Abstract
During B cell development, rearrangement and expression of Ig heavy chain (HC) genes promote development and expansion of pre-B cells accompanied by the onset of Ig light chain (LC) variable region gene assembly. To elucidate the signaling pathways that control these events, we have tested the ability of activated Ras expression to promote B cell differentiation to the stage of LC gene rearrangement in the absence of Ig HC gene expression. For this purpose, we introduced an activated Ras expression construct into JH-deleted embryonic stem cells that lack the ability to assemble HC variable region genes and assayed differentiation potential by recombination activating gene (RAG) 2-deficient blastocyst complementation. We found that activated Ras expression induces the progression of B lineage cells beyond the developmental checkpoint ordinarily controlled by μ HC. Such Ras/JH-deleted B cells accumulate in the periphery but continue to express markers associated with precursor B cells including RAG gene products. These peripheral Ras/JH-deleted B cell populations show extensive Ig LC gene rearrangement but maintain an extent of κ LC gene rearrangement and a preference for κ over λ LC gene rearrangement similar to that of wild-type B cells. We discuss these findings in the context of potential mechanisms that may regulate Ig LC gene rearrangement.
B lymphopoiesis progresses through a developmental program that links the ordered rearrangement of Ig gene segments with cellular expansion and differentiation events (1). During this program, B220+ CD43+ pro-B cells undergo rearrangement of Ig heavy chain (HC) D to JH gene segments, followed by VH to DJH rearrangement. The expression of μ HC proteins encoded by a functionally rearranged HC gene represents an important developmental checkpoint during B lymphopoiesis, in which μ HC proteins complex with the surrogate light chain (LC) proteins (VpreB and λ5) to form the pre-B cell receptor (pre-BCR) (2). The expression of the pre-BCR activates a number of processes, including differentiation of pro-B cells to the B220+ CD43− pre-B stage at which most Ig LC variable region gene assembly occurs. Although some LC gene rearrangements can occur in pro-B cells before expression of Ig HC (3, 4), it is clear that expression of a productively rearranged μ HC gene in some way leads to the transcriptional activation and rearrangement of the Ig LC loci in pre-B cells (5–9). Expression of an Ig LC protein that can functionally pair with the pre-existing HC leads to BCR expression and differentiation of pre-B cells to immature B lymphocytes, which may then move to the periphery.
The role of HC in promoting pro-B cell differentiation and Ig LC gene rearrangement has been studied by complementation of mutant mice with HC transgenes. Expression of a rearranged μ HC transgene in the recombination activating gene 1 (RAG-1)- or RAG-2-deficient (RAG-1Δ or RAG-2Δ) backgrounds, in which B cell development is arrested at the pro-B cell stage because of the inability to initiate V(D)J recombination, resulted in progression of RAGΔ pro-B cells to the pre-B cell stage whereas LC gene rearrangement remained blocked due to the V(D)J recombination defect (10, 11). However, expression of a rearranged μ HC transgene in the JH-deleted (JHΔ) background, which also arrests B cell development at the pro-B cell stage, resulted in peripheral BCR+ B cell populations with diverse, endogenous Ig LC gene rearrangements (12). Although these studies clearly demonstrated that the μ HC can signal the differentiation events associated with the pro-B to pre-B transition, the nature of the downstream effector pathways remains to be elucidated.
During normal B cell differentiation, κ LC gene rearrangement normally precedes that of λ LC genes. Thus, in normal B lineage cells, λ LC gene rearrangement usually does not occur unless both κ genes are aberrantly rearranged. Correspondingly, κ gene rearrangements occur in nearly all peripheral B lineage cells, whereas λ gene rearrangements occur in only ≈5% (13). The preferential rearrangement of κ vs. λ LC genes has been argued to be due to either an ordered sequence of κ vs. λ gene rearrangement or a stochastic process in which there is a much higher probability of κ vs. λ gene rearrangement (14, 15). Ig LC gene rearrangement also is subject to allelic and isotype exclusion; only one of the multiple κ and λ LC alleles is functionally rearranged in a given B cell to form a LC that associates with the HC to form a BCR (14, 16–18). In this regard, assembly of the BCR has been postulated to signal down-regulation of V(D)J recombination activity (RAG gene expression) and, potentially, LC gene accessibility to ensure the shut-down of further Ig gene rearrangement and maintain allelic exclusion (13). In addition, formation of a stable BCR appears to be crucial for mature B lymphocyte survival (19).
