Abstract
The inappropriate immune response to foods, such as peanut, wheat and milk may be the basis in the pathogenesis of enteropathies like coeliac and Crohn disease, which present small intestinal malabsorption. A number of recent studies have utilized d-xylose absorption as an investigative tool to study small intestinal function in a variety of clinical settings. Thus, the aim of this experimental study was to evaluate the intestinal absorption of d-xylose in an antigen-specific gut inflammatory reaction rat model. Animals of the experimental group were inoculated with peanut protein extract before their exposure to a challenge diet containing exclusively peanut seeds to induce the gut inflammatory reaction caused by peanut allergy. Our results show that systemic inoculation with peanut protein extract renders significantly higher antibody titres (5.085 ± 0.126 units) (P < 0.0001) than control rats (0.905 ± 0.053 units) and that the antibody titres correlate positively to an inflammatory alteration of the gut morphology (P < 0.0001). Animals pertaining to the experimental group showed an intestinal absorption of d-xylose lower than control rats (P < 0.0001). We also observed that d-xylose absorption correlates negatively with IgG titres and positively with morphometric parameters (Pearson correlation). In conclusion, the use of serum d-xylose test was useful to identify the presence of small intestinal malabsorption in our antigen specific gut inflammatory reaction rat model.
Keywords: gut inflammation, malabsorption, peanut allergy, rat, xylose
Inflammatory bowel disease (IBD), like ulcerative colitis, Crohn's disease and others non-infectious inflammation of the gut, have posed an enigma to gastroenterologists and immunologists alike since their first modern description some 75–100 years ago, consisting of one of the major causes of lifetime morbidity (Strober et al. 2007;Weber & Turner 2007). Recently, it has been estimated that IBD affects approximately one million people in North America alone (Bamias et al. 2005). The idea that the immunological environment of the gut seems to make great efforts to ensure that tolerance is the default response to antigen is often challenged on the basis that this would be a dangerous strategy for host survival in the face of continuous exposure to pathogens. The inappropriate immune response to foods and commensal bacteria responsible for coeliac and Crohn's disease is because of deregulation of crucial control processes of the gut (Mowat 2003). The use of animal models of mucosal inflammation as a means to probe the pathogenesis of IBD extends to almost a half century (Strober et al. 2002) and several animal models are available. These models have been used to evaluate pharmacological molecules or agents and have contributed greatly to important advances in our current understanding of the underlying mechanisms of inflammation and disease pathogenesis as well as treatment (Jurjus et al. 2004).
A number of recent studies have utilized d-xylose absorption as an investigative tool to study small intestinal function in a variety of clinical settings. d-xylose is a pentose found naturally in plants and its incomplete metabolization allows it to be used as an absorptive test (Craig & Ehrenpreis 1999). A majority of studies appear to favour serum d-xylose measurements over urinary d-xylose excretion to screen adult and paediatric patients for small intestinal malabsorption (Craig & Atkinson 1988) which is a simple and low-cost method (Ehrenpreis et al. 2001).
In an earlier study, we demonstrated the reproducibility of a peanut allergy model, originally developed in C57BL/6J mice by Teixeira (2003) and Teixeira et al. (2008), in BALB/c mice and Lou-M rats (Antunes et al. 2008). Peanut seeds (Arachis hypogea) were chosen as the source of antigen as peanuts are considered to be one of the most potent food allergens causing severe diseases (Fries1982; Burks et al. 1998; Van Wijk et al.2005). The main features of this model of gut inflammation are villous atrophy, crypt hiperplasia, prominent mononuclear leukocyte infiltrate and lamina propria oedema. These signs are similar to food induced enteropathies in humans such as coeliac disease, a gluten enteropathy.
Enterocyte damage, perhaps secondary to T cell activation and release of cytokines, may lead to malabsorption of monosaccharides and increased small intestinal permeability. While malabsorption may contribute to weight loss and malnutrition, increased intestinal permeability may lead to an inflammatory enteropathy (Sharpstone et al.1999). For these reasons, non-invasive investigations are useful to monitor patients gut alterations.
