Abstract
Naturally occurring coumarins possess anti-carcinogenic activities in part by inducing carcinogen-detoxifying enzymes glutathione S-transferase (GST) and/or NAD(P)H quinone oxidoreductase (NQO1). Our goal was to determine whether citrus coumarins induce hepatic GST and/or NQO1 via activation of Nrf2 and the antioxidant response element. First, HepG2 cells stably transfected with the ARE and a green fluorescent protein (GFP) reporter were treated with increasing concentrations of coumarins and compared to positive controls. tert-butylhydroquinone (TBHQ) and oltipraz increased GFP fluorescence, as did coumarin, limettin, auraptene, imperatorin, and 7,8-benzoflavone, suggesting that they activate the ARE, whereas isopimpinellin did not increase GFP fluorescence. Next, the effects of orally-administered coumarins and oltipraz on hepatic GST and NQO1 activities were compared in Nrf2 knockout mice or Nrf2 heterozygous mice exhibiting the wild-type phenotype. Oltipraz, auraptene, imperatorin, isopimpinellin, and auraptene all significantly increased liver cytosolic GST activities in Nrf2 heterozygous mice. This effect was abrogated in Nrf2(−/−) mice dosed with oltipraz, attenuated in mice Nrf2(−/−) mice treated with auraptene and imperatorin, and still significant in Nrf2(−/−) mice treated with isopimpinellin. Of these compounds, only isopimpinellin significantly increased liver cytosolic NQO1 activities, and this effect was not attenuated in Nrf2(−/−) mice. These results strongly suggest that imperatorin and auraptene induce murine liver cytosolic GST activities via the Nrf2/ARE mechanism. Although structurally similar, isopimpinellin did not appear to activate HepG2-ARE-GFP and the Nrf2 knockout mouse study suggests that isopimpinellin may induce GST and NQO1 via additional mechanisms.
Keywords: Coumarins, Antioxidant Response Element, Nrf2, GST, Chemoprevention, Natural Products
Introduction
Naturally occurring coumarins, including those found in citrus fruits, possess a range of anticarcinogenic activities in rodent models. We previously demonstrated that orally administered coumarin, limettin, imperatorin and isopimpinellin blocked 7,12-dimethylbenz[a]anthracene (DMBA) DNA adduct formation in mouse mammary glands (Prince et al., 2006). Two mechanisms for this effect have been proposed: First, the linear furanocoumarins imperatorin and isopimpinellin suppress cytochrome P450 1A1 and 1B1 mediated bioactivation of DMBA into electrophilic diol epoxides that bind to DNA and lead to mutations (Kleiner et al., 2002a; Kleiner et al., 2002b). Second, a structurally diverse range of coumarins (both simple and furanocoumarin type) induce the activities of carcinogen-detoxifying enzymes such as glutathione S-transferase (GST) and NAD(P)H quinone oxidoreductase (NQO1) (Hayes and Pulford, 1995; Kleiner et al., 2001; Kleiner et al., 2008; Murakami et al., 2000). Moreover, coumarin (0.05% in the diet) inhibited aflatoxin B1 initiated hepatic preneoplastic foci and decreased the number and size of aflatoxin B1 induced tumors of rats (Kelly et al., 2000). This chemopreventive activity was attributed to the increased mRNA expression, protein expression, and enzyme activities of aflatoxin B1 aldehyde reductase (AFAR), GSTA5, GSTP1, and NQO1 in rat liver (Kelly et al., 2000). GST induction facilitates detoxification of polycyclic aromatic hydrocarbons (Jaiswal, 2000) and aflatoxin (Kelly et al., 2000), and induction of NQO1 can prevent the formation of free radicals (Jaiswal, 2000).
As seen with many classic P450 inhibitors, imperatorin and isopimpinellin not only suppress P450 activities, but increase hepatic P450 expression, particularly P450 2B9/10 and 3A11, and increased liver weight following repeated oral administration to mice (Kleiner et al., 2001; Kleiner et al., 2008). On the other hand, little effect on P450 expression was observed with the simple coumarins, coumarin, limettin, and auraptene (Kleiner et al., 2008). Use of transfection reporter assays demonstrated that isopimpinellin interacts with both the pregnane X receptor (PXR) and the constitutive androstane receptor (CAR) (Kleiner et al., 2008). Furthermore, the induction of hepatic Cyp2b10 mRNA by isopimpinellin was attenuated in CAR(−/−) knockout mice, whereas Cyp3a11, GSTa1 and GSTp1 induction persisted (Kleiner et al., 2008). These results suggested that induction of Cyp2b10 mRNA by isopimpinellin was mediated by CAR, but that induction of Cyp3a11, GSTa1, and GSTp1 occur via other mechanisms.
It is known that GST induction can occur through different mechanisms, such as via the xenobiotic response element (XRE) or the antioxidant response element (ARE). Regulatory elements in the 5′-flanking region of the GST Ya subunit gene contain a core sequence for the ARE (Rushmore et al., 1990; Rushmore et al., 1991; Rushmore and Pickett, 1990). It was shown that hydrogen peroxide and tert-butylhydroquinone transcriptionally activated the Ya subunit gene in HepG2 cells transiently transfected with the ARE sequence (Rushmore et al., 1991). Monofunctional inducers elevate Phase II enzymes via the ARE without significantly elevating Phase I enzyme activities [reviewed in (Hayes and Pulford, 1995)]. Many monofunctional inducers possess an electrophilic center and are Michael reaction acceptors (Hayes and Pulford, 1995). Nuclear factor-erythroid 2-related factor 2 (Nrf2) is a member of the Cap’n’Collar family of basic region leucine zipper (bZIP) transcription factors (Itoh et al., 1997). The ARE is a 41 bp element in the 5′-flanking region of the rat GST Ya gene, that is positively regulated by Nrf2 (Itoh et al., 1997). Nrf2 activity is repressed by Keap 1 (Kelch-like ECH-associated protein). The Keap1/Nrf2 complex is a cytoplasmic sensor system for enzyme induction by electrophiles and oxidants [reviewed in (Zhang, 2006)]. Evidence has shown that inducers react covalently with thiols on the Keap1/Nrf2 complex (Dinkova-Kostova et al., 2002). Upon release from Keap1, Nrf2 translocates to the nucleus where Nrf2 heterodimerizes with other transcription factors and binds to the 5′-upstream regulatory regions of the ARE to induce Phase II genes [discussed in (Dinkova-Kostova et al., 2002)]. Mice that have a functional deletion in the nrf2 gene have lower basal and inducible GST and other Phase II enzymes (McMahon et al., 2001), are more susceptible to toxic and carcinogenic compounds, and are less protected by the anticarcinogenic effects of Phase II enzyme inducers such as oltipraz [reviewed in (Dinkova-Kostova et al., 2002)]. It has been previously demonstrated that butylated hydroxyanisole induces GST in liver of mice heterozygous for Nrf2 but not in mice with homozygous deletions of Nrf2 (Itoh et al., 1997). Coumarin, 3-hydroxycoumarin, 7-hydroxycoumarin, limettin, and angelicin (an angular furanocoumarin) were previously tested for their ability to induce GST and NQO1 in either Nrf2(+/+) wild-type or Nrf2(−/−) mutant mouse small intestine when administered in the diet (McMahon et al., 2001). Although the basal and inducible levels of GST and NQO1 were lower in the Nrf2(−/−) mutant mice, these coumarins were still able to induce GST and NQO1.