To further elucidate the relative functions of Ig HC, Ig LC, the BCR, and potential downstream effectors in signaling various events associated with B cell differentiation, it would be of interest to have a system in which peripheral B cell development and LC gene rearrangement could be reproducibly induced in the absence of HC or BCR expression. In this context, we have focused on the potential role of Ras-dependent signaling, which has been shown to be induced upon cross-linking of the mature BCR in lymphocyte cell lines (20, 21). Recently, we have demonstrated that activated Ras expressed in the RAG-1Δ background could mediate developmental progression of RAG-1Δ pro-B cells to B lineage cells that share characteristics of the pre-B and mature B cell stages (22). Therefore, we hypothesized that in JHΔ pro-B cells, which are unable to synthesize μ HC but are V(D)J recombination-competent, expression of activated Ras might result in the generation of B lineage cells that have progressed to a stage in which they were competent for Ig LC gene rearrangement in the absence of μ HC. To test this possibility, we have used the RAG-2Δ blastocyst complementation method (12) to assay the B lineage differentiation potential of JHΔ embryonic stem (ES) cells transfected with a B lineage expression construct encoding activated Ras.
MATERIALS AND METHODS
ES Cell Transfection and Generation and Analysis of RAG-2Δ Chimeras.
Approximately 107 ES cells JH Δ CCE ES cells (23) were transfected with 10 μg of NotI-linearized pEμRasV12 (22) together with 1 μg of linearized pGK-hygro essentially as described (12). Cells were selected in 170 μg/ml hygromycin B (Boehringer Mannheim). Drug-resistant ES colonies were picked and subcloned for injection into RAG-2Δ blastocysts as described (12). RAG-2Δ chimeras were maintained in a pathogen-free environment and were analyzed at 4–6 weeks of age. Fluorescence-activated cells sorter analysis of bone marrow, spleen, and lymph nodes was carried out as described (22, 24). Reverse transcription–PCR analyses were performed as described (22). Western blot analyses of protein lysates prepared from single-cell splenocyte suspensions were performed as described (22) by using an anti-Ha-ras mAb (clone F235; Calbiochem).
Analyses of LC Rearrangement.
DNA was isolated from spleen, kidney, murine embryonic fibroblasts, or sorted B220+ Ly 9.1+ B lineage cells by using proteinase K digestion as described (25), except that the DNA was subjected to phenol extraction before ethanol precipitation.
For PCR analyses of κ LC gene rearrangement, the total amount of input DNA was held constant at 50 ng, with murine embryonic fibroblast DNA added to decreasing amounts of B cell DNA to maintain equivalent total DNA concentrations in a given reaction. PCR reactions (50 μl) contained: 1× PCR buffer, 200 μM dNTPs, 2 μM sense and antisense oligonucleotide primers, 100 μg/ml BSA, and 0.5 units of Taq polymerase (Qiagen, Chatsworth, CA). The PCR conditions were as follows: 96°C for 2 min, followed by cycling at 94°C for 30 sec, 60°C for 1 min, and 72°C for 1.5 min. The number of cycles was varied from 15 to 30 to determine the linear range of amplification for each primer pair. A degenerate Vκ primer was employed with a Jκ2 primer (26) for 20 cycles to detect a 536-bp VκJκ1 rearrangement; a VκJκ2 rearrangement of 120 bp was also detected. A glyceraldehyde-3-phosphate dehydrogenase oligonucleotide primer pair was employed in 15 cycles of amplification to standardize DNA quantity (27). PCR products were resolved on 2% agarose gels and subjected to Southern analysis as above using a HindIII germline Jκ probe (28) and a glyceraldehyde-3-phosphate dehydrogenase cDNA probe. PCR primers to detect VλJλ1 rearrangements (29) were as follows: Vλ, 5′-GCCATTTCCCAGGCTGTTGTGACTCAGG-3′; and Jλ1, 5′-ACTCACCTAGGACAGTCAGTTTGGTTCC-3′. PCR conditions were as above, for 24 cycles. An XbaI/HindIII Vλ1 germline fragment, which cross-hybridizes to Vλ2, was used for Southern blot analysis (30). Densitometry was performed by using a Molecular Dynamics PhosphorImager with quantitation using imagequant software.
