Abstract
Background and Purpose
Stroke is emerging as a major public health problem for women, as it is for men. Controversy persists regarding gender differences in stroke incidence, severity and post-stroke disability.
Methods
Participants in the Framingham Original (N=5,119; 2,829 women) and Offspring (N= 4,957, 2,565 women) cohorts who were 45 years and stroke-free were followed to first incident stroke. Gender-specific outcome measures were adjusted for the Framingham Stroke Risk Profile components.
Results
We observed 1136 incident strokes (638 in women) over 56 years of follow-up. Women were significantly (p<0.001) older (75.1 vs. 71.1 years for men) at their first-ever stroke, had a higher stroke incidence above 85 years of age, lower at all other ages and a higher lifetime risk of stroke at all ages. There was no significant difference in stroke subtype, stroke severity and case fatality rates between genders. Women were significantly (p<0.01) more disabled prior to stroke and in the acute phase of stroke in dressing (59 % vs. 37%), grooming (57% vs. 34%) and transfer from bed to chair (59% vs. 35%). At 3 to 6 months post-stroke women were more disabled, more likely to be single, and 3.5 times more likely to be institutionalized (p<0.01).
Conclusions
These results from the Framingham Heart Study (FHS) support the existence of gender-differences in stroke incidence, lifetime risk (LTR) of stroke, age at first stroke, post-stroke disability and institutionalization rates. Pre-stroke disability and socio-demographic factors may contribute to the high rate of institutionalization and poorer outcome observed in women.
Keywords: gender, stroke, incidence, disability outcome
There are 780,000 new and recurrent strokes occurring each year in the United States, making stroke the major cause of disability, the third leading cause of death in the US and the second cause of death worldwide in both men and women. Recent data shows that 60,000 more women than men have a stroke each year in the US.1 The burden of stroke in women was often underestimated until the early 1980’s,2 and after being once considered primarily a disease of men, stroke is currently emerging as a major public health problem for women as well.
Prior reports on the LTR of stroke based on the Framingham Study cohort estimated that 1 in 5 women and 1 in 6 men who reach age 55 free of stroke will develop a stroke during their remaining lifetime.3 Thus elderly women, the fastest growing segment of the American population, face an increased likelihood of stroke, a troubling statistic since this group may have in addition the greatest risk of disability following stroke.4
There is increasing evidence of gender-specific differences in stroke symptoms, diagnosis, peri-procedural risk, treatment and preventive interventions.5-12 For example, results from the Women’s Health Study11 and the Physician’s Health Study,12 revealed that aspirin is an efficacious measure for primary stroke prevention in women but not in men. Surprisingly, the data on gender-specific rates of stroke incidence are still scarce13-15 and controversies continue regarding differences in stroke mortality and post-stroke disability outcomes.4,16-20
The aim of our study was to explore gender-specific differences in stroke incidence, cumulative incidence, severity and post-stroke disability in the FHS, based on data from 56 years of prospective follow-up in a community-based sample.
Subjects and Methods
Study population
The FHS is a longitudinal, community-based cohort study which was initiated in 1948 with the enrollment of 5209 participants aged 28 to 62 years. In 1971 the offspring and the offspring spouses of the Original cohort participants were enrolled as the Offspring cohort. Since the study’s inception, participants have had serial examinations including standardized interviews, physician examinations and laboratory testing. More details of the study design have been detailed elsewhere.21
FHS participants who survived free of clinical stroke to age 45 constituted the study sample for this investigation. These 10,076 participants (P5,119 from the Original cohort and 4,957 from the Offspring cohort), 5,394 of whom were women were followed for up to 56 years through 2005 to the first-ever stroke. This sample was used to estimate age-specific stroke incidence and remaining LTR of stroke. Incident stroke cases (n=1,136) were further investigated for pre-stroke co-morbidity and pre- and post-disability, stroke severity, and case fatality rates.
A subset of stroke cases was closely followed for disability as part of a separate study of the precursors and outcomes of stroke (PI: PA Wolf). Participants diagnosed with a stroke, alive, living in the geographic area, and able to participate in a disability evaluation in the acute phase of stroke and at 3 to 6 months post—stroke were included in the disability assessment. This assessment was performed on 594 stroke cases (364 women) in the acute phase of stroke and on 205 stroke cases (120 women) in the chronic phase of stroke, at 3 to 6 months post-stroke.