The aim of this study was to evaluate the intestinal absorption of the d-xylose in an antigen-specific gut inflammatory reaction rat model. Unlike for diagnosis in humans, we are unaware of any reports in the literature, in which d-xylose is utilized to evaluate the intestinal absorption in rat models of enteropathy.
Materials and methods
The study was conducted with the approval of the Ethics Committee in Research of the Medicine School of Fluminense Federal University (protocol 167/05).
Animals
In this study, 35 male adult Lou-M rats (8–12 weeks and 250–300 g body weight) bred and maintained at the Animal Facility of the Fluminense Federal University (Niteroi, Brazil) were used. These healthy animals were randomly divided into three groups. First, we used one group, denominated pilot group (n = 5) to perform the d-xylose absorption curve prior to the d-xylose test on the animals of this study. After the standardization of the xylose technique, the study was conducted with two groups: control group (n = 15) and experimental group (n = 15). They were individually numbered to enable paired statistical analysis.
Induction of the antigen-specific gut inflammation
To develop the antigen-specific gut inflammation, all rats of the experimental group (n = 15) were inoculated twice with 200 μg peanut protein extract (PPE) through the subcutaneous rout (sc). The primary inoculation was realized with 5 mg of adjuvant [Al(OH)3] and after an interval of 21 days, each animal received a booster inoculation without adjuvant. After this second inoculation, all rats were submitted to a challenge diet composed exclusively of peanuts in natura ad libitum over a 30-day period.
The control animals (n = 15) were inoculated with physiological saline plus 5 mg [Al (OH)3], and after the same interval of 21 days, they received a second inoculation with physiological saline. After the second inoculation, all control animals continued to receive commercial rodent chow (Nuvilab, NUVITAL, Brazil).
All animals were bled from the retrorbital plexus, prior to manipulation and 10 days after each inoculation, withdrawing 1 ml. The serum was collected and stored at −20 °C until analysis.
Determination of the specific anti-peanut protein antibody titres
The serum samples were evaluated by enzyme linked immuno sorbent assay (ELISA) to quantify specific anti-peanut protein antibody (Ab) titres. For antigen-specific IgG antibody, 96-well plates were coated with PPE at 10 μg/well in phosphate buffered saline (PBS) at 4 °C overnight. Unbound extract were discarded, plates were washed with 0.05% Tween-20 in PBS and then blocked with 100 μl of a 1% PBS/gelatin solution for 1 h at room temperature. Serum samples were placed in the first well at a 1% dilution (100 μl/well) followed by a threefold serial dilution (obtaining a final dilution of 1:218700) and incubated for 3 h at room temperature. Plates were then washed and received peroxidase-labelled goat anti-rat IgG (Southern Biotechnology, Birmingham, AL, USA) and incubated for 3 h at room temperature. Reactions were developed with 100 μl of solution containing H2O2 and o-phenylene-diamine (OPD; Sigma-Aldrich, Seelze, Germany). Plates were read at 492 nm on an automated ELISA reader (Anthos 2010, Krefeld, Germany). The results are reported as arbitrary units of ELISA corresponding to the value of the area under the dilution curve of each serum.
Anaesthesia
Rats received the equivalent of 100 mg/kg ketamine and 10 mg/kg xylazine body weight intraperitoneally.
Determination of D-xylose absorption curve in healthy animals
Pilot group rats (n = 5) were anaesthetized and administered an aqueous solution of 2 ml containing 1.23 g of d-xylose into the stomach by gavage. They were bled from the retrorbital plexus – withdrawing 1 ml – immediately prior to the gavage and at 1-h intervals after the d-xylose administration, until the fourth hour. The serum was collected and stored at −20 °C until analysis.