For genomic Southern analysis of κ LC gene rearrangements, 7–10 μg of sorted B220+ Ly 9.1+ genomic DNA was digested with HindIII. Digestion products were resolved on a 0.9% agarose gel for Southern analysis with the germline Jκ probe described above. A c-myc exon 2 PstI fragment (31) was used to standardize for DNA content. Densitometry and quantitation were performed as above. The percentage of germline Jκ retention was calculated by determining the ratio of Jκ to c-myc intensity. For nonrearranging tissue (kidney), the ratio was set to 100% retention. For B lineage cell samples, the Jκ to c-myc ratio in sample was divided by the ratio in kidney, and the result expressed as a percentage of germline observed in kidney.
Amplified Vκ–Jκ sequences were cloned into pT7blue (Novagen) and sequenced by using the T7 primer. Sequences were compared with the GenBank database to identify the most homologous germline (or rearranged) counterpart.
RESULTS
Developmental Progression of JH-Deleted Pro-B Cells by Activated Ras.
RAG-1Δ or RAG-2Δ mice lack both B and T cells because of the inability to initiate V(D)J recombination in progenitor lymphocytes (32, 33). In contrast, germline JHΔ mice, as well as somatic chimeric mice made by injecting JHΔ ES cells into RAG-2Δ blastocysts, have normal T cell development but blocked B cell development at the pro-B cell stage as a result of the inability to generate a HC protein needed to drive further differentiation (3, 12, 23). To assay the capacity of B lineage cells to undergo differentiation in the absence of HC expression, we used RAG-2Δ blastocyst complementation (12) to assay JHΔ ES cells that had been transfected with pEμRasV12, a construct containing an Ig variable region promoter and HC enhancer that drive expression of an activated c-Ha-Ras V12 cDNA (34). The resulting chimeric mice are referred to as RAG-2Δ/Ras-JHΔ mice. In such mice, B lineage cells beyond the pro-B cell stage must derive from the Ras-JHΔ ES cells; for the studies described, expression of the Ly 9.1 surface marker was used to ensure the ES cell origin of putative Ras-JHΔ B lineage cells (data not shown). In addition, because Ha-ras is not normally expressed in wild-type lymphocytes (35), we were able to use an anti-Ha-ras mAb in Western blot analyses of splenocytes from these chimeras to verify the expected expression of the activated Ras transgene (data not shown).
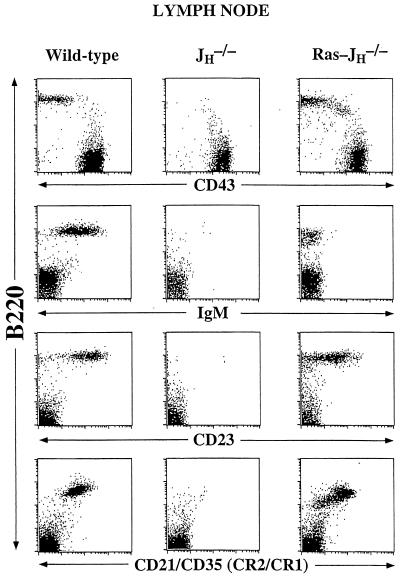
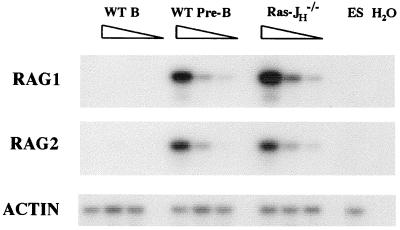
Flow cytometry analyses of bone marrow from RAG-2Δ/Ras-JHΔ chimeras revealed a population of B220+ CD43− IgM− cells not observed in RAG-2Δ or JHΔ mice; such cells were also found in lymph nodes and spleens of the RAG-2Δ/Ras-JHΔ chimeras in quantities that approached those found for mature Ig-expressing B lymphocytes in wild-type mice (Fig. 1 and data not shown). In the bone marrow and periphery, Ras-JHΔ B cells up-regulated certain surface markers often associated with more mature stages of B cell development, such as CD22, CD23, and CD21/CD35 (Fig. 1 and data not shown). To further delineate the stage of maturation of Ras-JHΔ B cells, we used a semiquantitative PCR assay to estimate expression levels of the λ5, RAG-1, and RAG-2 genes, which normally are expressed in pro-B and pre-B cells but generally down-regulated in mature B lymphocytes (4, 36). This assay indicated that both RAG-1 and RAG-2, as well as λ5, were expressed in peripheral Ras-JHΔ B lineage cells at levels comparable with those in wild-type pre-B cells (Fig. 2 and data not shown).