Written informed consent was obtained from all participants. The consent form and the study design were approved by the Institutional Review Board of Boston University Medical Center.
Methods
Details of our protocol for stroke surveillance, diagnosis and type of stroke, assessment of stroke severity and disability after stroke have been published previously.4,13
Stroke was clinically defined as a sudden onset of a focal neurological deficit of a presumed vascular etiology and lasting more than 24 hours. The surveillance for new stroke events involved screening at consecutive cohort examinations, including a medical history that specifically enquired about stroke symptoms and a physical examination. Persons with suspected stroke underwent evaluation by a study stroke neurologist. There was also ongoing daily monitoring of local hospital admissions and emergency room visits, tracking of records for interim hospitalizations, questioning of participants for stroke symptoms in annual telephone health updates, and referrals from ancillary study examinations including those from an ongoing study of brain magnetic resonance imaging in all eligible participants from the Original and Offspring cohorts.
The diagnosis of stroke and stroke subtype was based on pre-established criteria that included clinical, laboratory and noninvasive imaging and vascular studies, cardiac evaluations and information from autopsy studies, when available. Potential stroke cases were reviewed and adjudicated by a physician review panel of 3 investigators (with a minimum of 2 neurologists).
All ischemic and hemorrhagic strokes were included. Ischemic strokes were classified as atherothrombotic brain infarctions (ABI) or cardioembolic infarctions (CE). Hemorrhagic strokes were classified as intracerebral hemorrhages (ICH) or subarachnoid hemorrhages (SH). Stroke severity was defined according to the neurological deficits identified on examination during the acute phase of stroke and was further classified into 4 categories: none (no deficit), mild (deficit present in visual, motor, sensory or language domains but without functional impairment), moderate (deficit requiring assistance in 1 of the domains mentioned above), and severe (deficit requiring assistance in at least 2 of the domains). Case fatality rates were defined as the percentage of patients with stroke who died within 30, 90 and 180 days from the onset of stroke.
Disability assessment
Disability assessment was performed pre-stroke as part of the ongoing Framingham cycle examinations (done within the 5 years preceeding the stroke) and post-stroke (as part of a larger post-stroke assessment battery) in the acute phase and at 3 to 6 months, on a subset of cases as described above. We defined physical disability utilizing modified Katz activities of daily living (ADL) scale.22 This scale has been used in other large population-based studies with high test-retest reliability.23,24 The assessment included 5 domains of activity which were eating, dressing, grooming, transfer from bed to chair and walking. Information about institutionalization was obtained pre-stroke and at the time of disability evaluation in the acute and 3 to 6 month assessment.
Statistical analysis
We calculated gender-specific annual stroke incidence per 1,000 person-years within 10 year age groups and overall, and used direct standardization to estimate gender-specific age-adjusted stroke incidence. We compared stroke incidence in men and women within age groups and overall using Poisson regression.
Gender-specific estimates of remaining LTR of stroke were calculated using a survival analytical technique adjusting for the competing risk of death (described in detail elsewhere)3,25,26. Seshadri et al.3 estimated the remaining LTR of stroke in stroke-free men and women at the index age of 45; we updated those results here using slightly longer follow-up.
Estimates of age-specific stroke incidence and remaining LTR of stroke were calculated using participants aged 45-94 only; persons remaining free of stroke provided information until the date when they were last interviewed or examined to determine if they suffered a stroke, until they died or reached age 95 years, or until December, 2005.
Gender-specific crude prevalence of pre-stroke co-morbidity and disability, and stroke outcomes are presented, with comparisons made using age-adjusted logistic regression. Stroke outcomes were additionally adjusted for pre-stroke disability and the components of the Framingham Stroke Risk Profile:27 systolic blood pressure (SBP), antihypertensive treatment, atrial fibrillation (AF), current smoking, prevalent cardiovascular disease (CVD) and diabetes mellitus (DM).
Results
Gender-specific Stroke Incidence and LTR of Stroke
We observed a total of 1,136 strokes (638 in women) during the 56 year follow-up period. Of these, 1,117 incident strokes (625 in women) that occurred between ages 45-94 constituted the basis for the incidence and LTR analyses. Gender-specific stroke incidence by 10 year age group is shown in Table 1 with a graphic representation displayed in Figure 1. We found that stroke incidence increased with each decade of life in both women and men. Among those aged 45-84, stroke incidence was higher in men than in women (p<0.001). The gender effect reversed in the oldest group, with stroke incidence higher in women than in men among those aged 85-94. However, the number of cases in this age group was small and the difference did not reach statistical significance (p=0.087).
