Summary
The LKB1 serine/threonine kinase is a tumor suppressor responsible for the inherited familial cancer disorder Peutz-Jeghers syndrome and is inactivated in a large percentage of human lung cancers. LKB1 acts a master kinase, directly phosphorylating and activating a family of 14 AMPK-related kinases which control cell metabolism, cell growth, and cell polarity. In this issue of Biochemical Journal, Hardie and colleagues discover an alternative splice form of LKB1 that alters the C-terminus of the protein containing a few known sites of post-translational regulation. Though widely expressed, the short isoform (LKB1s) is the sole splice isoform expressed in testes and its expression peaks at the time of spermatid maturation. Male mice lacking the LKB1s isoform have dramatic defects in spermatozoa resulting in sterility.
Keywords: LKB1, AMPK, kinase, alternative splicing, spermatogenesis
How the cell reprograms its metabolism and cell growth/cell fate decisions under conditions of low nutrients is a highly conserved process, which in all eukaryotes involves an ortholog of the AMP-activated protein kinase (AMPK). Across species, AMPK orthologs are activated under conditions when intracellular ATP levels fall, such as under nutrient limited conditions [1]. Two molecular events are thought to control AMPK activation in most species studied to date – AMP binding to CBS nucleotide-binding domains in the AMPK gamma regulatory subunit, and a required phoshorylation event in the activation loop threonine of the AMPK alpha kinase subunit. Convergent approaches from a number of labs in 2003 revealed that the kinase responsible for phosphorylating the activation loop threonine of AMPK is encoded in metazoans by the LKB1/STK11 serine/threonine kinase and its orthologs. Subsequent studies from the Alessi laboratory revealed that LKB1 is a master upstream kinase directly phosphorylating the activation loop threonine of 12 additional kinases in the AMPK family [2], including the Par-1/MARK family of kinases, which were genetically linked to LKB1 previously in a screen for genes required for embryonic patterning and polarity in C. elegans, a relationship also observed in Drosophila embryogenesis (refs) (see Fig. 1).
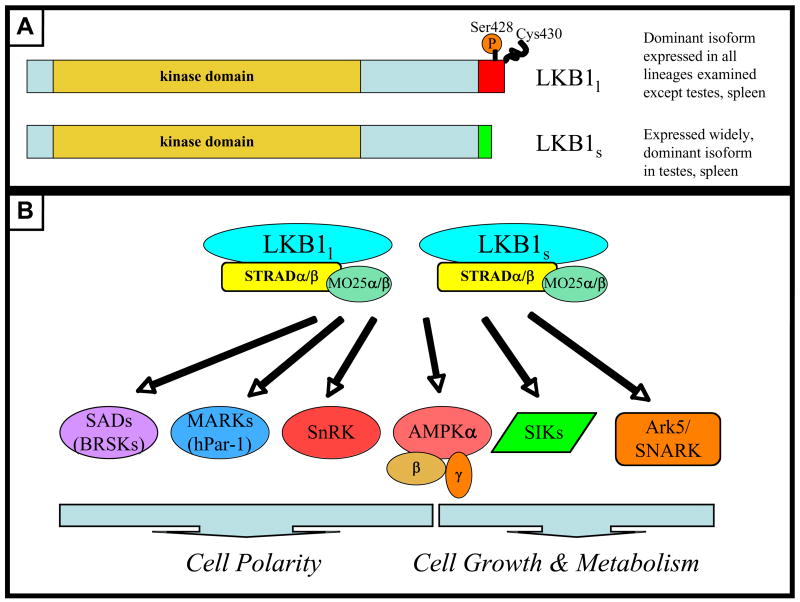
Figure 1.
A. Schematic of the two alternative splice forms of LKB1 (numbering according to human LKB1). LKB1l contains exon 9B which includes the well-documented Ser428 phosphorylation site which is a target for PKA and Rsk (and perhaps aPKCs), as well as the Cys430 farnesylation site, both of which are conserved back to Drosophila. B. Both isoforms of LKB1 appear to activate 14 members of the AMPK-related family of kinases, many of which have well-established roles in the control of cell polarity (SAD, MARK subgroups) as well as in cell growth and/or the control of glucose metabolism (AMPK, SIKs). Little is known about the function SnRK or the Ark5/SNARK family and many of these kinases may share overlapping substrates so designating some as regulating cell polarity and others as regulating cell growth/metabolism is somewhat arbitrary.