Figure 1.
Surface phenotype of Ras-JHΔ B lineage cells. Fluorescence-activated cell sorter analysis of lymph node cells derived from a RAG-2Δ/Ras-JHΔ mouse, with lymph node compartments from untransfected JHΔ RAG-2Δ chimera and wild-type 129 Sv/Ev. Depicted are log-scale dot plots of B220 vs. CD43, IgM, CD23, and CD21/35 (CR2/CR1). The plots reflect 20,000 collected events, with dead cells excluded by forward and side scatter. Similar results were found in the spleen. The number of B220+ Ly 9.1+ cells in Ras-JHΔ chimeras ranged from 3.4 × 105 to 1.2 × 107 in lymph node and from 4.9 × 105 to 2.1 × 107 in spleen. The B220− CD43+ cells are T lymphocytes. Chimeric animals were analyzed at 4–6 weeks of age, and had no gross evidence of malignancy. Moreover, sequence analysis of κ LC joins revealed diversity in Vκ usage, arguing against an oligoclonal proliferation of B cells (Fig. 4). Some older chimeric mice do develop B lineage lymphomas, but such transformed cells are markedly larger and have substantially altered staining characteristics (not shown). Results shown are representative of those obtained in the analysis of nine chimeric mice derived from three independent transfected ES clones.
Figure 2.
RAG expression in Ras-JHΔ B lineage cells. Reverse transcription–PCR analyses of first-strand cDNA derived from sorted populations of peripheral B220+ Ly 9.1+ Ras-JHΔ (Ras-JH−/−) and mature wild-type B cells (WT B), together with purified bone marrow B220+ CD43− IgM− wild-type pre-B cells (WT Pre-B) are shown. Serial five-fold dilutions of cDNA are depicted, and ES cell cDNA was diluted into the 5- and 25-fold dilutions in each sample set to equalize template quantity.
Ig LC Gene Rearrangement in Ras-JHΔ B Lineage Cells.
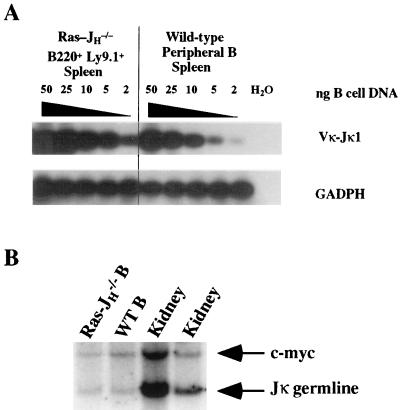
By expressing activated Ras in the JHΔ background, we were able to generate HC-deficient, recombination-competent B lineage cells that progressed beyond the μ HC developmental checkpoint when LC gene rearrangements are induced. Therefore, we were able to further examine whether activated Ras signaling in such cells resulted in rearrangement of Ig LC loci. Populations of Ras-JHΔ and wild-type mature B cells were purified by cell sorting from lymph nodes and spleen. Genomic DNA samples from these sorted populations and DNA from a nonlymphoid tissue (kidney) were then digested with HindIII and assayed by Southern blot analysis for hybridization to a probe that encompasses the germline Jκ genes. The germline HindIII DNA fragment harboring the Jκ segments is 2.8 kb; Vκ to Jκ rearrangements would result in the generation of novel-sized Jκ-hybridizing HindIII fragments. Therefore, the extent of Jκ rearrangement in a population of B lineage cells can be estimated by the extent to which the signal of the germline Jκ fragment is diminished. Consistent with previously reported observations for the range of Jκ rearrangement levels in mature B lymphocytes (37, 38), the Jκ germline signal was reduced to 45% of the kidney signal in purified mature peripheral wild-type B cells. Notably, Ras-JHΔ B lineage cell DNA yielded a similar reduction in the intensity of the germline Jκ signal, with reduction in intensity to ≈40% that in kidney (Fig. 3B).