In light of these observations, the goal of the current study was to examine a broader range of naturally occurring coumarins for their abilities to activate the ARE and to induce GST and NQO1. Two approaches were used: first, an assay has been previously developed in which human hepatoma HepG2 cells were stably transfected with a reporter gene consisting of green fluorescent protein (GFP) under the transcriptional control of a thymidine kinase (TK) promoter adjacent to concatamerized ARE regulatory elements (Zhu and Fahl, 2000). This assay was used to identify whether a panel of both simple and linear furanocoumarins activate the ARE. Next, the effects of both simple and linear furanocoumarins were tested for their abilities to induce hepatic GST, NQO1, and increased liver weight in Nrf2(−/−) mice.
Materials and Methods
Chemicals and reagents
Coumarin and glutathione (reduced form, GSH) were obtained from ICN Biomedicals, Inc. (Aurora, OH). 1-Chloro-2,4-dinitrobenzene (CDNB); 2,6-dichloroindophenolate (DCPIP); 1,2-dichloro-4-nitrobenzene (DCNB), dicoumarol, flavin adenine dinucleotide disodium salt hydrate (FAD), β-nicotinamide adenine dinucleotoide, reduced (NADH), limettin (5,7-dimethoxycoumarin), were purchased from Sigma-Aldrich, Inc. (St. Louis, MO). Bovine serum albumin (BSA, fraction V) was obtained from MP Biomedicals, LLC (Solon, OH). Imperatorin and isopimpinellin were supplied by Indofine Chemical Co., Inc. (Hillsborough, NJ). Auraptene and oltipraz were purchased from LKT Laboratories, Inc. (West St. Paul, MN).
HepG2-ARE-GFP cells
Human hepatoma HepG2 cells stably transfected with TK-GFP/ARE were received as a generous gift from Drs. Ming Zhu and William Fahl (ProCertus BioPharm, Inc. Madison, WI). Cells were maintained on 75 cm2 tissue culture treated flasks from Falcon BD (Franklin Lakes, NJ) with 12 mL growth media [DMEM containing 10% FBS, 4 mM L-glutamine, 0.1 mM non-essential amino acids, 10 mL/L antibiotic antimycotic Mixture (10,000 I.U. penicillin, 10,000 μg/mL streptomycin, 25 μg/mL amphotericin B), and 1 mM sodium pyruvate]. Cells were passaged after attaining 70–80% confluency in 1 X trypsin-EDTA.
For the fluorescence experiments, cells were seeded onto 24-well plates and grown to 70–80% confluency. Cells were treated with simple coumarin (1, 2-benzopyrone), auraptene, limettin, and linear furanocoumarins (imperatorin and isopimpinellin). Tert-butylhydroquinone (tBHQ) was used as a positive control. HepG2 cells were treated with DMSO (vehicle control) or test compounds at 6.25 – 200 μM for 24 h. The cells were stained with ethidium bromide (EtBr) to normalize data by DNA content. Data were expressed as a ratio of the fluorescent intensity of GFP/EtBr in each well.
For the western blot procedures, HepG2 cells were grown to ~70–80% confluency in duplicate 6-well plates. Cells were treated with 25 μM of each compound, or vehicle as the control (0.1% DMSO, v/v) for 24 h. Duplicate wells of cells were combined, harvested using 6x sample buffer (0.25 mL), 10 μL of sample was loaded onto precast 10% acrylamide mini-gels (Bio-Rad Ready Gel, Hercules, CA), and proteins were immunoblotted onto PVDF membranes as previously described (Campbell et al., 2007). Nrf2 protein expression was probed using rabbit anti-Nrf2 as the primary antibody (1:500 dilution, C-20, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and horseradish peroxidase labeled goat anti-rabbit IgG (H+L) as the secondary antibody (1: 10,000 dilution, Thermo Scientific, Rockford, IL). β-actin (used as a loading control) was probed using goat anti-actin (I-19, 1:500 dilution) and horseradish peroxidase labeled donkey anti-goat IgG (1:5000 dilution) as the primary and secondary antibodies, respectively (Santa Cruz Biotechnology, Inc.). Immunoreactive proteins were visualized using Pierce enhanced chemiluminescence substrate (Thermo Scientific). Integrated density of the scanned autoradiograph films was determined using Image J 1.33u software (http://rsb.info.nih.gov/ij/)
Nrf2 knockout mice
All mice were housed in a temperature- and humidity-controlled AAALAC facility with a 12 h light/dark cycle and allowed access to food and water ad libitum. All procedures were approved by the LSUHSC Institutional Animal Care and Use Committee in accordance with NIH guidelines. Nrf2(−/−) mice, originally on an ICR/129sv background, were back-crossed onto an ICR background. Mice were bred and genotyped by PCR using the following primers sequences as previously described (Itoh et al., 1997): Nrf2: 5′-TGGACGGGACTATTGAAGGCTG-3′; Nrf2AS: 5′-GCGGCCTTTTCAGTAGATGGAGG-3′; LacZ: 5′-GCGGATTGACCGTAATGGGATAGG-3′. Initially, mice were bred as Nrf2(+/−) x Nrf2(+/−) crosses. However, only 25% of the offspring result as Nrf2(+/+) wild-type and Nrf2(−/−) knockout mice each, leaving 50% of the mice as Nrf2(+/−). Therefore, initial studies were performed to compare Nrf2 wild-type to Nrf2 heterozygous mice (data not shown). Because similar results were obtained in GST induction by imperatorin and isopimpinellin, subsequent studies were done using Nrf2(+/−) heterozygous mice as the wild-type controls, and mice were bred as Nrf2(+/−) × Nrf2(−/−) crosses (resulting in ~50% yield each of Nrf2(+/−) and Nrf2(−/− mice).
