Abstract
Objective:
To investigate if loss of extracellular matrix metalloprotease inducer (EMMPRIN) will inhibit the growth of head and neck squamous cell carcinoma (HNSCC) tumor cell lines in vivo. Tumor cell–derived EMMPRIN is highly overexpressed in HNSCC and is thought to be induced by surrounding fibroblasts to stimulate matrix metalloproteases, which modulate tumor cell invasion, growth, and angiogenesis.
Design:
In vivo study using FaDu tumor xenografts.
Setting:
Academic research facility.
Subjects:
Severe combined immunodeficiency (SCID) mice.
Interventions:
The HNSCC cell line FaDu was transfected with EMMPRIN (FaDu/E), control vector (FaDu), or plasmid-expressing small-interfering RNA against EMMPRIN (FaDu/siE). Tumor cells combined with fibroblast cells were xenografted onto the flank of SCID mice. Tumors were measured biweekly over 4 weeks, at which time the mice were killed, and tumor samples were analyzed for proliferation (Ki-67 immunohistochemical analysis), vascularization (factor VIII staining), and apoptosis (TUNEL [terminal deoxynucleotidyl transferase– mediated deoxyuridine triphosphate–biotin nick end labeling] assay).
Main Outcome Measure:
Growth of head and neck cancer cell lines genetically engineered to express variable levels of EMMPRIN.
Results:
Tumor growth positively correlated and animal survival negatively correlated with increasing EMMPRIN expression. FaDu/E tumor growth was significantly larger at 4 weeks compared with FaDu tumors (P = .006). Similarly, the control vector–transfected FaDu tumors were significantly larger than FaDu/siE (P<.001). Immunohistochemical analysis demonstrated increased Ki-67 in EMMPRIN-transfected cells, without a significant change in the rate of apoptosis between groups. Vascular density and tumor formation rate also increased significantly with EMMPRIN expression.
Conclusion:
This study suggests that anti-EMMPRIN– targeted therapy may prove to be a novel treatment option in HNSCC.
Extracellular matrix metalloprotease inducer (EMMPRIN) is a cell surface glycoprotein that has been recognized as an important modulator of tumor-stromal communication and a mediator of tumor cell invasion, multidrug resistance, and tumor cell–induced angiogenesis.1 Also known as CD147 or basigin,2 EMMPRIN has been shown to be overexpressed in numerous malignant neoplasms such as bladder, skin, lung, and breast carcinomas.3 Head and neck squamous cell carcinoma (HNSCC) tumors, including tumors of the oral cavity4 and larynx,5 express very high levels of EMMPRIN compared with these other tumor types. Overexpression of EMMPRIN has been shown to correlate with poor outcome and increased lymph node metastasis in HNSCC.4,5
Tumor invasion and metastasis is a multistep process carried out in a well-orchestrated fashion involving interaction and collaboration of multiple cell types.3,6 Initially, mechanical barriers to tumor outgrowth, such as parenchyma and basement membranes, prevent tumor cell migration. Once tumor cells dissolve these rigid matrices and progress to invasive cancer, it becomes necessary that they have access to a dedicated vascular supply.7 Angiogenesis occurs in response to release of angiogenic growth factors, such as vascular endothelial growth factor (VEGF). Such growth factors cause proliferation of existing endothelial cells, which migrate into the neoplasm and eventually form a reliable blood supply. Matrix metalloproteases (MMPs) are a group of extracellular-degrading enzymes that are critical to tumor cell invasion and endothelial cell migration into the tumor edges. These enzymes have been shown to influence tumor angiogenesis, growth, local invasion, and distant metastasis.6,8,9 In extracellular matrix (ECM) degradation, MMPs are considered a rate-limiting step, which must occur for both angiogenesis and tumor proliferation to proceed. Because EMMPRIN has been shown to induce expression of both MMPs and VEGF, we hypothesize that EMMPRIN is critical to the growth of HNSCC tumors in vivo.
The role of EMMPRIN in angiogenesis and ECM degradation implies that it may serve as a therapeutic target. We have previously demonstrated that high levels of EMMPRIN expression in HNSCC stimulates tumor growth in vivo.10 We investigate herein whether loss of EMMPRIN suppresses tumor growth and vascularization in HNSCC and thus proves to be a novel therapeutic target. We also evaluate the mechanism by which loss of EMMPRIN confers loss of growth.