Figure 3.
Extensive κ LC gene rearrangement in Ras-JHΔ B lineage cells. (A) PCR analysis was performed on DNA isolated from B220+ Ly 9.1+ Ras-JHΔ (Ras-JH−/−) and wild-type peripheral B lineage cells purified by cell sorting of lymph node and spleen. A titration of B cell DNA is shown after Southern blot analysis using a germline Jκ probe (28); the total amount of DNA was held constant at 50 ng in each sample through the addition of murine embryonic fibroblast DNA. (B) Genomic Southern analysis of κ LC gene rearrangement. DNAs isolated from purified B220+ Ly 9.1+ wild-type peripheral B cells (WT B) and from kidney, together with B220+ Ly 9.1+ B lineage cell DNA pooled from the lymph nodes and spleen of three Ras-JHΔ RAG-2Δ (Ras-JH−/−) chimeras were digested with HindIII for Southern blot analysis. Loss of signal from the germline Jκ band was employed to estimate the extent of kappa rearrangement, and a c-myc cDNA probe was used as a nonrearranging standard.
As an independent approach to assess Jκ rearrangement, we used a semiquantitative PCR assay to quantify the relative level of Vκ-Jκ1 joins in Ras-JHΔ vs. wild-type mature B cells. These analyses demonstrated that the levels of such rearrangements were similar in these two types of cell populations (Fig. 3A). In contrast, only a very low level of κ gene rearrangement (in only 1% of B220+ cells) was observed in PCR assays of DNA from total bone marrow in JHΔ germline mice (23). Finally, DNA sequencing of Vκ-Jκ joins from Ras-JHΔ peripheral B lineage cells revealed substantial diversity in Vκ usage, arguing against the presence of a monoclonal proliferation of B lineage cells (Fig. 4). We conclude that the κ LC locus in Ras-JHΔ B lineage cells is substantially, but not completely, rearranged, with the extent of rearrangement being similar to that of mature wild-type B cells.
Figure 4.
Vκ-Jκ sequence analysis from Ras-JHΔ B lineage cells purified from lymph node and spleen. Sequence at the Vκ-Jκ junction is depicted, with five Jκ1 joins (11–13, 15, 16) and one Jκ4 join (11.1) shown. The GenBank accession number of the most homologous Vκ germline sequence is indicated, and the sequence shown extends to the Vκ germline exon-intron border. Identity with the Vκ germline or Jκ sequence is indicated by dashes, and nucleotide differences are indicated. Note that for several Vκ sequences, substantially greater homology was found in the database for sequences of rearranged and/or expressed κ LC alleles (data not shown).
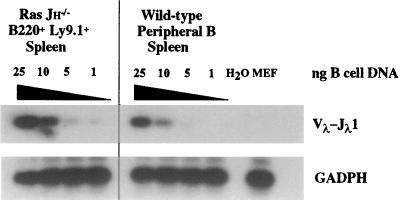
In normal B cell populations, only ≈ 5% of the cells have rearranged λ LC genes (13). To assess the effects of activated Ras expression on λ LC gene rearrangement, we used a semiquantitative PCR assay to estimate the relative level of Vλ–Jλ1 rearrangements in normal and Ras-JHΔ B lineage cells. Although these assays detected Vλ–Jλ1 rearrangements in Ras-JHΔ B lineage cells, the level of these rearrangements was not substantially above that observed in wild-type B lineage cell populations (Fig. 5).
Figure 5.