For experiments, male and female mice (7–10 weeks of age) were divided into groups of 3–4 per genotype per experimental group. For all but the oltipraz study, each experiment was performed twice and the results were combined. Mice were fed AIN76A semi-purified diet (Dyets, Bethlehem, PA) pellets 1 week prior to the study and maintained on AIN76A for the duration of the study to minimize lot-to-lot variations of chow diet. Mice were treated with a single dose of oltipraz (500 mg/kg bw) in 1% cremophor/25% glycerol and compared to vehicle control (1% cremophor/25% glycerol). In the other experiments, mice were treated with auraptene, imperatorin, and isopimpinellin (150 mg/kg bw in 0.1 ml/25 g bw corn oil, by oral gavage) once daily for 4 consecutive days. Control mice received vehicle only. Mice were sacrificed by cervical dislocation at 24 h after the last dose.
Preparation of liver cytosol
At 24 h after the final dose, livers were removed, weighed, minced, and homogenized at a 1:5 dilution (v/v) on ice in 0.05 M Tris buffer, pH 7.4 containing 0.25 M sucrose using a tissue homogenizer (Omni International Inc., Marietta, GA). All tissue handling after this point was conducted at 4 °C. Liver cytosol-enriched fractions were isolated by differential centrifugation as described previously (Kleiner et al., 2008). Protein concentration (Bio-Rad Laboratories, Inc., Hercules, CA) was estimated using the Bradford method (Bradford, 1976) with BSA as a standard.
GST assays
Liver cytosolic GST activities were assessed spectrophotometrically (Shimadzu, Columbia, MD, kinetic mode) as described previously (Habig and Jakoby, 1981; Habig et al., 1974). Briefly, GST activity using the general substrate, CDNB, was determined by incubating liver cytosol (5 μL of a 1:5 dilution) in 0.1 M KH2PO4 buffer, pH 6.5, containing 1 mM CDNB and 1 mM GSH at 25°C in a final volume of 1 mL. GST activity using DCNB as a substrate was determined by incubating liver cytosol (25– 50 μL undiluted) in 0.1 M KH2PO4 buffer, pH 7.5, containing 1 mM DCNB and 5 mM GSH at 25°C in a final volume of 1 mL. The change in absorbance was measured over six intervals of 10 s each following a 10 s lag time at 340 nm and 345 nm (CDNB and DCNB, respectively). The complete system in the absence of cytosol was used as a background control and subtracted from experimental values. Activities were calculated using extinction coefficients of 9.6 mM−1/cm−1 and 8.5 mM−1/cm−1, respectively.
NQO1 assay
Liver cytosolic NQO1 activity was assayed spectrophotometrically at 600 nm using 2,6-dichloroindophenol as a substrate and an extinction coefficient of 21 nM−1/cm−1 as previously described (Prochaska and Talalay, 1986). In this regard, aliquots of liver cytosol (25–50 μL, undiluted) were incubated in 0.05 M Tris buffer, pH 7.4, containing 0.5 mg/mL BSA, 0.05 mM FAD, 0.01% (v/v) Tween 20, 0.2 mM NADH, 0.05 mM 2,6-DCPIP, and 0.02 mM dicoumarol in a final volume of 1 mL at 24 ° C.
Statistical analysis
Experimental values were compared statistically using ANOVA followed by Fischer’s PLSD test using GBStat v6.5 software. Differences were considered significant at p ≤ 0.05.
Results
Effects of naturally occurring coumarins in HepG2-ARE-GFP cells
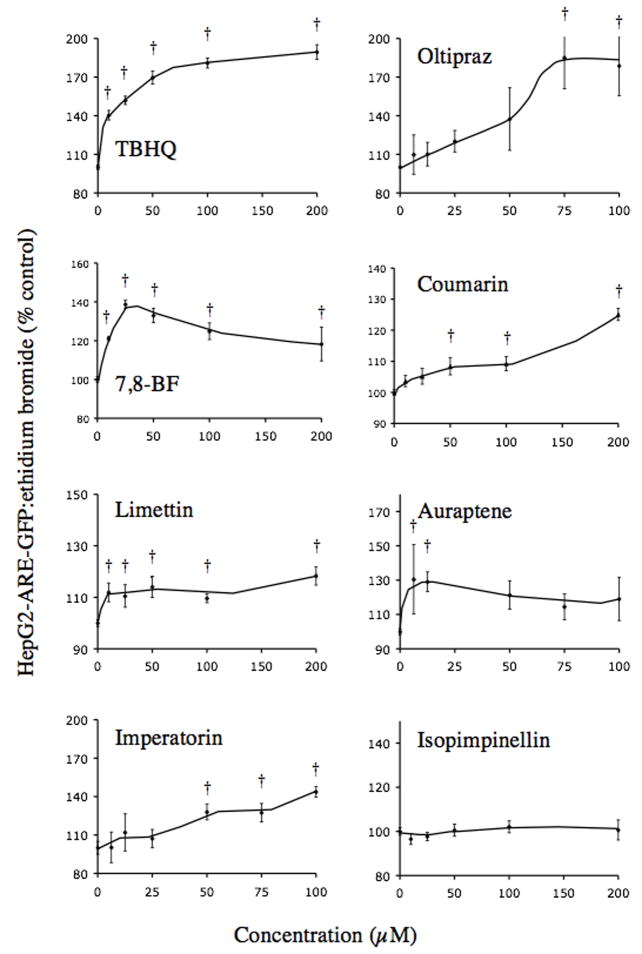
To determine whether a panel of naturally occurring coumarins activate ARE, human hepatoma HepG2 cells stably transfected with TK-GFP/ARE were treated with TBHQ, oltipraz, and 7,8-benzoflavone as controls, and with naturally occurring coumarins: coumarin, limettin, imperatorin, isopimpinellin, and auraptene. As shown in Figure 1, TBHQ increased the ratio of GFP:ethidium bromide fluorescence from 40% at 12.5 μM up to 89% at 200 μM compared to vehicle treated cells. Oltipraz also increased GFP:ethidium bromide fluorescence by a maximum 85% at 75μM. Thus, these positive controls exhibited similar properties as previously reported (Zhu and Fahl, 2000). Of the three simple coumarins tested, coumarin and limettin modestly increased GFP:ethidium bromide fluorescence by 18–25% at the highest doses (200 μM), with little effect at lower doses. Interestingly, the maximum effect of the 7-geranyloxy-substituted simple coumarin, auraptene, was observed as a 30% increase in fluorescence between 6.25–12.5 μM with no further increases at higher doses. Of the two linear furanocoumarins, imperatorin steadily increased GFP:ethidium bromide fluorescence up to 43% of vehicle control at 100 μM, but isopimpinellin had no apparent effect on GFP:ethidium bromide fluorescence at concentrations up to 200 μM. We included 7,8-BF in the study as a control that is known to modulate cytochrome(s) P450 (Kleiner et al., 2003) but not known to have effects on ARE. This compound also increased fluorescence by up to 40% at 12.5 μM.