Table 1.
Gender-Specific Stroke Incidence by 10 Year Periods of Time
| All Incident Strokes | ||||
|---|---|---|---|---|
| Age (years) |
Women | Men | ||
| N strokes | Incidence/1000PY | N strokes | Incidence/1000PY | |
| 45 – 54 | 34 | 0.82 | 41 | 1.16 |
| 55 – 64 | 76 | 1.76 | 93 | 2.58 |
| 65 – 74 | 161 | 5.04 | 182 | 7.59 |
| 75 – 84 | 226 | 12.09 | 145 | 13.40 |
| 85 – 94 | 128 | 21.57 | 34 | 15.51 |
| Crude | 625 | 4.42 | 495 | 4.56 |
| Age-adjusted | 4.07 | 4.96 | ||
Figure 1.
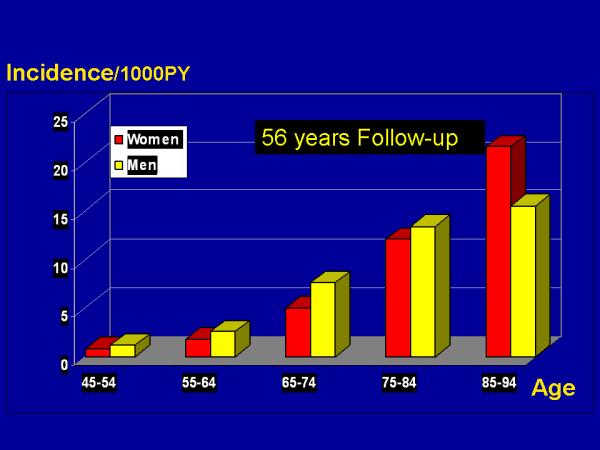
Incidence of Stroke by Age and Sex Over 56 Years of Follow-Up
The remaining LTR of stroke for individuals who survive stroke-free to age 45 was estimated as 1 in 6 for men and 1 in 5, for women (Figure 2). A similar pattern to that seen in the age-specific incidence analyses is noticed again with the mortality-adjusted cumulative incidence higher in men until age 85; beyond age 85 the LTR of stroke curve for women surpassed the rate for men. Age and Gender-Specific 10 -, 20 -, 30 -, 40 - year and LTR (through age 95) estimates for incident stroke are shown in Table A at http://stroke.ahajournals.org.
Figure 2.
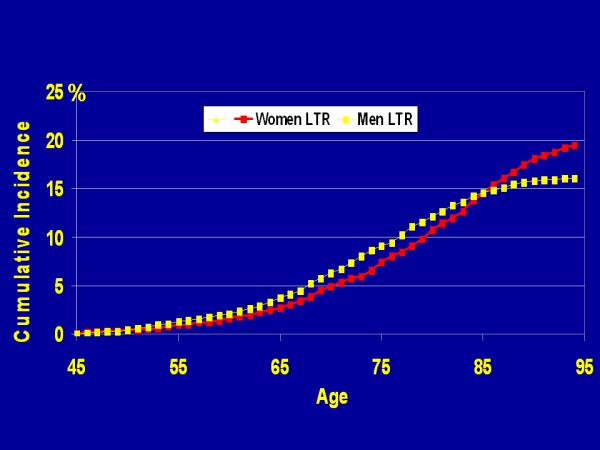
Gender-Specific Mortality-Adjusted Cumulative Incidence (Lifetime Risk) of Stroke
Table A.
“online only” - Age and Gender-Specific 10 -, 20 -, 30 -, 40 - year and lifetime risk (through age 95) estimates* for incident stroke
| Gender | Index Age | N Strokes | 10-year | 20-year | 30-year | 40-year | LTR |
|---|---|---|---|---|---|---|---|
| Women | 45 | 625 | 0.79 | 2.43 | 6.57 | 13.85 | 19.47 |
| 55 | 591 | 1.70 | 5.99 | 13.55 | 19.39 | ||
| 65 | 515 | 4.64 | 12.82 | 19.13 | |||
| 75 | 354 | 9.92 | 17.58 | ||||
| 85 | 128 | 12.87 | |||||
| Men | 45 | 495 | 1.08 | 3.36 | 8.69 | 14.23 | 16.10 |
| 55 | 454 | 2.42 | 8.10 | 13.99 | 15.97 | ||
| 65 | 361 | 6.61 | 13.46 | 15.77 | |||
| 75 | 179 | 9.78 | 13.08 | ||||
| 85 | 34 | 7.84 | |||||
Risks expressed in percentages over specified period of observation
Subtypes of All Incident Strokes
Table 2 presents data on subtypes of all incident strokes. Less than 1% of strokes remained unspecified and there was no significant difference between women and men with regard to stroke subtype.
