Abstract
The objective was to assess the effect of doxycycline treatment on a magnetic resonance imaging (MRI) biomarker of cartilage volume loss, and on matrix metalloproteinase (MMP) activity in a guinea pig osteoarthritis model. Guinea pigs (9 months old) were dosed with vehicle or doxycycline, 0.6, 3.0 mg/kg/day for 66 days. Fat-suppressed 3D gradient-echo MRI of the left knee was acquired pre- and post dosing. Change in medial tibial plateau (MTP) cartilage volume (MT.VC) was determined using image analysis. At termination, MTP cartilage was removed from knees and proteolytic MMP activity determined using a fluorescent peptide substrate assay. Vehicle-treated animals lost 20.5% (95% CI mean 25.6–15.1) MT.VC. The doxycycline (0.6 mg/kg/day) group lost 8.6% (P < 0.05, 95% CI 20.6 to −5.3) whilst the 3.0 mg/kg/day group lost 10.0% (P < 0.05, 95% CI 13.9–6.0%). Endogenous levels of active MMPs were below limits of detection in all samples. However, doxycycline treatment ablated amino phenyl mercuric acid activated MMP-13 and MMP-8 levels, reduced MMP-9 levels by 65% and MMP-1 levels by 24%. Doxycycline treatment resulted in partial protection from MT.VC loss and was associated with complete reduction in MMP-13 and MMP-8, and partial reduction in MMP-9 activity. These data imply a role of MMPs in cartilage degeneration but incomplete protection suggests that additional doxycycline insensitive mechanisms are important in this model. The protective effect of doxycycline correlates with the clinical finding of lessened joint space narrowing, strengthens the utility of this animal model in identifying disease-modifying osteoarthritic drugs and supports the use of MRI biomarkers of cartilage loss.
Keywords: cartilage volume, doxycycline, guinea pig, magnetic resonance imaging, matrix metalloproteinases, osteoarthritis
Osteoarthritis (OA) is the most common form of joint disease. It leads to pain and disability particularly in the elderly and is an economic burden on society (Elders 2000). It is a slowly progressive disease of the whole joint, characterized morphologically by destruction of cartilage, formation of bone cysts, sclerosis of subchondral bone, presence of osteophytes, thickening of the joint capsule and mild synovitis. There is a large unmet medical need for therapeutics to treat OA. Treatment options for patients with OA are limited and comprise mainly analgesia, or in severe disease, arthroplasty. The structural progression of the disease is little affected by existing medical therapy and there is an urgent need for safe and effective therapies that slow or prevent progression of the disease. However, drugs that preserve the structure of the joint have been difficult to develop, due in part to the difficulties in measuring and documenting such a benefit, but also to the lack of validated animal models of disease in which to assess new medical entities’ efficacy prior to evaluation in humans.
Cartilage loss is one of the cardinal pathological features of OA. X-ray joint-space width traditionally has been regarded as a biomarker for cartilage loss in OA. However, for knee OA it lacks statistical power for clinical trials. It has been estimated that assessment of a structure-modifying drug for knee OA using X-ray, is likely to require 2 years treatment in a large population (2001a). In addition, the damage it purports to measure may be as much in the meniscus as in the hyaline cartilage (Hunter et al. 2006). More recently magnetic resonance imaging (MRI) measurements of medial tibiofemoral cartilage thickness and volume have been shown in several centres (Eckstein et al. 2006; Wluka et al. 2006) to decline typically by 4% per annum in OA patients. Reproducibility varies between different centres and in different settings, but coefficients of variation of 2–4% are typical (Eckstein et al. 2006). This suggests that MRI biomarkers of cartilage loss can be used with adequate statistical power to test agents that delay or prevent cartilage loss in small clinical studies of 12 months duration. However, there are no data showing that pharmacologic intervention can affect this biomarker of cartilage loss.
We previously have reported our ability to acquire 3D MR image and segmentation of guinea pig knee cartilage in this spontaneous model of OA (Tessier et al. 2003). These results indicate this method to provide a robust and reproducible intra-animal assessment of the longitudinal loss of cartilage from the medial tibial plateau.