Figure 1.
Effect of naturally occurring coumarins and controls on activation of the ARE in HepG2-ARE-GFP cells. Increasing concentrations of each compound (indicated in the figure) were incubated with HepG2-ARE-GFP cells for 24 h, stained with ethidium bromide, and then analyzed for both GFP and ethidium bromide fluorescence on a plate reader. Data are expressed as a percent of the vehicle control ratio of GFP:ethidium bromide fluorescence (means ± SE of 6–9 determinations). † Denotes significantly different from vehicle control (p ≤ 0.05).
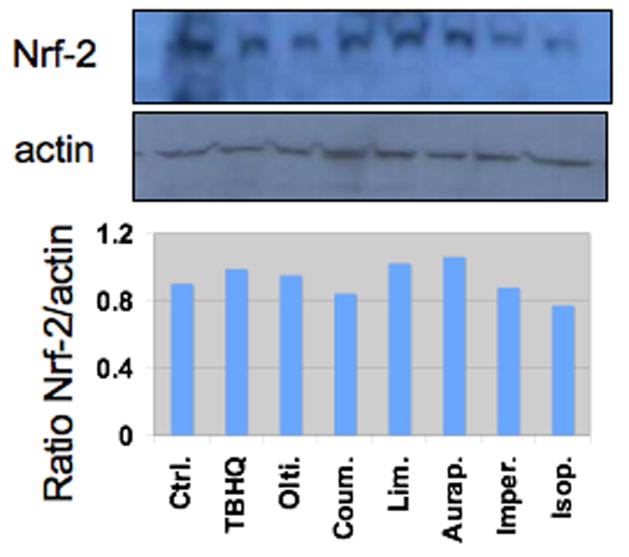
A western blot assay of Nrf2 protein expression in whole cell lysates of HepG2 cells treated with coumarins revealed very little effect on total Nrf2 expression (Figure 2). Although certain compounds (e.g. sulforaphane) are known to induce Nrf2 protein expression (Keum et al., 2006), this did not appear to be the case with the coumarins under the conditions of the current experiment.
Figure 2.
Western blot analysis of Nrf2 protein in HepG2 cells. HepG2 cells were treated with vehicle (control, ctrl., DMSO 0.1% v/v), or 25 μM of tBHQ, oltipraz (olti.), coumarin (coum.), limettin (lim.), auraptene (aurap.), imperatorin (imper.) and isopimpinellin (isop.) for 24 h. Whole cell lysates were immunoblotted for Nrf2 and compared to β-actin as a loading control. The lower panel shows the ratio of the integrated density of Nrf2:actin.
Effects of citrus coumarins and oltipraz on GST and NQO1 activities in Nrf2(−/−) mice
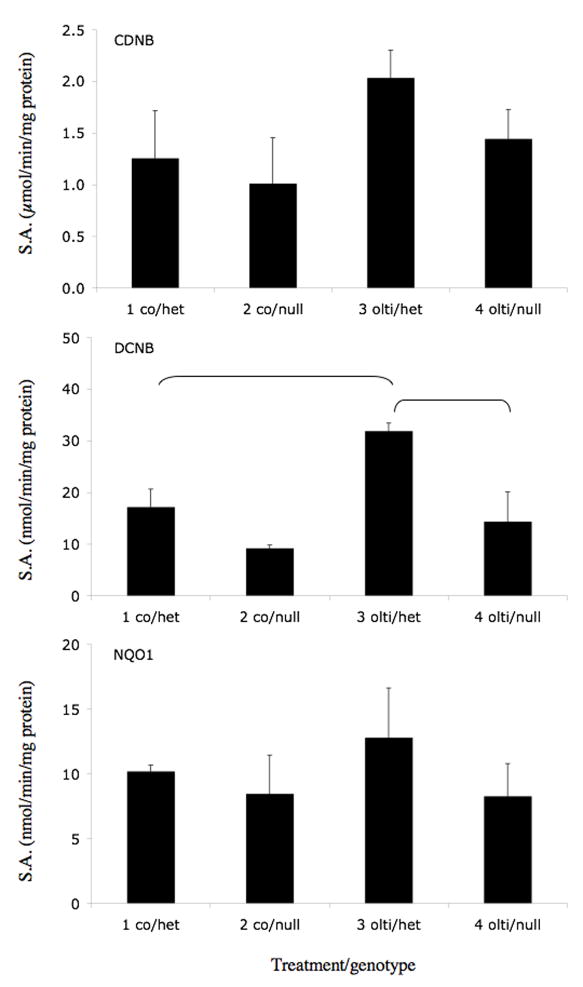
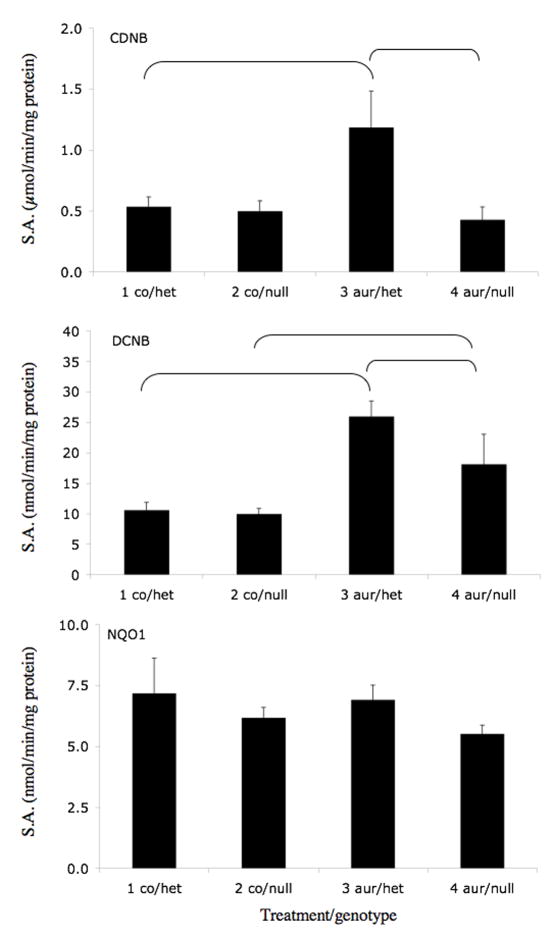
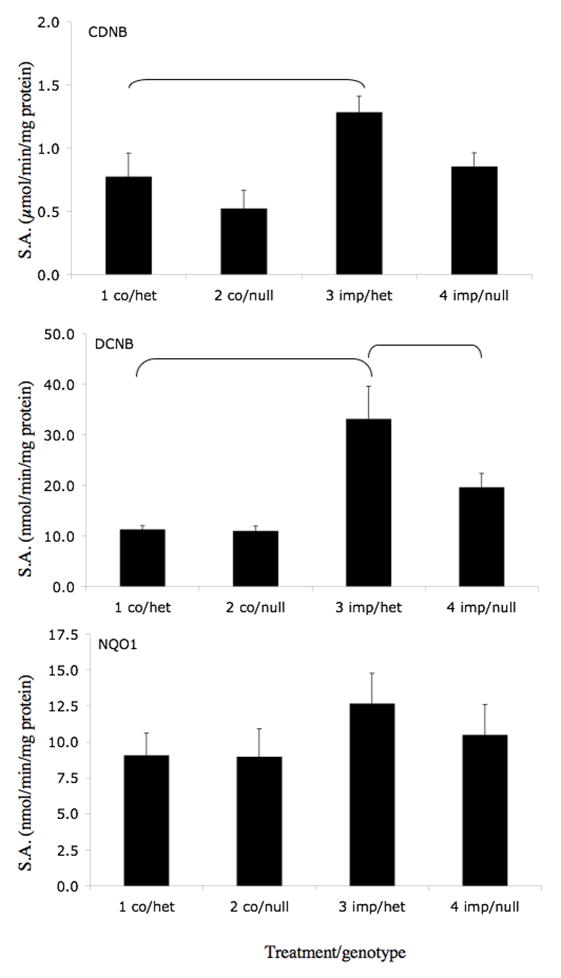
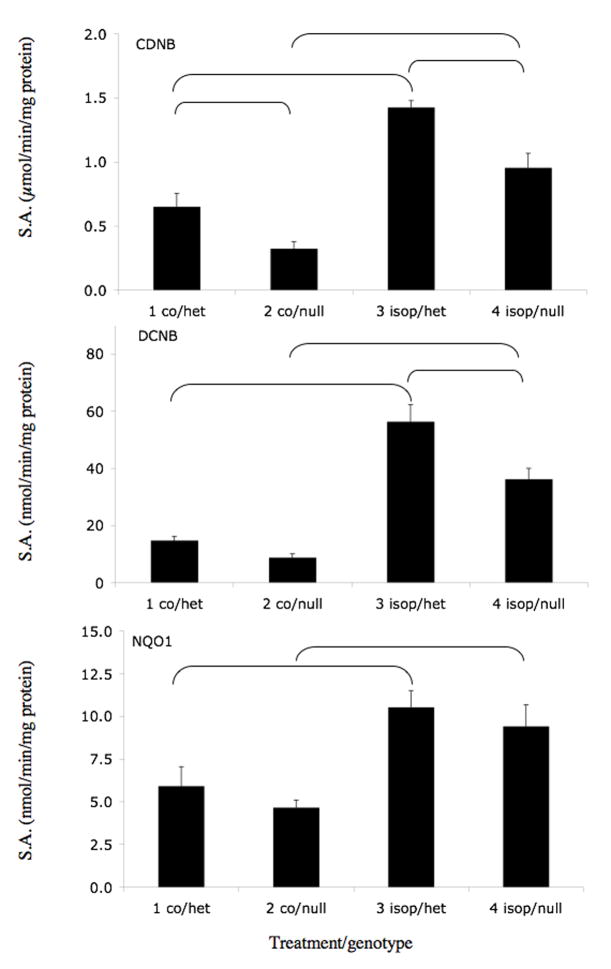
We selected three coumarins that are present in citrus fruits (auraptene, imperatorin, and isopimpinellin) for further study. The effects of orally administered olitpraz, auraptene, imperatorin, and isopimpinellin on GST activities (using CDNB and DCNB as substrates) and on NQO1 activity (using 2,6-DCPIP as a substrate) were compared in