The amount of d-xylose in each serum sample was determined using the spectrophotometric method described by Eberts et al. (1979), with some adaptation. In short, 60 μl of serum was added to the 2 ml of chromogen (phloroglucinol, chloridric acid and acetic acid) and reacted in a boiling water bath at 100 °C for 4 min. After cooling at room temperature, optical density was measured using a Pelkin Elmer spectrophotometer at 554 nm.
d-xylose absorption test in experimental and control animals
To determine if inflammation interferes in d-xylose absorption, the test was performed at the end of the 30-day period of challenge diet in the same fashion as described above using the first and third hour postgavage.
Necropsy and gut segments collection
After the d-xylose test, all animals were euthanized to collect 1–3 cm segments of gastro-duodenum junction and jejunum. These segments were fixed with 10% buffered formaldehyde and stained with haematoxilin–eosin (HE). The histological parameters evaluated were the general description of the slide (integrity of the intestinal structure, number of villi per field, oedema, congestion, and leucocyte infiltrate). The villi height/width ratio, villi-height/cript-height ratio and intestinal epithelial cells/intraepithelial leucocytes (IEC/IEL) ratio were obtained from the previous parameters (Teixeira 2003; Antunes et al. 2008).
Statistical analysis
The data were expressed as mean ± SD. Statistical analysis performed was anova with Tukey's or Fisher posttest to determine the minimum significance difference (MSD), using graphpad instat Program by graphpad Software Inc®. Interaction between variables was studied using Pearson correlation. A P-value less than 0.05 was considered statistically significant.
Results
The systemic inoculation with crude peanut extract renders high antibody titres
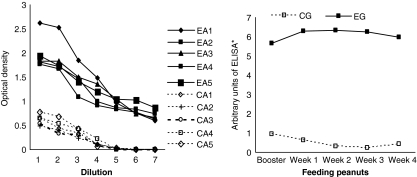
Confirming previous results in mice (Teixeira 2003), experimental rats (submitted to systemic inoculation with crude peanut extract) presented significantly higher (P < 0.0001) IgG antibody titres (5.085 ± 0.126 units) than control rats (inoculated with saline) (0.905 ± 0.053 units) (Figure 1).
Figure 1.
Total antipeanut IgG antibody titres in experimental and control rats. Left panel, the titration curve of anti-peanut protein of experimental animals (EA 1-5) and control animals (CA 1-5). Right panel, arbitrary units as the result of the mean of the area under the curves for each point of experimental and control animals. Experimental group (EG-submitted to systemic inoculation with crude peanut extract) presented significantly higher (P < 0.0001) IgG antibody titres than control group (CG-inoculated with saline).
Intestinal absorption of d-xylose
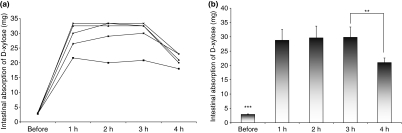
After gavage, all healthy animals (pilot group rats) were bled at hourly intervals for the first 4 h to determine the absorption and excretion curve of d-xylose. Evaluating the individual absorption curves of these first 4 h after d-xylose gavage, we found that as of the first and until the third hour there are no statistical differences in blood levels of d-xylose between animals (Figure 2a) Evaluating the mean values of these five healthy animals, we observed a statistical difference between before and after gavage, but not during the 3 h following gavage. When compared the third with the fourth hour, we observed a significant difference (P < 0.05), showing a d-xylose clearance at the fourth hour (Figure 2b).
Figure 2.
Determination of the absorption curve of d-xylose in healthy rats (pilot rats group). (a) Individual absorption curve during the first 4 h after gavage. There are no statistical differences in d-xylose blood levels between animals (P > 0.05). (b) Mean values of the five rats showing a statistical difference (***P < 0.0001) between before and after gavage. There is no significant difference between the first and the third hour, but we observed a d-xylose clearance at the fourth hour. There is a statistical difference when compared the third with the fourth hour (**P < 0.05).