Recently, the tetracycline antibiotic, doxycycline, in a randomized, placebo-controlled, double-blind trial reported by Brandt et al., showed some modest clinical benefit and slowed the rate of joint space narrowing (JSN) in knees with established OA (Brandt et al. 2005). The mechanism of action is believed to be in part mediated by the inhibition of metalloproteinase activity as an ex vivo analysis of cartilage taken from hip OA patients dosed for 5 days with doxycycline prior to arthroplasty indicated reduction of both collagenase and gelatinase activity (Smith et al. 1998).
The disease-modifying effect of doxycycline has been assessed in the Dunkin Hartley guinea pig spontaneous OA model. Under a prophylactic regimen doxycycline administered in chow, did not alter cartilage destruction as assessed by histological and biochemical end-points (de Bri et al. 1998); however, when administered orally for 12 or 18 months it has been shown to be effective in protecting cartilage pathology and osteopyhtosis (Greenwald 1994).
Bendele et al. (1996) published that diacerhein protected against cartilage damage in this guinea pig model. This was subsequently followed by the ECHODIAH clinical trial (Dougados et al. 2001), which reported that, among completers, diacerhein slowed joint space loss in humans with hip OA. Thus, the guinea pig model can predict results in a clinical trial of a putative disease-modifying osteoarthritic drug (DMOAD) in humans and furthermore indicates that this model shares similar risk factors (e.g. age-related OA; incidence of the disease is increased by body weight, mechanical load and posture) and pathological features (e.g. loss of cartilage, followed by osteophyte formation) to humans (Bendele & Hulman 1988; Wei et al. 1998; Bendele & Hulman 1991).
Given the need to improve our clinical assessment of cartilage loss in human OA and the effect of disease-modifying agents on this, we sought to investigate the therapeutic chondro-protective ability of doxycycline in the Dunkin Hartley guinea pigs spontaneous OA model using a MRI biomarker of medial tibial cartilage volume (MT.VC). A secondary aim of this study was to confirm if doxycycline's mode of action was related to suppression of gelatinase and collagenase activity by matrix metalloproteinases (MMPs) as observed in human OA tissue (Brandt 1995). If disease modification via an inhibition of gelatinase or collagenase activity with this agent could be demonstrated, this would also help to strengthen the validity of the model and therefore provide greater confidence in developing novel pharmacological agents targeted towards the degenerative pathway.
Materials and methods
Animals
All work was performed in full compliance with licenses issued under the UK animals (Scientific Procedures) Act, 1986 following local ethical committee review. Thirty-one male Dunkin Hartley guinea pigs (Harlan, Bicester, UK) were entered into the study at 9 months of age (900–1300 g), were housed either singly or in pairs with age-matched mates and were allowed food and water ad libitum.
MR image acquisition and processing
Image acquisition and cartilage segmentation measurements were performed as described previously (Tessier et al. 2003). MR images of the left knee were acquired under halothane inhalation anaesthesia (3% v/v induction, 1% v/v maintenance) 7 days pre- and 66 days postdosing. Animals were placed on a polymethylmethacrylate platform with the left hind leg extending through a radio-frequency coil. To prevent motion, the footpad was secured to a secondary lower platform by means of a soft strap. Respiration was monitored using a transducer linked to a water-filled balloon placed under the abdomen. Temperature was monitored by rectal probe and maintained at 38 °C ± 0.1 by a continuous flow of thermostatically heated air.
Postimaging, guinea pigs were placed on a warm mat until recovery was complete. Imaging was carried out using a spectrometer operating at 200 MHz (Inova, Varian, Palo Alto, CA USA), a 400-mm diameter horizontal bore 4.7 T magnet equipped with room temperature shim coils and 155 mm bore gradient coils (Oxford Instruments, Oxford, UK) providing gradients up to 200 mT/m, a double-balance-matched 30 mm diameter copper sheet solenoid, 10 mm in length constructed in this laboratory (180° pulse length = 50 μs at 50 W, coil unloaded). Following pilot scans, spoiled 3D gradient echo (TR 75 ms, TE 2.7 ms, nutation π/6) MRI was acquired with fat saturation. The image matrix was 512 × 192 × 96 points with a field of view (30 × 30 × 30 mm), zero-filled to 512 × 256 × 128, providing an effective image resolution of 0.06 mm through the cartilage thickness × 0.18 mm anterior–posterior × 0.23 mm medial–lateral. Manual segmentation of the cartilage of the tibial plateau was performed on sagittal slices covering the medial side only, because in this animal model cartilage lesions develop preferentially at this location. Our previous study had identified the main source of variability as inter-animal and inter-operator variability. For this study, we used one segmenter, blinded to time-point and treatment but not to subject number. Slices at one time-point were matched as close as possible to those obtained at subsequent time-points based on preselected image references. For any individual animal, the number of slices analysed was equal for all time-points (range 7–9 depending on the overall size of the tibial plateau). The end-point was thus MT.VC (in the nomenclature of Eckstein et al. 2006).