Nrf2(+/−) heterozygous mice, which exhibit similar phenotype as Nrf2(+/+) wild-type mice (Itoh et al., 1997), and in Nrf2(−/−) knockout mice, which have an impaired response to ARE inducers. As shown in Figure 3A, oltipraz increased liver cytosolic GST activity in Nrf2(+/−) mice by 86% using DCNB as a substrate. This effect was abrogated in the Nrf2(−/−) mice. Although not statistically significant, oltipraz appeared to produce similar effects on GST activity using CDNB as a substrate and on NQO1 activity. Auraptene (Figure 3B) also significantly increased GST activities in Nrf2(+/−) mice using both CDNB and DCNB as substrates, by 121% and 145%, respectively. In contrast, the effect of auraptene on GST activity in Nrf2(−/−) mice was eliminated when using CDNB as a substrate, and was attenuated when using DCNB as a substrate. Interestingly, auraptene did not increase hepatic NQO1 activities in Nrf2(+/−) or Nrf2(−/−) mice. Imperatorin (Figure 3C) significantly increased CDNB and DCNB activities in Nrf2(+/−) mice by 66% and 193%, respectively. There was no significant induction of GST activity using either substrate in Nrf2(−/−) mice treated with imperatorin. Also, imperatorin had little effect on NQO1 activities in either genotype. Isopimpinellin (Figure 3D) significantly increased GST activities using CDNB and DCNB as substrates, by 119% and 282% in the Nrf2(+/−) mice. Isopimpinellin also increased NQO1 activity by 78% in the Nrf2(+/−) mice. However, compared to the Nrf2(−/−) mice treated with vehicle, isopimpinellin still significantly increased GST and NQO1 activities in Nrf2(−/−) mice. Although not always statistically significant, basal GST and NQO1 activities appeared to be decreased in vehicle treated Nrf2(−/−) mice compared to Nrf2(+/−) mice. Thus, even though GST activities were significantly lower in Nrf2(−/−) mice treated with isopimpinellin compared to Nrf2(+/−) mice treated with imperatorin, the percent increase in GST activities compared to the respective controls were not much different.