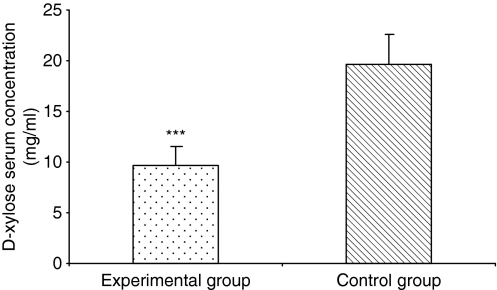
On the basis of absorption curve, we determined that each experimental and control animal should be bled on the first and third hour after gavage. Absorption values are expressed as the mean of both bleedings. As shown in Figure 3, experimental animals showed an intestinal absorption of xylose (9.66 ± 1.79 mg) significantly lower (P < 0.0001) than control rats (19.63 ± 2.89 mg).
Figure 3.
Comparison of mean absorption of d-xylose (mg/ml) in experimental and control rats, expressed as the mean of the first and the third hour bleedings. Experimental animals showed an intestinal absorption of xylose significantly lower (***) than control rats. ***P < 0.0001.
Intestinal analysis
Animals of the experimental group presented a frail consistency of the intestinal tissue observed in the macroscopic analysis in contrast to the intestinal tissue of the control group.
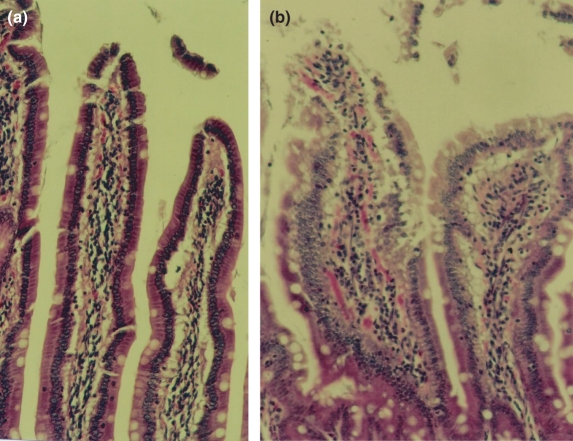
The microscopic analysis agrees with the macroscopic in which a preserved intestinal structure in control animals was seen while in the experimental animals, villi presented oedema, congestion and a high leucocyte infiltration in duodenum (Figure 4).
Figure 4.
Differences in duodenum analysis between experimental and control animals (200×– HE). (a) Villi from a control rat presenting normal aspects. (b) Villi from an experimental rat presenting alteration of the normal morphology (oedema, congestion and a high leucocyte infiltration).
Other criteria used to evaluate the inflammatory process were the villi height/width ratio, villi-height/cript-height ratio and IEC/IEL ratio. Table 1 shows the difference between control and experimental group after the duodenum segment analysis (P < 0.05).
Table 1.
Ratios established after the duodenum segment analysis
| Ratio | Control group | Experimental group |
|---|---|---|
| Villi height/width | 3.54 ± 1.10 | 4.55 ± 0.74 |
| Villi-height/cript-height | 1.97 ± 0.11 | 1.80 ± 0.07 |
| Intestinal epithelial cells/intraepithelial leucocytes | 22.95 ± 2.93 | 9.09 ± 1.43 |
The differences between groups were statistically significant (P < 0.05).
Correlating d-xylose absorption with IgG antibody titres of animals exposed to challenge diet, we found a negative correlation (r = −0.7773; P < 0.0001). On the other hand, correlating d-xylose absorption with villi height/width ratio (r = 0.7645; P < 0.0001), villi-height/crypt-height ratio (r = 0.9763; P < 0.0001) and IEC/IEL ratio (r = 0.8628; P < 0.0001).