Doxycycline administration
Guinea pigs were anaesthetized with approximately 3% (v/v) halothane 7 days post first image acquisition. An area of approximately 10 × 8 cm2 down the length of the lower back and flank was shaved and the area swabbed with a diluted solution of pevidine (National Vet Supplies, Stoke-on-Trent, UK) to remove any loose hair and debris. Under aseptic conditions, a small incision was made through the skin in the lumbar region of the back parallel to the spine using a scalpel blade. A pair of blunt scissors was inserted into the incision to provide a subcutaneous channel and an osmotic mini-pump (Alzet 2 ML 4) containing vehicle [20% v/v DMSO (Fisher chemicals, Loughborough, UK) 60% v/v polyethylene glycol 400 (Fisher chemicals), 20% H2O, (n = 13)] or doxycycline (doxycycline HCl; Sigma Aldrich, Gillingham, UK; 0.6 mg/kg/day, n = 9, 3.0 mg/kg/day, n = 9) was inserted in the subcutaneous layer.
Wounds were sealed using 2/0 Vicryl absorbable braided polyglactin-coated suture thread with a straight needle (60 mm cutting/0.84 mm diameter, Vet Tech Solutions, Congleton, UK). Implanted guinea pigs were inspected daily to check that surgery wounds were healing satisfactorily, and that there were no signs of infection present at the surgical site. Empty mini-pumps were removed under inhalation anaesthetic [3% (v/v) halothane] 33 days later and replaced with a full osmotic mini-pump at a different insertion site as described above.
Pharmacokinetics
Blood samples were taken from the saphenous vein of non-anaesthetized doxycycline-dosed animals at 1 and 28 days using capillary action into lithium–heparin-coated tubes (Sarstedt, Leicester, UK). A bleed under terminal anaesthesia at 66 days post mini-pump implantation and post the second MRI was taken via cardiac heart puncture into lithium–heparin-coated tubes. Samples were centrifuged at 12,000 g for 3 min and the supernatant (serum) was frozen at −20 °C until analysed.
Determination of MMP activity in cartilage
At study termination, MTP cartilage was removed from both knees of vehicle- and doxycycline- (3 mg/kg/day) treated animals. Cartilage was dissected at the junction of the calcified cartilage zone and the underlying bone, and then extracted overnight in phosphate-buffered saline containing a broad spectrum protease inhibitor cocktail [4-(2-aminoethyl) benzenesulfonyl fluoride, pepstatin A, E-64, bestatin, leupeptin and aprotinin, excluding EDTA, (Sigma Aldrich)]. The fluorokine Assay (R&D Systems, Europe, Ltd, Abingdon, UK) was used to quantify MMP-13, MMP-9, MMP-8 and MMP-1 enzyme activity as follows. Samples and standards were applied to a microtitre plate precoated with monoclonal antibody specific for the MMP and unbound material was removed by washing. Amino phenyl mercuric acid (APMA) (final concentration 20 mm) was added to all standards and samples to activate endogenous MMPs, because pilot studies had shown that endogenous MMP levels in guinea pig cartilage were below the limits of detection in the absence of APMA (data not shown). Following MMP activation with APMA, the fluorogenic substrate linked to a quencher molecule was added for between 17 and 20 h. Active enzyme cleaves the peptide linker between the fluorophore and the quencher molecule, resulting in a fluorescent signal proportional to the amount of enzyme activity in the sample (excitation λ 320 nm, emission λ 405 nm). A standard curve was created using computer software (versamax, Molecular Devices, Sunnyvale, California, USA) and the sample concentrations calculated. Total MMP-13, 1, 8 and 9 activities were expressed as ng/ml per 200 μg protein, measured by BCA protein assay (Pierce, Rockford, IL, USA).