Analysis of λ LC gene rearrangement in Ras-JHΔ B lineage cells. PCR analysis was performed on DNA isolated from B220+ Ly 9.1+ Ras-JHΔ (Ras-JH−/−) and wild-type peripheral B lineage cells purified by cell sorting of lymph node and spleen. A titration of B cell DNA is shown after Southern blot analysis using a germline Vλ1 probe that cross-hybridizes to Vλ2; the total amount of DNA was held constant at 25 ng in each sample through the addition of murine embryonic fibroblast (MEF) DNA.
DISCUSSION
Activated Ras-Induced Differentiation of JHΔ Pro-B Cells.
We show that expression of activated Ras in JHΔ pro-B cells promotes the differentiation and peripheral accumulation of B lineage cells that largely resemble pre-B cells but also have activated expression of certain markers associated with more mature stages. This phenotype of Ras-JHΔ B lineage cells appears to be indistinguishable from that which we recently observed in Ras-RAG-1Δ B lineage cells (22). Thus, we conclude that activated Ras expression results in developmental progression of the mutant pro-B lymphocytes in the absence of Ig HC gene expression in two independent genetic backgrounds in which blocks at the pro-B stage of development are imposed through distinct mechanisms. The major difference is that the differentiated JHΔ B lineage cells, unlike RAGΔ B lineage cells, retain the ability to undergo V(D)J recombination and, thereby, show extensive Ig LC gene rearrangement in association with their activated Ras-induced differentiation.
At present, we do not know whether activated Ras plays a direct role in the control of Ig LC gene rearrangement, or whether activated Ras instead mediates developmental progression to a stage(s) of B lymphopoiesis in which the promotion and regulation of LC gene rearrangements are initiated in a Ras-independent fashion. However, our data are consistent with the possibility that Ras may link intracellular signaling pathways to the regulation of Ig LC gene rearrangement in B lymphocyte progenitors. Further studies employing activated alleles of downstream Ras effectors, such as the mitogen- or stress-activated protein kinases, should provide additional insights into the specific downstream effectors and indicate if different pathways are involved in effecting different types of differentiation events (e.g., rearrangement control vs. expansion). Regardless, activated Ras-induced differentiation of JHΔ pro-B cells provides a method to study Ig LC gene rearrangement in the absence of potential constraints imposed by expression of Ig HC, including Ig HC/LC pairing. In this context, it is striking that Ras-JHΔ B lineage cells accumulate to substantial levels in the periphery, as BCR expression is critical for peripheral B cell survival (19). It is possible that activated Ras may engage such survival signaling pathways; alternatively, the peripheral Ras-JHΔ B lineage may represent an earlier B cell developmental stage in which such BCR-generated survival signals are not yet required.
A System to Study Regulation of Ig LC Gene Rearrangement.
It has been proposed that signals generated from the BCR feedback to cause cessation of endogenous LC gene rearrangement (effecting allelic and isotype exclusion) by suppressing V(D)J recombinase (RAG) expression, making the endogenous LC variable region genes inaccessible, or both (14, 39). In this context, one potential prediction of the ability to activate Ig LC rearrangement in HC-deficient B lineage cells would be that the absence of a negative signal ordinarily generated by the BCR might lead to substantially increased extents of κ and/or λ locus rearrangements (and allelic inclusion). However, we find that rearrangement of both the κ and λ Ig LC loci in Ras-JHΔ B lineage cells appears to have proceeded to an extent similar to that observed in mature wild-type B lymphocytes. Therefore, these findings suggest that the differential regulation of the rearrangement of Ig κ vs. λ LC gene loci, and, at least approximately, the extent of rearrangement within these loci, are maintained in Ras-JHΔ B lineage cells and that HC expression is not required to effect this regulation. Clearly, the persistence of germline LC alleles in Ras-JHΔ B lineage cells shows that rearrangements did not proceed to completion in either LC locus, as might be expected if activated Ras expression resulted in completely deregulated rearrangement (Fig. 3B and data not shown). In this regard, it is significant that RAG-1 and RAG-2 expression levels in Ras-JHΔ B lineage cells appear comparable with those in wild-type pre-B lymphocytes, thus arguing against limiting amounts of V(D)J recombinase as an explanation for the apparent cessation of LC rearrangement.