Table 2.
Subtypes of All Incident Strokes
| All Strokes |
||
|---|---|---|
| Women | Men | |
| N Incident Strokes | 638 | 498 |
| ABI | 367 (58%) | 310 (62%) |
| CE | 170 (27%) | 116 (23%) |
| SH | 33 (5%) | 21 (4%) |
| ICH | 59 (9%) | 46 (9%) |
ABI = atherothrombotic brain infarctions, CE = cardioembolic infarctions, SH = subarachnoid hemorrhage, ICH = intracerebral hemorrhage
Co-morbidities, Age, Stroke Severity, Case Fatality Rates at 30, 90 and 180 days after First-Ever Stroke
Socio-demographic factors, chronic medical conditions prior to stroke, stroke severity and case fatality rates at 30, 90 and 180 days are shown in Table 3. Women were significantly older at the first-ever stroke with an average age of 75 years in women vs. 71 years in men (p< 0.001). Prior to stroke, women had a significantly lower prevalence of cancer (p<0.001) than men; men and women did not differ with respect to the presence of CVD, DM, AF, smoking or hypertension. Stroke severity and case fatality rates at 30, 90 and 180 days did not differ significantly between women and men.
Table 3.
Co-morbidities, Age, Stroke Severity at First-Ever Stroke and Case Fatality Rates at 30, 90 and 180 days among participants who attended an exam within 5 years prior to stroke
| N Incident Strokes | Women | Men |
|---|---|---|
| 519 | 413 | |
| Pre-Stroke Morbidity | ||
| Diabetes mellitus | 25% | 28% |
| Cardiovascular disease | 34% | 39% |
| Atrial Fibrillation | 16% | 14% |
| Systolic blood pressure (mmHg) | 155 ± 27 | 149 ±25 |
| Antihypertensive treatment | 49% | 41% |
| Smoking | 22% | 28% |
| Cancer * | 16% | 18% |
| Age at stroke** (years) | 75 ± 11 | 71 ± 10 |
| Stroke severity: | ||
| Fatal/severe | 29% | 21% |
| Death in 30 days | 21% | 15% |
| Death in 90 days | 27% | 20% |
| Death in 180 days | 30% | 23% |
age-adjusted p<0.01
p < 0.001
Gender-Specific Disability and Institutionalization Rates
The pre and post-stroke disability and institutionalization rates were calculated in the acute phase of stroke on all incident strokes included in the disability assessment. In those who survived and attended a 3 to 6 months post-stroke visit, gender-specific disability and institutionalization rates were obtained in the acute phase of stroke and at 3 to 6 months post-stroke, as shown in Table 4.
Table 4.