Statistical analysis
Log cartilage MT.VC was analysed. Baseline MT.VC values were initially used in a covariance analysis which verified that the relationship with baseline was similar for each doxycycline group and vehicle controls (P > 0.05). The relationship was so strong that assessment of compound effects were done on the change in log MT.VC from baseline using a Student's t-test (two-sided) on a pooled between animal variability from the anova allowing for groups. The MMP activities were analysed using a Student's t-test, (two-sided) assuming unequal variability between vehicle and doxycycline groups.
Results
Animal health and welfare
All animals completed the study except for one guinea pig, which died under anaesthesia prior to first MR image acquisition. Body weights commonly decreased by 5–10% postimaging and after mini-pump implant, but generally recovered within a few days. Body weights at termination were similar to prestudy weights (± 10%). No adverse effects of doxycycline administration were observed.
MRI
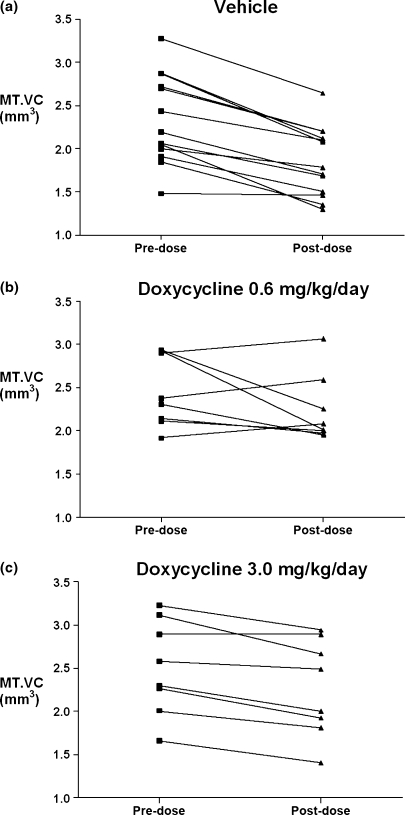
The mean pre- and postdose MT.VC and % changes from predose are shown in Table 1. The mean MT.VC loss for vehicle-treated animals was 20.5% (P < 0.0001, compared with predose volume). With the exception of one animal, all vehicle animals lost MTP cartilage (Figure 1(a)). The doxycycline (0.6 mg/kg/day) group lost 8.6% (P < 0.05, compared with vehicle). Within this group, there was greater variation. Five animals lost cartilage, however, three animals gained cartilage (Figure 1(b)). The group treated with 3.0 mg/kg/day doxycycline lost 10.0% (P < 0.05, compared with vehicle), however, there was less variation with one animal maintaining and seven animals losing MT.VC, but to a lesser extent than the vehicle-treated group.
Table 1.
Pre and postdose MT.VC (mm3) and % loss from predose volume (mean, 95% CI)
| Dose | ||||
|---|---|---|---|---|
| mg/kg/day | n | Predose MT.VC | Postdose MT.VC | % loss from pre |
| Vehicle | 13 | 2.288 (1.998–2.443) | 1.818 (1.594–2.075) | 20.5 (25.69–15.1) |
| 0.6 | 8 | 2.423 (2.786–0.795) | 2.215 (2.537–0.953) | 8.6 (−20.6 to −5.3)* |
| 3.0 | 9 | 2.582 (1.100–0.736) | 2.323 (1.338–0.858) | 10.0 (13.9–6.0)* |
P < 0.05 compared with vehicle treated.
Figure 1.
Individual animal MT.VC (medial tibial cartilage volume) pre- and postdose for vehicle (a), 0.6 mg/kg/day doxycycline (b) and 3.0 mg/kg/day doxycycline (c) treated animals.
Pharmacokinetics
Blood was taken from the saphenous vein of representative animals 1, 28 and 66 days after doxycycline administration and serum concentrations determined. Exposure at 3 mg/kg/day was reasonably consistent between days 1, 28 and 66 (Table 2, mean 138 ng/ml across all days) and at 0.6 mg/kg (Table 2, mean 55.8 ng/ml mean days 1 and 28) but was below the limit of detection at termination (day 66) (Table 2).
Table 2.
Mean serum doxycycline concentrations on days 1, 28 and 66. Mean nm (SEM, n)
| Dose (mg/kg/day) | Day 1 | Day 28 | Day 66 |
|---|---|---|---|
| 0.6 | 61.2 (10.1, 4) | 50.4 (1) | <lod |
| 3.0 | 107.6 (9.0, 9) | 166.7 (11.3, 9) | 140.5 (2) |
lod, limit of detection.