Like AMPK itself, LKB1 is part of a heterotrimeric complex with two associated subunits, here a STE20-like pseudokinase named STRAD and a third protein named Mo25 [3]. These proteins are well-conserved throughout metazoans [4], though while Mo25 orthologs are clearly identified in fission and budding yeast, it is unclear whether they regulate LKB1 or AMPK orthologs. Importantly, in mammalian cells LKB1 kinase does not appear to be regulated in response to stress stimuli as measured by IP-kinase assays from a large number of groups, though a number of post-translational modifications including phosphorylation, acetylation, and farnesylation has been documented [3, 5, 6]. To date, no specific function has been attributed to the phosphorylation events on LKB1, although non-phosphorylatable alleles at several sites appear to behave similar to kinase inactive mutants in some assays [7–9]. Consistent with the LKB1 complex being constitutively active, heterotrimeric LKB1/STRAD/MO25 complex purified from bacteria is active [10]. This also fits with recent data from yeast and mammalian cells demonstrating that the regulation of the LKB1-phosphorylation site in AMPK in response to cellular stress is mediated by suppression of phosphatase activity rather than induction of LKB1 kinase activity [11–12]. This is also supported by the fact that all of the other AMPK-related kinases examined so far appear to be constitutively phosphorylated on their activation loop threonines, except in cells deficient for LKB1 [2]. Nontheless, the mere existence of 14 downstream substrates for LKB1 which demonstrate varied subcellular locations suggests possible regulation though relocalization of LKB1/STRAD/MO25 complexes. This idea is reinforced by the finding that overexpression of the STRAD subunit results in cytoplasmic redistribution of LKB1, an effect which was recently shown to be due to altered interaction with the Crm1 nuclear import machinery [13].
In addition to these potential regulatory mechanisms, during their biochemical identification of LKB1 as the major AMPKK in rat liver, Hardie and colleagues noted a band with altered mobility reacting with the LKB1 antibody and stoichiometrically coming down in the LKB1-containing complex, suggestive of a LKB1 splice form [14]. In this issue of Biochemical Journal, these authors describe the discovery of an alternative splice form of LKB1, which alters the final coding exon [15]. Existing cDNAs in the database indicated the existence of an alternate exon 9A. Messages including exon 9A lack exon 9B, resulting in a shorter polypeptide which also lacked a well-established and highly conserved PKA/Rsk phosphorylation site and farnesylation site at the very C-terminus of the originally defined “long” isoform of LKB1 (LKB1l) – see Figure 1. The authors here create antibodies recognizing isoform specific peptides and combining these tools with the immunoprecipitation of both endogenous isoforms using an antibody directed against the amino-terminus, they examine their tissue distribution. Strikingly, while most rodent tissues examined express a mixture of both isoforms, the LKB1s isoform is the predominant isoform expressed in both testes and spleen [15].
The authors next examine whether the short isoform displays altered function relative to the standard long isoform in a number of assays. First, they examine whether the short isoform is altered in its ability to activate AMPK, or several of the AMPK related kinases. The authors take advantage of the fact that HeLa cells lack LKB1 to introduce each isoform in the absence of the other. No significant difference was observed, although in vitro kinase assays using purified recombinant AMPKα 1 or the BRSK/SAD kinases as substrates both revealed increased catalytic activity of the LKB1s isoform. Strikingly, despite lacking the farnesylation site and C-terminal phosphorylation site, no difference was observed for the subcellular localization of each isoform in HeLa cells. Further studies will be required to determine if in some cell types there may be a differential localization for either isoform in response to specific stimuli, particularly those that result in increased phosphorylation of Serine428 or farnesylation of Cysteine 430.
To directly investigate a requirement for the LKB1s isoform, the authors take advantage of a genetically-engineered mouse bearing a conditional floxed allele of LKB1 in which the last 6 exons were replaced by a cDNA encoding only the long isoform. Thus when homozygous for the floxed allele, these animals completely lack the short isoform for LKB1 but retain expression of the long isoform. Male mice homozygous for the floxed allele are sterile, which given their observation that the short isoform is highly expressed in testes, hinted that LKB1s may play a role in spermatogenesis. A detailed timecourse of LKB1s expression in testes revealed it to be dramatically upregulated coincident with the first appearance of mature, haploid spermatids, and upon fractionation of the various cell populations in the testes, was most consistent with expression in developing germ cells after meiosis. Histological examination of mice lacking LKB1s revealed a near complete absence of mature spermatozoa. Isolated spermatids from LKB1s -deficient mice were completely non-motile and displayed abnormal acrosome morphology, as visualized by scanning electron microscopy, perhaps suggestive of a cell polarity defect.
