immunol. 2001;166:7082–7089. doi: 10.4049/jimmunol.166.12.7082. [DOI] [PubMed] [Google Scholar]
- Davidson NJ, Hudak SA, Lesley RE, Menon S, Leach MW, Rennick DM. IL-12, but not IFN-gamma, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J. Immunol. 1998;161:3143–3149. [PubMed] [Google Scholar]
- Dianda L, Hanby AM, Wright NA, Sebesteny A, Hayday AC, Owen MJ. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am. J. Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Chen D, Tarcsafalvi A, Su R, Qin L, Bromberg JS. Suppressor of cytokine signaling 1 inhibits IL-10-mediated immune responses. J. Immunol. 2003;170:1383–1391. doi: 10.4049/jimmunol.170.3.1383. [DOI] [PubMed] [Google Scholar]
- Ewaschuk JB, Dieleman LA. Probiotics and prebiotics in chronic inflammatory bowel diseases. World J Gastroenterol. 2006;12:5941–5950. doi: 10.3748/wjg.v12.i37.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl.):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- He F, Morita H, Ouwehand AC, et al. Stimulation of the secretion of pro-inflammatory cytokines by Bifidobacterium strains. Microbiol. Immunol. 2002;46:781–785. doi: 10.1111/j.1348-0421.2002.tb02765.x. [DOI] [PubMed] [Google Scholar]
- Hoarau C, Lagaraine C, Martin L, Velge-Roussel F, Lebranchu Y. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J. Allergy Clin. Immunol. 2006;117:696–702. doi: 10.1016/j.jaci.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Imaoka A, Shima T, Kato K, et al. Anti-inflammatory activity of probiotic Bifidobacterium: Enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J Gastroenterol. 2008;14:2511–2516. doi: 10.3748/wjg.14.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi N, Takahashi N, Xiao JZ, Miyaji K, Iwatsuki K. In vitro Th1 cytokine-independent Th2 suppressive effects of bifidobacteria. Microbiol. Immunol. 2007;51:649–660. doi: 10.1111/j.1348-0421.2007.tb03953.x. [DOI] [PubMed] [Google Scholar]
- Kato S, Hokari R, Matsuzaki K, et al. Amelioration of murine experimental colitis by inhibition of mucosal addressin cell adhesion molecule-1. J. Pharmacol. Exp. Ther. 2000;295:183–189. [PubMed] [Google Scholar]
- Kinjyo I, Hanada T, Inagaki-Ohara K, et al. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity. 2002;17:583–591. doi: 10.1016/s1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Mansell A, Smith R, Doyle SL, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz J, Ciszek M, Bobek M, et al. Differential inflammatory mediator response in vitro from murine macrophages to lactobacilli and pathogenic intestinal bacteria. Int. J. Exp. Pathol. 2007;88:155–164. doi: 10.1111/j.1365-2613.2007.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Tsuzuki Y, Kurose I, et al. Endotoxin stimulates lymphocyte-endothelial interactions in rat intestinal Peyer's patches and villus mucosa. Am. J. Physiol. 1996;271:G282–G292. doi: 10.1152/ajpgi.1996.271.2.G282. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Biancone L, Marasco R, et al. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parronchi P, Romagnani P, Annunziato F, et al. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am. J. Pathol. 1997;150:823–832. [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibolet O, Podolsky DK. TLRs in the Gut. IV. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1469–G1473. doi: 10.1152/ajpgi.00531.2006. [DOI] [PubMed] [Google Scholar]
- Stoiber D, Kovarik P, Cohney S, Johnston JA, Steinlein P, Decker T. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-gamma. J. Immunol. 1999;163:2640–2647. [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]