MMP activity
No endogenous MMP activity was detected in cartilage extracts from either vehicle- or doxycycline-treated animals, which indicated active enzymes were absent or below the limits of detection. However, on activation of the pro-enzyme forms, doxycycline ablated MMP-13 and MMP-8 levels (100% reduction), reduced MMP-9 activity (65% reduction) but had minimal effect on MMP-1 activity (24% reduction) (Table 3). Both MMP-2 and MMP-9 were detected by gelatin zymography in both vehicle- and doxycycline-treated animals, with a trend towards decreased levels of both gelatinases in doxycycline cartilage extracts (result not shown).
Table 3.
Cartilage MMP activities from tibial cartilage extracts
| MMP ng/ml/ 200 μg | ||
|---|---|---|
| protein | ||
| MMP | Treatment (n) | Mean (SEM) |
| MMP-1 | Vehicle (11) | 0.33 (0.05) |
| Doxycycline (9) | 0.25 (0.02) | |
| MMP-13 | Vehicle (11) | 0.74 (0.16) |
| Doxycycline (10) | 0.03 (0.01)*** | |
| MMP-8 | Vehicle (5) | 0.41 (0.08) |
| Doxycycline (5) | <lod*** | |
| MMP-9 | Vehicle (5) | 0.37 (0.04) |
| Doxycycline (5) | 0.13 (0.06)*** |
Mean cartilage MMP activities from tibial cartilage extracts analysed by fluorokine ELISA.
P < 0.001 compared with vehicle treated.
Discussion
Given the need to improve our clinical assessment of cartilage loss in human OA and develop DMOADs, we sought to investigate the therapeutic chondro-protective ability of doxycycline in the Dunkin Hartley guinea pig spontaneous OA model using an MRI biomarker (MT.VC) and to confirm that doxycycline's mode of action was related to suppression of gelatinase and collagenase activity by MMPs.
No animal model can faithfully recapitulate every aspect of human disease, but models can offer valuable insights into the mechanisms underlying human pathology. In addition, they can be used to predict the response to drug intervention trials in humans; although the predictive value is dependent on the degree of validation of the model, e.g. whether therapies with proven human clinical efficacy are also effective in the model. Validation of such models enables exploration of other translational activities, for instance, investigation of the utility/refinement or identification of new outcome measures, especially those for disease modification.
We report that therapeutic administration of the tetracycline antibiotic, doxycycline, can slow the rate of cartilage volume loss, as assessed by in MRI, in the guinea pig model of spontaneous OA. This effect is associated in part by the reduction of activity, specifically MMPs 8 and 13 and to a lesser extent MMP-9. These results are of importance as they add to the existing literature that supports that the guinea pig spontaneous model of OA is an appropriate model for assessing the impact of potential disease-modifying osteoarthritic drugs. This includes the observations that pathological changes in bone and cartilage follow a similar time course as seen in human OA (Dieppe et al. 1993; Buckland-Wright 2004) and that risk factors that contribute to human disease progression in OA also impact disease progression in this model. These include weight and diet restriction (Bendele & Hulman 1991), ligament laxity (Quasnichka et al. 2006) and elevated subchondral bone turnover (Anderson-MacKenzie et al. 2005; Huebner et al. 2002).
de Bri et al. (1998) examined doxycycline in the guinea pig model of OA noting in contrast to the modified tetracycline analogue (CMT-7) and the results presented in this paper; it did not appear to offer any protection to cartilage loss when assessed by histological techniques. Protocol differences between our studies could contribute to the differences in efficacy including the age of the animals at study start, the route of compound administration and the outcome measure.
We utilized animals at 9 months of age for two reasons: first, animals at this age are skeletally mature as evidence by the degree of closure of the growth plate, which minimizes confound effects on compounds which might impact growing bones; secondly, the maximal rate of cartilage loss occurs between 9 and 12 months of age (Tessier et al. 2003). Animals in the de Bri et al.'s (1998) report were treated from a much younger age. It is possible that collagenase and gelatinase activity may be less important than other proteinases, such as the aggrecanase, in the disease progression at this time. In fact we have preliminary data that indicates that aggrecanase levels and aggrecan-specific neo-epitope generated upon cleavage of aggrecan by aggrecanase are increased early in this model (2001b) and maximal cleavage precedes the period of rapid cartilage loss utilized in this study (Flannelly et al. 2006). However, it is not known whether doxycycline or indeed CMT-7 modulate aggrecanase activity as well as metalloproteinase activity.