Figure 3.
Effects of orally administered oltipraz (panel A), auraptene (panel B), imperatorin (panel C), and isopimpinellin (panel D) on hepatic GST and NQO1 activity in Nrf2(+/−) heterozygous and Nrf2(−/−) knockout mice. GST activity was assessed using CDNB (top panel) and DCNB (middle panel) as substrates. NQO1 activity was assessed using 2,6-dichloroindophenol as a substrate. Figures represent mean specific activities ± SE (n=3–8). Brackets indicate statistical significance at p ≤ 0.05. co, vehicle control, olti, oltipraz, imp, imperatorin, isopimp, isopimpinellin, aur, auraptene.
Effects of citrus coumarins and oltipraz on liver:body weight%
Orally administered citrus coumarins (particularly the linear furanocoumarins) have previously been observed to significantly increase average liver weights and liver:body weight ratios in mice (Kleiner et al., 2001; Kleiner et al., 2008). In the current study, we assessed the effects of citrus coumarins and oltipraz on liver:body weight % in Nrf2(+/−) vs Nrf2(−/−) mice. As shown in Table 1, oltipraz, imperatorin, and isopimpinellin significantly increased liver:body weight % in the Nrf2(+/−) heterozygous mice. These effects were primarily due to an increase in liver weight (data not shown). There were no statistically significant effects of oltipraz, imperatorin, isopimpinellin, or auraptene on body weights in Nrf2(+/−) or Nrf2(−/−) mice (data not shown). The effect on liver:body weight % was partially attenuated in the Nrf2(−/−) knockout mice treated with oltipraz and imperatorin, but not isopimpinellin. Auraptene did not significantly increase liver:body weight % in the current study. There appeared to be a slight increase in liver:body weight % in the Nrf2(−/−) corn oil control mice compared to the Nrf2(+/−) mice (p = 0.07). The data revealed that there was a slight decrease in overall body weight in the vehicle control Nrf2(−/−) mice compared to the Nrf2(+/−) mice, but no increase in liver weight. Taken together, these results suggest that the increase in liver:body weight % by isopimpinellin is not likely to be mediated by activation of Nrf2. In contrast, the increase in liver:body weight % may be mediated, at least in part, by Nrf2 activation in imperatorin and oltipraz treated mice.
Table 1.
Effects of Citrus Coumarins and Oltipraz on Liver:Body weight %
| Group | Nrf-2 (+/−) | Nrf-2 (−/−) |
|---|---|---|
| Cremophor 1 | 4.56 ± 0.24 | 5.22 ± 0.52 |
| Oltipraz 1 | 6.42 ± 0.39a | 5.60 ± 0.26 |
| Corn oil 2 | 4.80 ± 0.24 | 6.07 ± 0.58 |
| Imperatorin 2 | 8.15 ± 0.18a | 7.14 ± 0.48 |
| Corn oil 3 | 5.52 ± 0.26 | 5.68 ± 0.32 |
| Isopimp. 3 | 8.22 ± 0.59a | 8.57 ± 0.52a |
| Corn oil 4 | 5.11 ± 0.17 | 5.87 ± 0.60 |
| Auraptene 4 | 5.88 ± 0.54 | 5.35 ± 0.72 |
|
| ||
| Avg all corn oil | 5.16 ± 0.20 | 5.86 ± 0.31 |
Significantly different from vehicle control of the same genotype (P<0.05). Figures represent liver wt (g) divided by body wt (g) × 100% (means ± S.E.)
Discussion
Many cancer chemopreventive agents, in particular those that are naturally occurring, boost cellular antioxidant defenses (Kwon et al., 2007). Evidence is mounting that many of these phytochemicals activate the ARE through Nrf2. Activation of the ARE is generally recognized as a good strategy for cancer chemoprevention, particularly for natural produces/dietary agents (Surh, 2003). In the current study, we have analyzed a panel of structurally diverse naturally occurring coumarins for their abilities to activate the ARE and to induce GST and NQO1 activities. The HepG2-ARE-GFP cell line was developed by William Fahl and Ming Zhu as a screen to test chemopreventive compounds. Several inducers in addition to TBHQ and oltipraz have produced positive effects in this screen, including the broccoli-derived compound, sulforaphane (Zhu and Fahl, 2000). In the current study, the naturally occurring coumarins exhibited varying degrees of activation of ARE in HepG2-ARE-GFP cells, with the furanocoumarin imperatorin displaying the greatest effects. To our surprise, isopimpinellin, which, along with many other naturally occurring coumarins, is known to induce liver cytosolic GST and NQO1 activities when administered orally to mice (Kleiner et al., 2008) did not have any effects in the HepG2-ARE-GFP cells. It was also interesting to note that 7,8-BF apparently activated the ARE in HepG2-ARE-GFP cells in our results. Previously, we found that orally administered 7,8-BF (70–150 mg/kg bw, p.o.) increased liver cytosolic NQO1 and GST activities (using 2,6-DCPIP and CDNB as substrates, respectively), but had no effect on GST α, π, or μ protein expression (Prince et al., 2006). None of the coumarins appeared to affect total Nrf2 protein expression in whole cell lysates, suggesting coumarins do not activate the ARE via induction of Nrf2. Future studies are planned to further investigate other possibilities for how coumarins activate the ARE.