Our current analyses of Ras-JHΔ B lineage cells suggest a role for signaling pathways downstream of activated Ras in effecting Ig LC gene rearrangement and also suggest the possibility of unanticipated accessibility-related mechanisms that may govern the extent of κ and λ LC gene rearrangements. One potential scenario to explain our data is that activated Ras expression has somehow led to the activation of LC gene accessibility, but that peripheral Ras-JHΔ B lineage cells, perhaps in association with factors related to movement to the periphery, may have then down-regulated accessibility of endogenous LC loci even though activated Ras and RAG gene expression are retained. Clearly, additional analyses of Ras-JHΔ B lineage cells will be necessary to clarify such possibilities. For example, studies of κ and λ gene rearrangement in individual Ras-JHΔ peripheral B lineage cells may provide insights into the role of the HC and BCR in effecting the LC allelic and isotype exclusion processes, as well as testing the role of HC-LC compatibility (40) in shaping the peripheral Ig LC variable region repertoire.
Acknowledgments
We thank Drs. Raif Geha and Jianzhu Chen for critical review of the manuscript. We also thank Drs. Barbara Malynn and Craig Bassing for helpful comments and discussions. F.W.A. is an investigator of the Howard Hughes Medical Institute. This work was also supported by National Institutes of Health Grants AI-20047 (F.W.A.) and AI-01532-01 (A.C.S.). A.C.S. was a recipient of a Howard Hughes Medical Institute Postdoctoral Research Fellowship for Physicians. W.S. is a recipient of the Arthritis Foundation Hulda Irene Duggan Investigator Award.
ABBREVIATIONS
- HC
heavy chain
- LC
light chain
- RAG
recombination activating gene
- RAG-1Δ and RAG-2Δ
RAG-1- and RAG-2-deficient
- JHΔ
JH-deleted
- ES
embryonic stem
- BCR
B cell receptor
References
- 1.Willerford D M, Swat W, Alt F W. Curr Opin Genet Dev. 1996;6:603–609. doi: 10.1016/s0959-437x(96)80090-6. [DOI] [PubMed] [Google Scholar]
- 2.Karasuyama H, Rolink A, Melchers F. Adv Immunol. 1996;63:1–41. doi: 10.1016/s0065-2776(08)60853-6. [DOI] [PubMed] [Google Scholar]
- 3.Ehlich A, Schaal S, Gu H, Kitamura D, Muller W, Rajewsky K. Cell. 1993;72:695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- 4.Grawunder U, Leu T M J, Schatz D G, Werner A, Rolink A G, Melchers F, Winkler T H. Immunity. 1995;3:601–608. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 5.Alt F, Rosenberg N, Lewis S, Thomas E, Baltimore D. Cell. 1981;27:381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie K A, Brinster R L, Storb U. Nature (London) 1984;312:517–520. doi: 10.1038/312517a0. [DOI] [PubMed] [Google Scholar]
- 7.Reth M G, Ammirati P, Jackson S, Alt F W. Nature (London) 1985;317:353–355. doi: 10.1038/317353a0. [DOI] [PubMed] [Google Scholar]
- 8.Reth M, Petrac E, Wiese P, Lobel L, Alt F W. EMBO J. 1987;6:3299–3305. doi: 10.1002/j.1460-2075.1987.tb02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nussenzweig M C, Shaw A C, Sinn E, Danner D B, Holmes K L, Morse H C, Leder P. Science. 1987;236:816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- 10.Young F, Ardman B, Shinkai Y, Lansford R, Blackwell T K, Mendelsohn M, Rolink A, Melchers F, Alt F W. Genes Dev. 1994;8:1043–1057. doi: 10.1101/gad.8.9.1043. [DOI] [PubMed] [Google Scholar]
- 11.Spanopoulou E, Roman C A, Corcoran L M, Schlissel M S, Silver D P, Nemazee D, Nussenzweig M C, Shinton S A, Hardy R R, Baltimore D. Genes Dev. 1994;8:1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Lansford R, Stewart V, Young F, Alt F W. Proc Natl Acad Sci USA. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorman J R, Alt F W. Adv Immunol. 1998;69:113–181. doi: 10.1016/s0065-2776(08)60607-0. [DOI] [PubMed] [Google Scholar]