Discussion
The objective of this study was to evaluate the intestinal absorption of rats with an antigen specific gut inflammatory reaction, using the serum d-xylose absorption test, which consists of a standard method of diagnosing small intestinal bowel malabsorption (Ehrenpreis et al. 2001). We chose the serum d-xylose test rather than urine test because in some human studies, a reduced specificity of the urine test was observed due to false-positives. In paediatric patients, especially in very young children and infants, the use of serum rather than urinary d-xylose testing is favoured because of difficulties in obtaining accurately timed urine collections (Craig & Ehrenpreis 1999). Ehrenpreis et al.(2001) recommend the use of both 1 and 3 h serum d-xylose determination to screen patients with symptoms of malabsorption. In literature, there are a few studies on the use of d-xylose in rats and they are related to disease models different from IBD, such as trauma haemorrhage, sepsis (Singh et al.1991) and iron-deficiency anaemia (Wayhs et al.2004). In the first study, authors measured the gut absorptive capacity using the 1-h d-xylose absorption test and found significant findings in portal blood (Singh et al.1991). On the other hand, in the study involving anaemia (Wayhs et al.2004), authors based the intestinal absorption of d-xylose on urinary excretion and did not find any difference between the anaemic and control groups. These results confirm earlier findings carried out by Sharma et al. (1973) in rats. We may argue that the differences observed in these studies may be because of the technical procedures or the underlying diseases. As a result, because of the difficulties involved in adequate urine collection in the rat, we chose to use serum determination of d-xylose absorption.
In this study, it was necessary to determine the best bleeding times after d-xylose gavage. Based on literature, animals were bled four times with 1 h intervals. Our results are in accordance with data presented by Ehrenpreis et al. (2001) who specified that patients should be tested at the first and third hour after d-xylose administration. We also demonstrated that there are no statistical differences in blood concentration of d-xylose between the first and third hour sharply declining thereafter. Thus, in accordance with human studies and on the basis of timing of d-xylose elimination from the blood, we defined as a rule to use the first and third hour after gavage to bleed the animals.
As observed in our previous work (Teixeira 2003; Antunes et al.2008; Teixeira et al.2008), systemic inoculation with crude peanut extract (experimental rats) renders antibody titres significantly higher than saline inoculated rats. Experimental animals that eat the challenge diet containing peanuts over a 30-day period, after inoculation, maintain the high antibody levels throughout the period and present significant alteration of the gut morphology typical of chronic inflammatory gut reactions demonstrating that this group presented peanut allergy. As shown by Mowat (2003) that deregulation of food tolerance in humans can provoke IBD like coeliac or Chron's disease, our results confirm that systemic inoculation to a food protein can lead to an enteropathy in the animal model.
Confirming our hypothesis, as the histomorphometric ratios observed in experimental animals decrease (indicative of a chronic inflammatory process), d-xylose absorption also decreases, which implies that inflammation reduces or impedes d-xylose absorption. Correlating d-xylose absorption with serological events, a negative correlation was observed in animals submitted to the challenge diet. On the other hand, sensitized animals that have not yet been submitted to the challenge diet did not present the same correlation P > 0.05 (data not shown) indicating that the challenge diet, which induces the inflammatory process, is essential for the malabsortion and is capable of maintaining high antibody titres over a 30-day period. Thus, in accordance with human findings (Ehrenpreis et al.2001), the presence of the antigen in the diet, a positive association between higher IgG anti-peanut antibody titres, presence of intestinal inflammation and the presence of small intestine malabsorption were demonstrated by the d-xylose test.
In conclusion, the 1 and 3 h serum d-xylose test was useful to identify the presence of small intestinal malabsorption in our antigen-specific gut inflammatory reaction rat model. This permits the use of fewer animals in long experiments carried out to understand human chronic inflammatory processes of the gut, allowing a clinical evaluation without having to euthanize the animals during the experimental period.
Acknowledgments
The present work was carried out with support of the CAPES, Institution of the Brazil Government for development of human resources. We are also indebted to UFF. We would also like to thank Ms. Maira Platais for her significant contribution in the revision of the language.