de Bri et al. (1998) administered doxycycline by incorporating it into the animal's chow. Variable absorption and intake may have contributed to lack of chondroprotective effect in their study. We chose to administer doxycycline by continuous subcutaneous infusion (mini-pumps) for two reasons: first, oral dosing guinea pigs twice a day can incur additional stress upon the animal, in addition to the stress arising from blood sampling and repeat MRI scans, under anaesthesia; and secondly, infusion by osmotic mini-pump allows in principle, greater controlled release at steady-state exposures. This enabled us to understand more easily the minimum exposure required for structural modification.
We chose to administer doses of 0.6 and 3.0 mg/kg/day as these dose levels were in the range of the dose used in the clinical study (e.g. 100 mg bid which equates to approximately 2.8 mg/kg/day for a 70 kg individual) (Brandt et al. 2005). However, these doses do not indicate whether we achieved the same exposure (total or unbound) in our study because of possible potency, protein binding and pharmacokinetic difference between guinea pig and human. We found that exposure at 3 mg/kg/day was reasonably consistent between days 1, 28 and 66 (Table 1, mean 138 ng/ml across all days), and at 0.6 mg/kg (Table 1, mean 55.8 ng/ml mean days 1 and 28), the concentration was below the limit of detection at termination (day 66). As de Bri et al. (1998) did not report any blood concentrations, it is difficult to interpret how drug exposures in their study compared with our own findings.
We also attempted to demonstrate a dose-related effect of doxycycline in this model. Although there was a fivefold difference in dose, there was only an approximate 2.5-fold difference in serum concentrations between doses. This is an insufficient difference to discriminate exposure dependant differences in the amount of cartilage lost.
In this study, we have used a robust methodology (MRI), to quantify intra-animal cartilage loss from medial tibial cartilage and to assess the effect of the clinically active doxycycline on this loss as contrasted with the use of histology used by de Bri et al. (1998). We observed the amelioration of cartilage loss by approximately 42% and 49% (0.6, 3.0 mg/kg/day respectively), which is consistent with that (100 mg) shown to reduce the rate of JSN in knees with established OA (Brandt et al. 2005). In addition. we were able to detect loss in group sizes as small as 8 over a short time period (66 days), which is advantageous in study design and execution, as well as contribute in the reduction, refinement and even eventual replacement of animal use. Analysing intra-animal MT.VC change by MRI allows us to assess individual animal disease progression and response to treatment (Figure 1). All vehicle-treated animals, with the exception of one animal, lost cartilage (Figure 1(a)); however, this animal had the lowest predose MT.VC, which suggested this animal had advanced disease and had reached maximal medial tibial cartilage loss. Individual animal response to 0.6 mg/kg/day doxycycline treatment was variable as we observed animals that lost cartilage and in three instances, animals which actually gained MT.VC (Figure 1(b)). In these instances, it may be that in the presence of doxycycline, the balance between degradative processes and anabolic reparative processes are shifted in favour of matrix synthesis. Individual animal responses to the 3.0 mg/kg/day dose (Figure 1(c)) were less variable, consistent with higher exposures. While X-ray joint-space width traditionally has been regarded as the biomarker for cartilage loss in OA, these results suggest that MRI biomarkers of cartilage loss may be used with adequate statistical power to test agents that delay or prevent cartilage loss in small clinical studies of short duration.
Doxycycline has been reported to have pleiotropic effects in vitro (Zetner & Rothmueller 2002; Yu et al. 1991; Amin et al. 1996; Lotz 1999) but has no direct inhibitory activity against MMPs, when tested in vitro using a synthetic substrate (data not shown). To gain further understanding of the mechanism of doxycycline mode of action in the guinea pig model of OA and to corroborate previous data in human tissue demonstrating reduction of gelatinase and collagenase activity using in vitro and ex vivo human cartilage (Smith et al. 1998), medial tibial plateau cartilage was harvested from guinea pigs at study termination and assayed for MMP activity. The amount of tissue was limited and therefore analysis was restricted to the collagenases (MMP-1, MMP-8, MMP-13) and one gelatinase (MMP-9). As both doses had resulted in similar levels of protection from cartilage loss, MMP analysis was performed only on the vehicle-treated and 3.0 mg/kg/day treated groups.