ADL Modified Katz Disability Scale and Rate of Institutionalization in the Acute Phase of Stroke and at 3 or 6 Months Post-Stroke
| Among those who survived and attended a 3 to 6 month visit | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Acute Phase of Stroke | Acute Phase of Stroke | 3 to 6 Months Post-Stroke | |||||||
| Women | Men | Age-adjusted OR | Women | Men | Age-adjusted OR | Women | Men | Age-adjusted OR | |
| N Incident Strokes | 276 | 182 | 106 | 77 | 106 | 77 | |||
| Pre-Stroke dependent living | 27% | 6% | 4.03*** | 22% | 5% | 3.71* | |||
| Pre-Stroke Katz ADL scale | 16% | 6% | 2.31* | 11% | 3% | 4.33 | |||
| (1+dependent) | |||||||||
| Age at Stroke | 81±9 | 75±9 | 80±9 | 75±8 | |||||
| Married | 22% | 74% | 0.12*** | ||||||
| Post-Stroke Institutionalization | 86% | 81% | 1.16 | 82% | 74% | 1.45 | 35% | 10% | 3.50** |
| Post-Stroke Disability | |||||||||
| Domain of Activity | |||||||||
| Eating | 42% | 26% | 1.56* | 24% | 17% | 1.31 | 15% | 9% | 1.05 |
| Dressing | 59% | 37% | 1.91** | 48% | 33% | 1.59 | 37% | 20% | 1.79 |
| Grooming | 57% | 34% | 2.01*** | 44% | 22% | 2.26* | 32% | 17% | 1.64 |
| Transfer bed to chair | 59% | 35% | 2.16*** | 48% | 30% | 1.82 | 32% | 13% | 2.37* |
| Walking | 64% | 48% | 1.48 | 57% | 49% | 0.98 | 37% | 18% | 1.91 |
p<0.05
p<0.01
p<0.001
In a sample of 276 women and 182 men, we observed that prior to stroke women were significantly more disabled as assessed by the Katz ADL scale (16% vs. 6%, OR=2.31, p<0.05) and 4 times more likely to be dependent in their living situation (27% vs. 6%, OR=4.03, p<0.001). In the acute phase of stroke, women were also significantly more disabled than men (p<0.01) in eating (42% vs. 26%, OR=1.56), dressing (59% vs. 37%, OR=1.91), grooming (57% vs. 34%, OR = 2.01) and transfer from bed to chair (59% vs. 35%, OR = 2.16). After adjusting for all the components of the Framingham Stroke Risk Profile27(SBP, antihypertensive treatment, AF, current smoking, prevalent CVD and DM) and for the pre-stroke disability, women were still significantly more disabled than men (p<0.01) in dressing (OR=1.87), grooming (OR=2.11) and transfer from bed to chair (OR=2.31).
In a subsample of 106 women and 77 men, who survived the acute phase of stroke and attended the 3 to 6 months post-stroke visit, women were again almost 4 times more likely to be dependent in their living situation prior to stroke (22% vs. 5%, OR = 3.71, p<0.05), were less likely to be married at the time of the acute stroke (22% vs. 74%, OR = 0.12, p<0.001) and showed a trend towards greater disability in all ADLs both in the acute phase of stroke and at 3 to 6 months post-stroke evaluation. However, this trend reached statistical significance only for grooming (44% vs. 22%, OR = 2.26, p<0.05) in the acute phase and for transfer from bed to chair (32% vs. 13%, OR = 2.37, p<0.05) at 3 to 6 months post-stroke. At 3 to 6 months post-stroke, women were 3.5 times more likely to be institutionalized than men (35% vs. 10%, OR = 3.50, p<0.01).
Discussions
In our community-based longitudinal study, we observed that women develop a first-ever stroke an average of 5 years later than men, that they have an overall higher LTR of stroke due to their greater life-expectancy and that their pre-stroke and post-stroke disability and institutionalization rates were significantly higher. At the same time, we did not observe a significant difference in the stroke severity or case fatality rates between women and men. Because women have a greater life expectancy advantage, a stroke occurring later in life at a time when a women’s health and ability to function independently are already compromised, compounds the disability observed in stroke survivors.
The biological and social explanations for these observations require further study. Since sex differences in stroke began to be recognized, the particular influence of estrogen and testosterone on the endothelium and the vascular system, the role of risk factors unique to women such as the use of oral contraceptives, hormone replacement therapy and pregnancy, systemic delays in the recognition and insufficient treatment of conventional stroke risk factors in women have all been considered as probable explanations. Efforts to discuss the possible role of these different factors have been hampered by the paucity of data on gender-differences in age-specific stroke incidence, as was recently outlined by Reeves et al.28 The inherent difficulties in conducting long-term longitudinal follow-up cohort incidence studies and the persistent misperception that stroke is a rarer disease in women may in part be responsible for the paucity of available data. Systematic data gathering on stroke incidence in women has largely occured only in the past 2-3 decades.29 Hence, our current data which are gathered over 56 years of follow-up in the Framingham Heart Study are unique.