- 14.Alt F W, Enea V, Bothwell A L M, Baltimore D. Cell. 1980;21:1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- 15.Coleclough C, Perry R P, Karjalainen K, Weigert M. Nature (London) 1981;290:372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- 16.Kuehl W M, Kaplan B A, Scharff M D. Cell. 1975;5:139–147. doi: 10.1016/0092-8674(75)90022-7. [DOI] [PubMed] [Google Scholar]
- 17.Kwan S P, Max E E, Seidman J G, Leder P, Scharff M D. Cell. 1981;26:57–66. doi: 10.1016/0092-8674(81)90033-7. [DOI] [PubMed] [Google Scholar]
- 18.Bernard O, Gough N M, Adams J M. Proc Natl Acad Sci USA. 1981;78:5812–5816. doi: 10.1073/pnas.78.9.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam K P, Kuhn R, Rajewsky K. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 20.Lazarus A H, Kawauchi K, Rapoport M J, Delovitch T J. J Exp Med. 1993;178:1765–1769. doi: 10.1084/jem.178.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harwood A E, Cambier J C. J Immunol. 1993;151:4513–4522. [PubMed] [Google Scholar]
- 22.Shaw A C, Swat W, Ferrini R, Davidson L, Alt F W. J Exp Med. 1999;189:123–129. doi: 10.1084/jem.189.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Trounstine M, Alt F W, Young F, Kurahara C, Loring J, Huszar D. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 24.Young F, Mizoguchi E, Bhan A K, Alt F W. Immunity. 1997;6:23–33. doi: 10.1016/s1074-7613(00)80239-3. [DOI] [PubMed] [Google Scholar]
- 25.Laird P W, Zijderveld A, Linders K, Rudnicki M A, Jaenisch R, Berns A. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlissel M S, Baltimore D. Cell. 1989;58:1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- 27.van der Stoep N, Gorman J R, Alt F W. Immunity. 1998;8:743–750. doi: 10.1016/s1074-7613(00)80579-8. [DOI] [PubMed] [Google Scholar]
- 28.Gorman J R, van der Stoep N, Monroe R, Cogne M, Davidson L, Alt F W. Immunity. 1996;5:241–252. doi: 10.1016/s1074-7613(00)80319-2. [DOI] [PubMed] [Google Scholar]
- 29.ten Boekel E, Melchers F, Rolink A. Int Immunol. 1995;7:1013–1019. doi: 10.1093/intimm/7.6.1013. [DOI] [PubMed] [Google Scholar]
- 30.Blackwell T K, Moore M W, Yancopoulos G D, Suh H, Lutzker S, Selsing E, Alt F W. Nature (London) 1986;324:585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- 31.Smith R K, Zimmerman K, Yancopoulos G D, Ma A, Alt F W. Mol Cell Biol. 1992;12:1578–1584. doi: 10.1128/mcb.12.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinkai Y, Rathbun G, Lam K P, Oltz E M, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall A, Alt F W. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 33.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 34.Shibuya E K, Polverino A J, Chang E, Wigler M, Ruderman J V. Proc Natl Acad Sci. USA. 1992;89:9831–9835. doi: 10.1073/pnas.89.20.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leon J, Guerrero I, Pellicer A. Mol Cell Biol. 1987;7:1535–1540. doi: 10.1128/mcb.7.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y-S, Hayakawa K, Hardy R R. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coffman R L, Weissman I L. J Mol Cell Immunol. 1983;1:31–38. [PubMed] [Google Scholar]
- 38.Arakawa H, Shimizu T, Takeda S. Int Immunol. 1996;8:91–99. doi: 10.1093/intimm/8.1.91. [DOI] [PubMed] [Google Scholar]
- 39.Ma A, Fisher P, Dildrop R, Olta E, Rathbun G, Achacoso P, Stall A, Alt F W. EMBO J. 1992;11:2727–2734. doi: 10.1002/j.1460-2075.1992.tb05338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ten Boekel E, Melchers F, Rolink A G. Immunity. 1997;7:357–368. doi: 10.1016/s1074-7613(00)80357-x. [DOI] [PubMed] [Google Scholar]