No endogenous MMP activity was detected in cartilage extracts from either vehicle- or doxycycline-treated animals, which indicated active enzymes were below the limits of detection. Upon activation of the pro-enzymes with APMA, measurable levels of MMP-13, MMP-8, MMP-1 and MMP-9 could be detected from vehicle-treated animals, in contrast only MMP-1 and MMP-9 activity was detected in cartilage extract from doxycycline-treated animals. Doxycycline treatment had completely abolished MMP-13 and MMP-8, and partially reduced MMP-9 levels. These data suggest that in the guinea pig, doxycycline possibly exerts its effect through selective inhibition of the collagenases, MMP-13 and MMP-8 but not MMP-1. This is slightly different to the effect of doxycycline in human OA chondrocytes, where pharmacologically relevant doses of doxycycline have been shown to reduce mRNA levels of all three collagenases (Shlopov et al. 1999). Because of insufficient cartilage, it was not possible to study the impact of doxycycline treatment on mRNA expression in this study. That MMP-1 was not inhibited in the guinea pig may be a species effect, a differential response from cartilage compared with chondrocytes, or in vivo vs. and in vitro effect. The role of MMP-13 (Mitchell et al. 1996; Billinghurst et al. 1997) in the pathogenesis of clinical OA previously has been reported. In addition, partial inhibition of MMP-9 activity suggests this enzyme may also play a role in cartilage breakdown, indeed, studies in MMP-9 knock-out mice suggest that this gelatinase plays a key role in inflammatory joint disease (Itoh et al. 2002). Finally, complete abrogation of MMP-13 and MMP-8 was evident but this did not result in complete protection from cartilage loss. This would suggest that additional biochemical mechanisms, e.g. aggrecanases (Glasson et al. 2005) might have a role in the development of OA in this model; however, activity of doxycycline against these enzymes was not determined.
Conclusions
The protective effect of doxycycline on MT.VC loss in the guinea pig correlates with the clinical data on reduction of JSN in the index knee of patients with OA. These data support the concept that doxycycline treatment, possibly through the inhibition of MMPs is of therapeutic benefit in the guinea pig model. Preclinical and clinical data on the same therapeutic agent, using the same translational methodology (MRI), strengthens the utility of the guinea pig model in the discovery of novel DMOADs.
Acknowledgments
The authors gratefully acknowledge Pauline Gargan and Brian Middleton for pharmacokinetic and statistical analysis respectively. We would also like to thank Nicola Greenwood for technical assistance.
References
- Amin AR, Attur MG, Thakker GD, et al. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14014–14019. doi: 10.1073/pnas.93.24.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson-MacKenzie JM, Quasnichka HL, Starr RL, et al. Fundamental subchondral bone changes in spontaneous knee osteoarthritis. Int. J. Biochem. Cell Biol. 2005;37:224–236. doi: 10.1016/j.biocel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Bendele AM, Hulman JF. Spontaneous cartilage degeneration in guinea pigs. Arthritis Rheum. 1988;31:561–565. doi: 10.1002/art.1780310416. [DOI] [PubMed] [Google Scholar]
- Bendele AM, Hulman JF. Effects of body weight restriction on the development and progression of spontaneous osteoarthritis in guinea pigs. Arthritis Rheum. 1991;34:1180–1184. doi: 10.1002/art.1780340916. [DOI] [PubMed] [Google Scholar]
- Bendele AM, Bendele RA, Hulman JF, et al. [Beneficial effects of treatment with diacerhein in guinea pigs with osteoarthritis] Rev. Prat. 1996;46:S35–S39. [PubMed] [Google Scholar]
- Billinghurst RC, Dahlberg L, Ionescu M, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J. Clin. Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt KD. Modification by oral doxycycline administration of articular cartilage breakdown in osteoarthritis. J. Rheumatol. Suppl. 1995;43:149–151. [PubMed] [Google Scholar]
- Brandt KD, Mazzuca SA, Katz BP, et al. Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2005;52:2015–2025. doi: 10.1002/art.21122. [DOI] [PubMed] [Google Scholar]