We observed a trend towards a higher incidence of first ever stroke in women than in men after age 85 years, and a lower risk at all other ages. Although the stroke incidence in both genders has shown a trend toward an increase with each decade of life, the main burden of stroke was seen in participants between 65 and 85 years of age. Our observation of a trend towards a higher incidence of stroke in women older than 85 years of age, even though it did not reach statistical significance (p=0.087), likely as a result of the small number of participants in this age group, is concordant with results from the Oxford Vascular Study,30 which showed a higher incidence of stroke for women aged 85 years and older and with a Swedish study,6 which also found an increased incidence of stroke in women aged 75 years or more. Data from the Rotterdam Study,31 another population based study, with a mean follow-up time of 6 years, found that, although stroke incidence increases with age in both sexes, it remained higher in men than in women over the entire age range studied. However, the Rotterdam estimates are based on only 28 events occurring at of after age 85, whereas the current Framingham estimates are based on data from 162 events in persons aged 85 or above.
Towfighi et al.,32 using data from the National Health and Nutrition Examination Surveys (NHANES) reported a midlife stroke surge among women in the United States; between the ages of 45 and 54 women were twice as likely as men to have had a stroke. The higher prevalence was largely explained by an increase in stroke incidence among women aged 45 to 54, a finding also supported by the Rochester Community Study33 and the Swedish Hospital Discharge Register.34 In our study we did not observe such a ‘midlife’ increase in risk among women but the number of events we observed in this age group was small.
The higher stroke incidence in elderly women (above age 85) that we observed has been supported by other studies.30,6 However, in addition to age and the common stroke risk factors, social isolation and loss of a spouse (which is more common among elderly women than men), could, as already shown by other studies,35-37 negatively affect overall health, and appears to account for the increased risk of institutionalization in our study.
The LTR of stroke in our study was higher in women than in men, consistent with our prior observations.3 The longer life expectancy in women is most likely responsible for this finding, but a higher incidence of stroke in women at older ages could be an additional explanation.
Our study did not find a significant gender-specific difference in stroke severity and case fatality rates at 30, 90 or 180 days. This is concordant with data from the Rotterdam study. However, higher case fatality rates were seen in women in almost all populations studied as part of the WHO MONICA Project38 as well as in the International Stroke Trial.39
Along with death, disability and institutionalization are the most feared and devastating post-stroke outcomes. Our current study explored short-term follow up disability outcomes at 3 to 6 months after stroke and observed a trend toward women being more disabled than men, and the rates of institutionalization significantly higher for women, almost 4 times as high as for men. The most intriguing finding was that although men had at the time of the stroke a higher prevalence of cardiovascular disease and cancer, women were more disabled in their ADLs and they were half as likely to be living independently even prior to stroke. Men were also almost 3 times more likely to be married at the time of their stroke as opposed to women, who were more likely to be widowed or unmarried and to be living alone. These results are concordant to other disability studies,40-42 including previous data from the FHS.4,17 Pre-stroke disability along with social isolation and lack of social support might prevent women from having the same recovery outcomes as men. Nevertheless, these findings differ from the findings of the few existing studies that evaluate long term survival and functional status after stroke.18,43 One of these studies, a prior publication from the Framingham study18, noted that women who survived for more than 20 years after a stroke retained good functional abilities although they had a greater mortality than age- and sex-matched control subjects. The difference between our current and prior observations may lie in the selective mortality of the most disabled women who sustained a stroke. In the prior study the average age at first stroke was only 56 years versus a mean age of over 75 years in the current sample.
The 2001 World Heath Organization International Classification of Functioning, Disability and Health, highlights the role of environmental and personal factors in the disablement process.44 The importance of age, family and social factors as a risk factor for institutionalization, one of the most dreaded consequences of stroke cannot be underestimated.
Limitations and Strengths
Our study was restricted to an ethnically homogenous sample, white of European descent, which limits the generalizability of our results. Its strengths are the availability of more than 5 decades of meticulously collected, prospective, follow-up data from the large community-based longitudinal Framingham cohort, the use of consistent standardized definitions, the ongoing stroke surveillance, the continuity of follow-up with the same investigators over more than 3 decades (MKH, CSK, PAW) and the serial disability assessments.
Public Health Significance
Results from the FHS and other studies supporting the presence of gender-differences in stroke incidence, lifetime risk of stroke, age at first stroke, post-stroke disability and institutionalization rates could influence new gender-specific stroke prevention and rehabilitation strategies, targeting women for enrollment in clinical stroke prevention trials. In regard to elderly women our data suggest a need for increased social support. A better understanding of social and medical factors explaining gender-specific disability related issues in people living with stroke could help reduce stroke-related declines in quality of life and increases in living costs in the rapidly aging populations, especially of the United States and Western Europe.