To further understand whether naturally occurring coumarins modulate GSTs and NQO1 via the ARE, we used Nrf2(−/−) knockout mice. Our results demonstrated that oltipraz (used as a positive control), auraptene, imperatorin, and isopimpinellin increased hepatic GST activities (using CDNB and/or DCNB as a substrate) in Nrf2(+/−) heterozygous mice. Interestingly, only isopimpinellin significantly increased NQO1 activities, although there was an apparent increase in NQO1 activities in the mice treated with oltipraz and imperatorin. We also noted that in most assays, basal GST and NQO1 activities were lower in Nrf2(−/−) mice compared to Nrf2(+/−) heterozygous mice. This is consistent with previous observations by (McMahon et al., 2001) in small intestine. Overall, the induction of GSTs and/or NQO1 were attenuated in Nrf2(−/−) mice treated with oltipraz, auraptene, and imperatorin. However, induction of GST and NQO1 activities persisted in Nrf2(−/−) mice treated with isopimpinellin. Taken together, the results of the Nrf2(−/−) knockout mouse study suggest that auraptene and imperatorin induce murine hepatic GST and NQO1 activities via the ARE/Nrf2 mechanism, whereas isopimpinellin appears to involve other mechanisms that cannot be suppressed in Nrf2(−/−) knockout mice. These results are consistent with the HepG2-ARE-GFP study, in which imperatorin and auraptene activated HepG2-ARE-GFP but isopimpinellin did not. Furthermore, although we did not test coumarin and limettin in the Nrf2(−/−) mice, a previous study by McMahon and co-workers determined that induction of GST and NQO1 activities in small intestine were not completely abrogated in Nrf2(−/−) mice (McMahon et al., 2001). In our study, coumarin and limettin had a fairly weak effect on HepG2-ARE-GFP expression. These results suggest that coumarin and limettin may act by factors other than or in addition to ARE/Nrf2.
In our experience, orally administered linear furanocoumarins exhibit a fairly pleiotropic effect in mice, reminiscent of Phenobarbital. Linear furanocoumarins, when administered orally, have previously been reported to increase liver:body weight %, along with their effect on increases in hepatic P450 1A1/2, 2B9/10, and 3A11, GST, and NQO1 activities and/or expression (Kleiner et al., 2001; Kleiner et al., 2002b; Kleiner et al., 2008). Despite these changes in liver weight, no overall adverse effects were observed, and no significant changes in plasma glutamic oxalacetic transaminase (GOT), glutamic pyruvate transaminase (GPT), blood urea nitrogen (BUN), or blood clotting time were reported in mice administered imperatorin or isopimpinellin (35–150 mg/kg bw, p.o.) (Kleiner et al., 2002b). The purpose of examining liver weight in the current study was to determine if the increase in liver weight was due to activation of Nrf2/ARE. Simple coumarins generally induce GST and NQO1 activities without increasing P450 1A1/2, 2B9/10, and 3A11 expression (Kleiner et al., 2008). Consistent with our previous findings, the increase in liver:body weight % was greatest in mice treated with imperatorin and isopimpinellin. Oltipraz, which also increased liver:body weight % in our study, has been shown to induce rat CYP1A and CYP2B1/2 mRNA when administered in the diet (0.075% w/w) (Langouet et al., 1997).
Auraptene did not increase liver:body weight% in our study. The effect of oltipraz and imperatorin on liver weight was somewhat reduced in Nrf2(−/−) mice, whereas the effects of isopimpinellin on liver weight was completely unaffected in Nrf2(−/−) mice. Taken together, these results suggest that the abilities of furanocoumarins compounds to increase liver weight appear to correspond more with the effects on P450 induction than with the abilities to increase GST activities/expression. From the data, it does not appear that activation of Nrf2/ARE was related to increases in liver weight.
Coumarins represent a diverse class of phytochemicals that are ubiquitous in the human diet. Isopimpinellin, which is present in celery, limes, and Angelica-containing traditional Chinese medicine, appears to possess different properties than imperatorin, which is found in lemons and also in Angelica-containing traditional Chinese medicine (Beier et al., 1983; Cardoso et al., 2006; Frerot and Decorzant, 2004; Ivie and Beier, 1996). These two linear furanocoumarins differ by the substitutions across the center ring, with isopimpinellin containing two methoxy groups, and imperatorin possessing one allyloxy group. Hence, we conclude that imperatorin and auraptene induce hepatic GST and/or NQO1 activities via activation of the ARE through Nrf2, isopimpinellin induces these enzymes (and liver weight) via additional mechanisms. Although our recent evidence indicates that isopimpinellin interacts with CAR and PXR, it is not clear whether these mechanisms or others are responsible for the effects of isopimpinellin on GSTs and NQO1. As coumarins continue to be studied for their cancer chemopreventive properties, it will be important to more fully delineate their mechanisms of action. Finally, the results of the current study should enable us to further define the role of the ARE/Nrf2 in the cancer chemopreventive effects of imperatorin and auraptene using Nrf2(−/−) knockout mice.
Acknowledgments
Grant Support: This research was supported by a National Cancer Institute grant 1K22CA102005-01A2. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Ms. Childers was supported by the Biomedical Research Foundation SMART Donor Account.
We acknowledge the help of our student interns, Amy Wells, Victor Kha, Eduardo Lopez, and Arianne Keller for assistance with the GST and NQO1 assays. We thank Dr. Chris Kevil for the use of his fluorescent plate reader.