- de Bri E, Lei W, Svensson O, et al. Effect of an inhibitor of matrix metalloproteinases on spontaneous osteoarthritis in guinea pigs. Adv. Dent. Res. 1998;12:82–85. doi: 10.1177/08959374980120012601. [DOI] [PubMed] [Google Scholar]
- Buckland-Wright C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthritis Cartilage. 2004;12(Suppl. A):S10–S19. doi: 10.1016/j.joca.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann. Rheum. Dis. 1993;52:557–563. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougados M, Nguyen M, Berdah L, Mazieres B, Vignon E, Lequesne M. Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial. Evaluation of the chondromodulating effect of diacerein in OA of the hip. Arthritis Rheum. 2001;44:2539–2547. doi: 10.1002/1529-0131(200111)44:11<2539::aid-art434>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14(Suppl. A):A46–A75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Elders MJ. The increasing impact of arthritis on public health. J. Rheumatol. Suppl. 2000;60:6–8. [PubMed] [Google Scholar]
- Flannelly JK, Day CL, Read SJ, Bowyer J. P64 characterisation of temporal changes of aggrecanase-dependant proteoglycan loss in the guinea pig model of spontaneous OA. Osteoarthritis Cartilage. 2006;14:S48–S49. [Google Scholar]
- Glasson SS, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- Greenwald RA. Treatment of destructive arthritic disorders with MMP inhibitors. Potential role of tetracyclines. Ann. N. Y. Acad. Sci. 1994;732:181–198. doi: 10.1111/j.1749-6632.1994.tb24734.x. [DOI] [PubMed] [Google Scholar]
- Huebner JL, Hanes MA, Beekman B, TeKoppele JM, Kraus VB. A comparative analysis of bone and cartilage metabolism in two strains of guinea-pig with varying degrees of naturally occurring osteoarthritis. Osteoarthritis Cartilage. 2002;10:758–767. doi: 10.1053/joca.2002.0821. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Zhang YQ, Tu X, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006;54:2488–2495. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]
- Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J. Immunol. 2002;169:2643–2647. doi: 10.4049/jimmunol.169.5.2643. [DOI] [PubMed] [Google Scholar]
- Lotz M. The role of nitric oxide in articular cartilage damage. Rheum. Dis. Clin. North Am. 1999;25:269–282. doi: 10.1016/s0889-857x(05)70067-3. [DOI] [PubMed] [Google Scholar]
- Mitchell PG, Magna HA, Reeves LM, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quasnichka HL, Anderson-MacKenzie JM, Bailey AJ. Subchondral bone and ligament changes precede cartilage degradation in guinea pig osteoarthritis. Biorheology. 2006;43:389–397. [PubMed] [Google Scholar]
- Shlopov BV, Smith GN, Jr, Cole AA, Hasty KA. Differential patterns of response to doxycycline and transforming growth factor beta1 in the down-regulation of collagenases in osteoarthritic and normal human chondrocytes. Arthritis Rheum. 1999;42:719–727. doi: 10.1002/1529-0131(199904)42:4<719::AID-ANR15>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Smith GN, Jr, Yu LP, Jr, Brandt KD, Capello WN. Oral administration of doxycycline reduces collagenase and gelatinase activities in extracts of human osteoarthritic cartilage. J. Rheumatol. 1998;25:532–535. [PubMed] [Google Scholar]
- Tessier JJ, Bowyer J, Brownrigg NJ, et al. Characterisation of the guinea pig model of osteoarthritis by in vivo three-dimensional magnetic resonance imaging. Osteoarthritis Cartilage. 2003;11:845–853. doi: 10.1016/s1063-4584(03)00162-6. [DOI] [PubMed] [Google Scholar]
- Wei L, de Bri E, Lundberg A, Svensson O. Mechanical load and primary guinea pig osteoarthrosis. Acta Orthop. Scand. 1998;69:351–357. doi: 10.3109/17453679808999046. [DOI] [PubMed] [Google Scholar]
- Wluka AE, Forbes A, Wang Y, Hanna F, Jones G, Cicuttini FM. Knee cartilage loss in symptomatic knee osteoarthritis over 4.5 years. Arthritis Res. Ther. 2006;8:R90. doi: 10.1186/ar1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LP, Jr, Smith GN, Jr, Hasty KA, Brandt KD. Doxycycline inhibits type XI collagenolytic activity of extracts from human osteoarthritic cartilage and of gelatinase. J. Rheumatol. 1991;18:1450–1452. [PubMed] [Google Scholar]
- Zetner K, Rothmueller G. Treatment of periodontal pockets with doxycycline in beagles. Vet. Ther. 2002;3:441–452. [PubMed] [Google Scholar]