Acknowledgments and funding page
Supported in part by the National Institute of Health/National Heart, Lung and Blood Institute’s Framingham Heart Study (NIH/NHLBI Contract #N01-HC-25195) and Grant from the National Institute of Neurological Disorders and Stroke (5R01-NS 17950)
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Bousser MG. Stroke in women: the 1997 Paul Dudley White International Lecture. Circulation. 1999;99:463–467. doi: 10.1161/01.cir.99.4.463. [DOI] [PubMed] [Google Scholar]
- 3.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 4.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12:119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 5.Di CA, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 6.Glader EL, Stegmayr B, Norrving B, Terent A, Hulter-Asberg K, Wester PO, Asplund K. Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke. 2003;34:1970–1975. doi: 10.1161/01.STR.0000083534.81284.C5. [DOI] [PubMed] [Google Scholar]
- 7.Savitz SI, Schlaug G, Caplan L, Selim M. Arterial occlusive lesions recanalize more frequently in women than in men after intravenous tissue plasminogen activator administration for acute stroke. Stroke. 2005;36:1447–1451. doi: 10.1161/01.STR.0000170647.42126.a8. [DOI] [PubMed] [Google Scholar]
- 8.Labiche LA, Chan W, Saldin KR, Morgenstern LB. Sex and acute stroke presentation. Ann Emerg Med. 2002;40:453–460. doi: 10.1067/mem.2002.128682. [DOI] [PubMed] [Google Scholar]
- 9.Pilote L, Dasgupta K, Guru V, Humphries KH, McGrath J, Norris C, Rabi D, Tremblay J, Alamian A, Barnett T, Cox J, Ghali WA, Grace S, Hamet P, Ho T, Kirkland S, Lambert M, Libersan D, O’Loughlin J, Paradis G, Petrovich M, Tagalakis V. A comprehensive view of sex-specific issues related to cardiovascular disease. CMAJ. 2007;176:S1–44. doi: 10.1503/cmaj.051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto T, Baba T, Ito A, Maekawa K, Koshiji T. Gender differences in stroke risk among the elderly after coronary artery surgery. Anesth Analg. 2007;104:1016–22. doi: 10.1213/01.ane.0000263279.07361.1f. tables. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 12.Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 13.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, Wolf PA. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006;296:2939–2946. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 14.Dyall L, Carter K, Bonita R, Anderson C, Feigin V, Kerse N, Brown P. Incidence of stroke in women in Auckland, New Zealand. Ethnic trends over two decades: 1981-2003. N Z Med J. 2006;119:U2309. [PubMed] [Google Scholar]
- 15.Eisenblatter D, Heinemann L, Classen E. Community-based stroke incidence trends from the 1970s through the 1980s in East Germany. Stroke. 1995;26:919–923. doi: 10.1161/01.str.26.6.919. [DOI] [PubMed] [Google Scholar]
- 16.Paolucci S, Bragoni M, Coiro P, De AD, Fusco FR, Morelli D, Venturiero V, Pratesi L. Is sex a prognostic factor in stroke rehabilitation? A matched comparison. Stroke. 2006;37:2989–2994. doi: 10.1161/01.STR.0000248456.41647.3d. [DOI] [PubMed] [Google Scholar]
- 17.Kelly-Hayes M, Wolf PA, Kannel WB, Sytkowski P, D’Agostino RB, Gresham GE. Factors influencing survival and need for institutionalization following stroke: the Framingham Study. Arch Phys Med Rehabil. 1988;69:415–418. [PubMed] [Google Scholar]
- 18.Gresham GE, Kelly-Hayes M, Wolf PA, Beiser AS, Kase CS, D’Agostino RB. Survival and functional status 20 or more years after first stroke: the Framingham Study. Stroke. 1998;29:793–797. doi: 10.1161/01.str.29.4.793. [DOI] [PubMed] [Google Scholar]
- 19.Chong JY, Lee HS, Boden-Albala B, Paik MC, Sacco RL. Gender differences in self-report of recovery after stroke: the Northern Manhattan Study. Neurology. 2006;67:1282–1284. doi: 10.1212/01.wnl.0000238161.71591.e9. [DOI] [PubMed] [Google Scholar]
- 20.Ayala C, Croft JB, Greenlund KJ, Keenan NL, Donehoo RS, Malarcher AM, Mensah GA. Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995-1998. Stroke. 2002;33:1197–1201. doi: 10.1161/01.str.0000015028.52771.d1. [DOI] [PubMed] [Google Scholar]