Abbreviations
- ARE
antioxidant response element
- CAR
constitutive androstane receptor
- CDNB
1-chloro-2,4-dinitrobenzene
- DCPIP
2, 6-dichloroindophenolate
- DCNB
1,2-dichloro-4-nitrobenzene
- FAD
flavin adenine dinucleotide
- GST
glutathione S-transferase
- Keap 1
Kelch-like ECH-associated protein
- NADH
β-nicotinamide adenine dinucleotide, reduced
- NQO1
NAD(P)H quinone oxidoreductase
- PXR
pregnane X receptor
- XRE
xenobiotic response element
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beier RC, Ivie GW, Oertli EH, Holt DL. HPLC analysis of linear furocoumarins (psoralens) in healthy celery (Apium graveolens) Food Chem Toxicol. 1983;21:163–165. doi: 10.1016/0278-6915(83)90231-4. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Campbell CT, Prince M, Landry GM, Kha V, Kleiner HE. Pro-apoptotic effects of 1′-acetoxychavicol acetate in human breast carcinoma cells. Toxicol Lett. 2007;173:151–160. doi: 10.1016/j.toxlet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Cardoso CA, Pires AE, Honda NK. A method for quantitative determination of furanocoumarins in capsules and tablets of phytochemical preparations. Chem Pharm Bull (Tokyo) 2006;54:442–447. doi: 10.1248/cpb.54.442. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerot E, Decorzant E. Quantification of total furocoumarins in citrus oils by HPLC coupled with UV, fluorescence, and mass detection. J Agric Food Chem. 2004;52:6879–6886. doi: 10.1021/jf040164p. [DOI] [PubMed] [Google Scholar]
- Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Ivie GW, Beier RC. Isopimpinellin is not phototoxic in a chick skin assay. Photochem Photobiol. 1996;63:306–307. doi: 10.1111/j.1751-1097.1996.tb03031.x. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Characterization and partial purification of microsomal NAD(P)H:quinone oxidoreductases. Arch Biochem Biophys. 2000;375:62–68. doi: 10.1006/abbi.1999.1650. [DOI] [PubMed] [Google Scholar]
- Kelly VP, Ellis EM, Manson MM, Chanas SA, Moffat GJ, McLeod R, Judah DJ, Neal GE, Hayes JD. Chemoprevention of aflatoxin B1 hepatocarcinogenesis by coumarin, a natural benzopyrone that is a potent inducer of aflatoxin B1-aldehyde reductase, the glutathione S-transferase A5 and P1 subunits, and NAD(P)H:quinone oxidoreductase in rat liver. Cancer Res. 2000;60:957–969. [PubMed] [Google Scholar]
- Keum YS, Yu S, Chang PP, Yuan X, Kim JH, Xu C, Han J, Agarwal A, Kong AN. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006;66:8804–8813. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- Kleiner HE, Reed MJ, DiGiovanni J. Naturally occurring coumarins inhibit human cytochromes P450 and block benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene DNA adduct formation in MCF-7 cells. Chem Res Toxicol. 2003;16:415–422. doi: 10.1021/tx025636d. [DOI] [PubMed] [Google Scholar]
- Kleiner HE, Vulimiri SV, Miller L, Johnson WH, Jr, Whitman CP, DiGiovanni J. Oral administration of naturally occurring coumarins leads to altered phase I and II enzyme activities and reduced DNA adduct formation by polycyclic aromatic hydrocarbons in various tissues of SENCAR mice. Carcinogenesis. 2001;22:73–82. doi: 10.1093/carcin/22.1.73. [DOI] [PubMed] [Google Scholar]
- Kleiner HE, Vulimiri SV, Reed MJ, Uberecken A, DiGiovanni J. Role of cytochrome P450 1a1 and 1b1 in the metabolic activation of 7,12-dimethylbenz[a]anthracene and the effects of naturally occurring furanocoumarins on skin tumor initiation. Chem Res Toxicol. 2002a;15:226–235. doi: 10.1021/tx010151v. [DOI] [PubMed] [Google Scholar]
- Kleiner HE, Vulimiri SV, Starost MF, Reed MJ, DiGiovanni J. Oral administration of the citrus coumarin, isopimpinellin, blocks DNA adduct formation and skin tumor initiation by 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis. 2002b;23:1667–1675. doi: 10.1093/carcin/23.10.1667. [DOI] [PubMed] [Google Scholar]
- Kleiner HE, Xia X, Sonoda J, Zhang J, Pontius E, Abey J, Evans RM, Moore DD, DiGiovanni J. Effects of naturally occurring coumarins on hepatic drug-metabolizing enzymes in mice. Toxicol Appl Pharmacol. 2008;232:337–350. doi: 10.1016/j.taap.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KH, Barve A, Yu S, Huang MT, Kong AN. Cancer chemoprevention by phytochemicals: potential molecular targets, biomarkers and animal models. Acta Pharmacol Sin. 2007;28:1409–1421. doi: 10.1111/j.1745-7254.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- Langouet S, Maheo K, Berthou F, Morel F, Lagadic-Gossman D, Glaise D, Coles B, Ketterer B, Guillouzo A. Effects of administration of the chemoprotective agent oltipraz on CYP1A and CYP2B in rat liver and rat hepatocytes in culture. Carcinogenesis. 1997;18:1343–1349. doi: 10.1093/carcin/18.7.1343. [DOI] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The Cap’n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- Murakami A, Wada K, Ueda N, Sasaki K, Haga M, Kuki W, Takahashi Y, Yonei H, Koshimizu K, Ohigashi H. In vitro absorption and metabolism of a citrus chemopreventive agent, auraptene, and its modifying effects on xenobiotic enzyme activities in mouse livers. Nutr Cancer. 2000;36:191–199. doi: 10.1207/S15327914NC3602_8. [DOI] [PubMed] [Google Scholar]
- Prince M, Campbell CT, Robertson TA, Wells AJ, Kleiner HE. Naturally occurring coumarins inhibit 7,12-dimethylbenz[a]anthracene DNA adduct formation in mouse mammary gland. Carcinogenesis. 2006;27:1204–1213. doi: 10.1093/carcin/bgi303. [DOI] [PubMed] [Google Scholar]
- Prochaska HJ, Talalay P. Purification and characterization of two isofunctional forms of NAD(P)H: quinone reductase from mouse liver. J Biol Chem. 1986;261:1372–1378. [PubMed] [Google Scholar]
- Rushmore TH, King RG, Paulson KE, Pickett CB. Regulation of glutathione S-transferase Ya subunit gene expression: identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc Natl Acad Sci U S A. 1990;87:3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- Spink DC, Eugster HP, Lincoln DW, 2nd, Schuetz JD, Schuetz EG, Johnson JA, Kaminsky LS, Gierthy JF. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1A1: a comparison of the activities induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in MCF-7 cells with those from heterologous expression of the cDNA. Arch Biochem Biophys. 1992;293:342–348. doi: 10.1016/0003-9861(92)90404-k. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Zhang DD. Mechanistic studies of the Nrf2\Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- Zhu M, Fahl WE. Development of a green fluorescent protein microplate assay for the screening of chemopreventive agents. Anal Biochem. 2000;287:210–217. doi: 10.1006/abio.2000.4875. [DOI] [PubMed] [Google Scholar]