References
- Antunes DMF, Castro Júnior AB, Campos SMN, et al. Induction of an antigen specific gut inflammatory reaction in mice and rats: a model for human inflammatory bowel disease. Braz. Arch. Biol. Technol. 2008 ??????, ??????.in press. [Google Scholar]
- Bamias G, Nyce MR, De La Rue SA, Cominelli F. New concepts in the pathophysiology of inflammatory bowel disease. Ann. Intern. Med. 2005;143:895–904. doi: 10.7326/0003-4819-143-12-200512200-00007. [DOI] [PubMed] [Google Scholar]
- Burks W, Sampson HA, Bannon GA. Peanut allergens. Allergy. 1998;53:725–730. doi: 10.1111/j.1398-9995.1998.tb03967.x. [DOI] [PubMed] [Google Scholar]
- Craig RM, Atkinson AJ., Jr D-xylose testing: a review. Gastroenterology. 1988;95:223–231. doi: 10.1016/0016-5085(88)90318-6. [DOI] [PubMed] [Google Scholar]
- Craig RM, Ehrenpreis ED. D-xylose testing [clinical review: the small intestine, nutrition, and malabsorption] J. Clin. Gastroenterol. 1999;29(2):143–150. doi: 10.1097/00004836-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Eberts TJ, Sample RHB, Glick MR, Ellis GH. A simplified, colorimetric micromethod for xylose in serum or urine, with phloroglucinol. Clin. Chemist. 1979;25:1440–1443. [PubMed] [Google Scholar]
- Ehrenpreis ED, Salvino M, Craig RM. Improving the serum d-xylose test for the identification of patients with small intestinal malabsorption. J. Clin. Gastroenterol. 2001;33(1):36–40. doi: 10.1097/00004836-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Fries JH. Peanuts: allergic and other untoward reactions. Ann. Allergy. 1982;48:220–226. [PubMed] [Google Scholar]
- Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J. Pharmacol. Toxicol. Meth. 2004;50:81–92. doi: 10.1016/j.vascn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- Sharma DC, Singh PP, Simlot MM. Small intestinal absorptive functions in iron deficient rats. Dig. Dis. 1973;18:73–74. doi: 10.1007/BF01072243. [DOI] [PubMed] [Google Scholar]
- Sharpstone D, Neild P, Crane R, et al. Small intestinal transit, absorption and permeability in patients with aids with and without diarrhoea. Gut. 1999;45:70–76. doi: 10.1136/gut.45.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Chaudry KI, Chudler LC, O′Neil PJ, Chaudry IH. Measurement of D-xylose gut absorptive capacity in conscious rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1991;261:R1313–R1320. doi: 10.1152/ajpregu.1991.261.5.R1313. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Ann. Rev. Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J. Clin. Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira GAPB. A Murine Model of Chronic Gut Inflammation – Tolerance and Oral Immunization: Antigenicity Versus Temporality. Rio de Janeiro: Graduation Program of Pathology, UFF; 2003. PhD Thesis. [Google Scholar]
- Teixeira GAPB, Paschoal PO, Oliveira VL, et al. Diet selection in immunologically manipulated mice. Immunobiology. 2008;213:1–12. doi: 10.1016/j.imbio.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Van Wijk F, Hoeks S, Nierkens S, et al. CTLA-4 signaling regulates the intensity of hypersensitivity responses to food antigens, but is not decisive in the induction of sensitization. J. Immunol. 2005;174:174–179. doi: 10.4049/jimmunol.174.1.174. [DOI] [PubMed] [Google Scholar]
- Wayhs MLC, Patrício FSR, Amâncio OMS, Pedroso MZ, Fagundes NU, Morais MB. Morphological and functional alterations of the intestine of rats with iron-deficiency anemia. Braz. J. Med. Biol. Res. 2004;37:1631–1635. doi: 10.1590/s0100-879x2004001100006. [DOI] [PubMed] [Google Scholar]
- Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6–8. doi: 10.1136/gut.2006.104182. [DOI] [PMC free article] [PubMed] [Google Scholar]