METHODS
CELLS AND CELL CULTURE CONDITIONS
FaDu cells were purchased from American Type Tissue Collection, Manassas, Virginia. Cells were maintained in Dulbecco modified Eagle medium (DMEM) (Mediatech, Manassas, Virginia) supplemented with 10% (volume to volume ratio) fetal bovine serum (FBS) (Hyclone, Logan, Utah) and 1% penicillin-streptomycin solution (10 000 U/mL of penicillin G potassium and 10 000 μg/mL of streptomycin sulfate; Mediatech) in a humidified atmosphere containing 5% carbon dioxide at 37°C. Normal dermal fibroblasts (NDFs) were isolated from primary culture and maintained in DMEM with 20% FBS and antibiotics. To obtain NDFs, normal skin specimens were minced, washed in 70% ethanol followed by phosphate-buffered saline, and then dried on 6-well culture plates in triplicate for 30 minutes before the addition of culture media. Specimens were incubated for 21 to 28 days in DMEM supplemented with 20% FBS, 584-mg/L L-glutamine, penicillin G potassium (100 U/mL), streptomycin sulfate (1 mg/mL), and amphotericin B (2 μg/L). Cells were then transferred by brief trypsinization (0.25% for 30 seconds with gentle agitation at room temperature) to 6-well dishes and grown to confluence (passage 0). Cells were again passaged by differential trypsinization and plated onto coverslips (passage 1) and subsequently 100-mm dishes. Cells grown on coverslips were used to perform immunohistochemical analysis for vimentin, as well as cytokeratins 8 and 14 on passages 0, 1, and 2, to determine the presence of tumor cells. Epithelial cells were not identified in fibroblast populations after passage 1. All experiments used fibroblasts between passages 1 and 4.
TRANSFECTION OF FaDu CELL LINE WITH EMMPRIN
Transfection of the CAL 27 cell line with EMMPRIN has been described previously1 and parallels that used in the transfection of the FaDu cell line. Briefly, the EMMPRIN expression vector that contains the 806–base pair (bp) EMMPRIN complementary DNA (cDNA) and the 700-bp cDNA for the 29-kDa green fluorescent protein has been previously used in tumor cell lines and found to express functional EMMPRIN on the cell surface of human breast cancer cell lines. FaDu cell lines were transfected with the plasmid using Lipofectamine 2000 (Invitrogen, Carlsbad, California). Under G418 selective conditions (0.9 mg/mL), resistant clones were screened by fluorescent appearance. Stably resistant clones were then selected from each cell population, propagated, and analyzed for EMMPRIN protein expression by immunoblotting. All experiments were performed in parallel with FaDu control cells stably transfected with the pcDNA 3.0 vector (Invitrogen). To assess transfection success, transfection cells were homogenized in radioimmunoprecipitation assay buffer with 15 μg/mL each of aprotinin and pepstatin. After protein determination using a bicinchoninic acid protein assay (Pierce, Rockford, Illinois), 20-μg total protein per lane was separated by sodium dodecyl lauryl sulfate–polyacrylamide gel protein electrophoresis under reducing conditions on a precast 12% Tris-glycine gel (Invitrogen) and then transferred to polyvinylidene difluoride membrane (Millipore, Bedford, Massachusetts). Immunoblotting was performed with anti-EMMPRIN monoclonal antibody (Zymed, San Francisco, California). Secondary antimouse antibody was used, followed by chemiluminescence detection. Mouse monoclonal antibody to β-actin (anti–β-actin antibody; Santa Cruz Biotechnologies, Santa Cruz, California) was then used to assess for uneven protein concentrations.
GENERATION OF EMMPRIN-SILENCED FaDu CELL LINE
To obtain an EMMPRIN stably silenced cell line, pSilencer 4.1-CMV puro (Ambion Inc, Austin, Texas) was used to construct the DNA-directed RNA interference plasmids. Oligo sequence pairs were designed to anneal to the linearized vector. Oligonucleo-tides were synthesized by Integrated DNA Technologies, Coral-ville, Iowa, and cloned into pSilencer 4.1 CMV puro vectors according to the manufacturer's instructions. Vectors containing the inserts were transformed into NovaBlue Singles competent cells (Novagen, Madison, Wisconsin). Colonies were analyzed by polymerase chain reaction and DNA sequencing. Confirmed plasmids were propagated and transfected into FaDu cells. After selection under puromycin (1.0 μg/mL), resistant cells were analyzed by Western blot for EMMPRIN expression (data not shown). Silenced cells were further selected by flow cytometry with Becton Dickinson FACStar (Becton Dickinson, Franklin Lakes, New Jersey). The target sequence selected for EMMPRIN silencing is GAGCTACACATTGAGAACCTG.