Much future work will be needed to define which of the 14 LKB1-dependent kinases are important for sperm maturation. The Par-1/MARK kinase subfamily has well-established roles in cell polarity and the related SAD kinases have been shown to be critical for cell polarity in neurons, where they are most highly expressed [3, 9,16]. Moreover, AMPK has been shown to play a role in cell polarity in Drosophila and some mammalian cell types so it could be involved in the LKB1s -deficient phenotype [17]. Strikingly, another of the 14 LKB1-dependent kinases, SnRK, is only expressed appreciably in the testes [18], making it an excellent candidate to be mediating some the effects of the LKB1s isoform in this tissue
Several intriguing questions remain. First, the prominent expression of LKB1s in spleen warrants a closer examination of whether specific haemopoetic lineage(s) predominantly express this isoform, and whether subtle immunological defects may exist which are revealed in response to specific immune challenges. Polarization is also critical in T-cell activation and stem cell differentiation so it would be interesting to examine whether LKB1s, or simply LKB1 in general, plays a role in this process. Another question raised by these findings is the nature of the signal leading to the dramatic upregulation of LKB1s protein at the time of sperm maturation.
Finally, while this is the first report of an alternative splicing event for LKB1, other pathway components are known to be alternatively spliced. For example, AMPK gamma subunits γ2 and γ3 each encode two different splice isoforms [1], and the key LKB1 regulatory subunits STRADα and STRADβ have three annotated splice isoforms in Uniprot. Opening a Pandora’s box of potential complexity is a recent study revealing 12 distinct splice isoforms of STRADα [19]. As more is discovered about the regulation of LKB1 and its subunits by alternative splicing and post-translational modification, the environmental signals that control formation of the LKB1 heterotrimeric complex and direct it towards subsets of its downstream kinases controlling cell proliferation, glucose metabolism, and cell polarity will be better illuminated. For now, we can add spermatogenesis to the list of physiological processes for which LKB1 is required for in mammals, alongside suppression of tumorigenesis, control of cell polarity, and control of glucose metabolism.
References
- 1.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 2.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessi DR, Sakamoto K, Bayascas JR. Lkb1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Izumi Y, Matsuzaki F. The GC kinase Fray and Mo25 regulate Drosophila asymmetric divisions. Biochem Biophys Res Commun. 2008;366:212–218. doi: 10.1016/j.bbrc.2007.11.128. [DOI] [PubMed] [Google Scholar]
- 5.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization and activity of LKB1; possible role in AMP-activated protein kinase activation. J Biol Chem. 2008 doi: 10.1074/jbc.M805711200. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song P, Xie Z, Wu Y, Xu J, Dong Y, Zou MH. Protein kinase Czeta-dependent LKB1 serine 428 phosphorylation increases LKB1 nucleus export and apoptosis in endothelial cells. J Biol Chem. 2008;283:12446–12455. doi: 10.1074/jbc.M708208200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JS, Williams MR, Morrice N, Deak M, Alessi DR. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell growth. J Biol Chem. 2001;276:19469–19482. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]
- 8.Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- 9.Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Neumann D, Suter M, Tuerk R, Riek U, Wallimann T. Co-expression of LKB1, MO25alpha and STRADalpha in bacteria yield the functional and active heterotrimeric complex. Mol Biotechnol. 2007;36:220–231. doi: 10.1007/s12033-007-0029-x. [DOI] [PubMed] [Google Scholar]
- 11.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubenstein EM, McCartney RR, Zhang C, Shokat KM, Shirra MK, Arndt KM, Schmidt MC. Access denied: Snf1 activation loop phosphorylation is controlled by availability of the phosphorylated threonine 210 to the PP1 phosphatase. J Biol Chem. 2008;283:222–230. doi: 10.1074/jbc.M707957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorfman J, Macara IG. STRADalpha regulates LKB1 localization by blocking access to importin-alpha, and by association with Crm1 and exportin-7. Mol Biol Cell. 2008;19:1614–1626. doi: 10.1091/mbc.E07-05-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Towler MC, Fogarty S, Hawley SA, Pan DA, Martin D, Morrice NA, McCarthy A, Galardo MN, Meroni SB, Cigorraga SB, et al. A novel short splice variant of the tumour suppressor LKB1 is required for spermiogenesis. Biochem J. 2008 doi: 10.1042/BJ20081447. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Williams T, Brenman JE. LKB1 and AMPK in cell polarity and division. Trends Cell Biol. 2008;18:193–198. doi: 10.1016/j.tcb.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Jaleel M, McBride A, Lizcano JM, Deak M, Toth R, Morrice NA, Alessi DR. Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Lett. 2005;579:1417–1423. doi: 10.1016/j.febslet.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 19.Marignani PA, Scott KD, Bagnulo R, Cannone D, Ferrari E, Stella A, Guanti G, Simone C, Resta N. Novel splice isoforms of STRADalpha differentially affect LKB1 activity, complex assembly and subcellular localization. Cancer Biol Ther. 2007;6:1627–31. doi: 10.4161/cbt.6.10.4787. [DOI] [PubMed] [Google Scholar]