- 21.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz S, Ford ab, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 23.Crawford SL, Jette AM, Tennstedt SL. Test-retest reliability of self-reported disability measures in older adults. J Am Geriatr Soc. 1997;45:338–341. doi: 10.1111/j.1532-5415.1997.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 24.Jette AM. The Functional Status Index: reliability and validity of a self-report functional disability measure. J Rheumatol Suppl. 1987;14(Suppl 15):15–21. [PubMed] [Google Scholar]
- 25.Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, D’Agostino RB. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 26.Beiser A, D’Agostino RB, Sr., Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19:1495–1522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 28.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI:10.1016/S1474-4422(08)70193-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bousser MG, Eschwege E, Haguenau M, Lefaucconnier JM, Thibult N, Touboul D, Touboul PJ. “AICLA” controlled trial of aspirin and dipyridamole in the secondary prevention of athero-thrombotic cerebral ischemia. Stroke. 1983;14:5–14. doi: 10.1161/01.str.14.1.5. [DOI] [PubMed] [Google Scholar]
- 30.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 31.Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2003;74:317–321. doi: 10.1136/jnnp.74.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurology. 2007;69:1898–1904. doi: 10.1212/01.wnl.0000268491.89956.c2. [DOI] [PubMed] [Google Scholar]
- 33.Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373–380. [PubMed] [Google Scholar]
- 34.Medin J, Nordlund A, Ekberg K. Increasing stroke incidence in Sweden between 1989 and 2000 among persons aged 30 to 65 years: evidence from the Swedish Hospital Discharge Register. Stroke. 2004;35:1047–1051. doi: 10.1161/01.STR.0000125866.78674.96. [DOI] [PubMed] [Google Scholar]
- 35.Rosengren A, Wilhelmsen L, Orth-Gomer K. Coronary disease in relation to social support and social class in Swedish men. A 15 year follow-up in the study of men born in 1933. Eur Heart J. 2004;25:56–63. doi: 10.1016/j.ehj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Brummett BH, Barefoot JC, Siegler IC, Clapp-Channing NE, Lytle BL, Bosworth HB, Williams RB, Jr., Mark DB. Characteristics of socially isolated patients with coronary artery disease who are at elevated risk for mortality. Psychosom Med. 2001;63:267–272. doi: 10.1097/00006842-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Boden-Albala B, Litwak E, Elkind MS, Rundek T, Sacco RL. Social isolation and outcomes post stroke. Neurology. 2005;64:1888–1892. doi: 10.1212/01.WNL.0000163510.79351.AF. [DOI] [PubMed] [Google Scholar]
- 38.Thorvaldsen P, Asplund K, Kuulasmaa K, Rajakangas AM, Schroll M. Stroke incidence, case fatality, and mortality in the WHO MONICA project. World Health Organization Monitoring Trends and Determinants in Cardiovascular Disease. Stroke. 1995;26:361–367. doi: 10.1161/01.str.26.3.361. [DOI] [PubMed] [Google Scholar]
- 39.Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24:123–128. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- 40.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcome. The Copenhagen Stroke Study. Stroke. 1994;25:808–813. doi: 10.1161/01.str.25.4.808. [DOI] [PubMed] [Google Scholar]
- 41.Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, Cheung AM. Sex differences in stroke care and outcomes: results from the Registry of the Canadian Stroke Network. Stroke. 2005;36:809–814. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- 42.Gray LJ, Sprigg N, Bath PM, Boysen G, De Deyn PP, Leys D, O’neill D, Ringelstein EB. Sex differences in quality of life in stroke survivors: data from the Tinzaparin in Acute Ischaemic Stroke Trial (TAIST) Stroke. 2007;38:2960–2964. doi: 10.1161/STROKEAHA.107.488304. [DOI] [PubMed] [Google Scholar]
- 43.Anderson CS, Carter KN, Brownlee WJ, Hackett ML, Broad JB, Bonita R. Very long-term outcome after stroke in Auckland, New Zealand. Stroke. 2004;35:1920–1924. doi: 10.1161/01.STR.0000133130.20322.9f. [DOI] [PubMed] [Google Scholar]
- 44.2001 World Heath Organization International Classification of Functioning, Disability and Health. 2001 [ http://www.who.int/classifications/icfbrowser]