ANIMAL MODELS
Severe combined immunodeficient (SCID) male mice, aged 4 to 6 weeks (Charles River Laboratories, Wilmington, Massachusetts), were obtained and housed in accordance with Institutional Animal Care and Use Committee guidelines of the University of Alabama at Birmingham. The mice were divided into 3 groups, each receiving bilateral flank xenografts of FaDu cells combined with fibroblast cells. Group A (n=5) received FaDu transfected with control vector (FaDu), group B (n = 5) received FaDu transfected with EMMPRIN (FaDu/E), and group C (n=5) received FaDu transfected with small-interfering RNA (siRNA) against EMMPRIN (FaDu/siE). Mice were assessed for tumor growth biweekly by caliper measurement of the greatest dimensions of visible tumor to allow for calculation of tumor area. Data were obtained over a period of 4 weeks, at which time the animals were killed. Following tumor resection, samples were sent for immunohistochemical analysis.
IMMUNOHISTOCHEMICAL ANALYSIS
Formalin-fixed, paraffin-embedded tissues were sectioned at 5 μm, placed on treated Fisher SuperFrost/Plus slides, and heated for 1 hour at 58°C to adhere the tissue sections onto the slides. The sections were then deparaffinized and rehydrated with three baths of xylene followed by absolute, 95%, and 70% ethanol for 5 minutes each and placed in Tris buffer (0.05M Tris base, 0.15M sodium chloride, and 0.1% TritonX-100 [pH 7.0]). After draining the buffer, endogenous peroxidase enzymes were quenched by addition of 3% aqueous hydrogen peroxide for 5 minutes. Each section was then incubated with 3% goat serum at room temperature for 20 minutes to reduce nonspecific immunostaining. The slides were incubated with the appropriate primary antibody for 1 hour at room temperature. Primary antibodies used were Ki-67 (Laboratory Vision Corporation, Fremont, California) and factor VIII (Cell Marque, Hot Springs, Alaska) at dilutions of 1:750 and 1:120, respectively. The slides for Ki-67 were heated in a 10mM EDTA solution at pH 8 in a pressure cooker for 10 minutes and then cooled slowly. Slides for factor VIII were incubated for 1 hour at room temperature with rabbit anti– factor VIII polyclonal antibody, and enzyme antigen retrieval was performed using pepsin 0.5 mg/mL in 0.01M hydrochloric acid at 37°C for 15 minutes. Negative controls were performed by omitting the primary antibodies. Secondary detection was accomplished using a multispecies detection system (Signet Laboratories Inc, Dedham, Massachusetts). The sections were exposed to a biotinylated antimouse/antirabbit antibody for 20 minutes and then peroxidase-labeled streptavidin was added for 20 minutes. A diaminobenzidine tetrachloride supersensitive substrate kit (BioGenex) was used to visualize the antibody-antigen complex. Each section was counterstained using a weak Myers hematoxylin, dehydrated using graded alcohols, and soaked in xylene baths.
IMMUNOHISTOCHEMICAL EVALUATIONS
Ki-67
The percentage of staining nuclei was estimated by counting the positive nuclei of proliferating cells and the negative nuclei of nonproliferating cells on 5 random high-power fields (original magnification ×400) from 4 tumor samples from each of the 3 groups (n=12). Careful attention was applied to avoid the inclusion of connective tissue cells, endothelial cells, and necrotic areas in the calculation of the unstained cells potentially confounding the data. A grid was constructed as an overlay on each field to allow for counting of both positive stained cells and negative unstained cells in 1 quadrant per field. Estimation of total field count was accomplished by multiplying by 4 for each field. Counts were confirmed independently by 2 of the authors (J.B.S. and W.E.G.).
TUNEL Assay
The formalin-fixed, paraffin-embedded 5-μm sections of representative tumor samples (n=12) were studied by TUNEL (terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate–biotin nick end labeling) staining by using the Apoptag Kit (Intergen, Purchase, New York). The extent of apoptosis was evaluated by counting the positive brown-stained cells as well as the total number of cells in 5 arbitrarily selected fields using the same counting method as described in the Ki-67 subsection. Necrotic areas of the tissue samples were avoided in the field selection. The number of apoptotic cells per ×400 field were reported.
Factor VIII
Endothelial staining for the presence of vessel growth was estimated by counting the number of vessels per high-power field (original magnification ×400). Positively staining vessels were counted from 5 randomly chosen fields for tumor samples (n=12) from each of the 3 tumor lines. As noted in the previous subsection, care was taken to avoid necrotic areas present in the tissue samples.
Preparation of Cell Membrane Extracts
FaDu, FaDu/E (overexpressing EMMPRIN), and FaDu/siE (EMMPRIN knockdown by RNA silencing) were cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin solution. Scraped cells were pelleted and resus-pended in homogenizing buffer (20mM Tris-hydrochloride [pH 8.0], 0.5mM calcium chloride, and 2mM dithiothreitol). Cells were homogenized in 10 mL of Potter-Elvehjem homogenizer for 30 strokes. Cell membranes were separated by centrifugation at 600g in homogenizing buffer containing 30% sucrose on a cushion of homogenizing buffer containing 45% sucrose. Membrane fraction was pelleted by dilute sucrose to 10% and centrifuged at 10 000g for 10 minutes. Membranes were dissolved in Tris-buffered saline Tween-20. Tris-buffered saline Tween-20 cold acetone was added 4 times and incubated for 1 hour at −20°C to precipitate the membranes. Membranes were pelleted by centrifugation at 15 000g for 10 minutes and washed twice with 4:1 acetone-water solution. Once the acetone evaporated, the membrane was dissolved in DMEM, and the protein concentration was determined by the bicinchoninic assay method.
Preparation of Conditioned Media
Normal dermal fibroblast cells were plated in 6-well plates at 2×105 per well and incubated at 37°C and 5% carbon dioxide overnight (16 hours). Cells were washed with Dulbecco phosphate-buffered saline. One milliliter of DMEM containing 0.1% bovine serum albumin complemented with 30 μg of cell membrane extract was added to each well, and the cells were incubated for 10 hours. Conditioned media were collected and centrifuged to remove cell debris (samples were applied to enzyme-linked immunosorbent assay [ELISA] assay immediately or kept at −80°C for later experiments).
ELISA Quantitation of VEGF Expression in Conditioned Media
A Quantikine ELISA kit for human VEGF was purchased from R&D Systems, Minneapolis, Minnesota. ELISA assay was conducted according to the manufacturers' instructions. Briefly, a series of standard of VEGF was prepared. Fifty microliters of assay diluent for culture supernatant was added to each well of the microplate. Then 50 μL of standard or conditioned media was added, mixed, sealed, and incubated for 2 hours at room temperature. The microplate was washed 3 times with wash buffer. Two hundred microliters of chemokine conjugate was added to each well, sealed with adhesive strip, and incubated for 2 hours at room temperature. The microplate was washed again 3 times, and 200 μL of substrate solution was added to each well and incubated for 20 minutes at room temperature (protected from light). The reaction was stopped by adding 50 μL of stop solution. Optical density (OD) was read with Multiskan Ascent (Thermo Scientific, Waltham, Massachusetts) at 450 nm and 570 nm. Optical density reading was corrected by subtracting OD 570 from OD 450, and chemokine concentration in conditioned media was calculated from the standard curve. Each sample was examined in triplicate.
STATISTICAL ANALYSIS
Pairwise comparisons of the means for tumor size at day 29 and factor VIII, Ki-67, and TUNEL expression were compared by using the least squares means with the Tukey adjustment to fit the general linear model. P<.05 was considered significant. Survival was compared by the Kaplan Meir method with the log-rank test. The bias present between the caliper measurements and measurements of immunohistochemical evaluation was expressed as standard error of the mean.
RESULTS
EMMPRIN TRANSFECTION AND INCREASED MMP-9 EXPRESSION
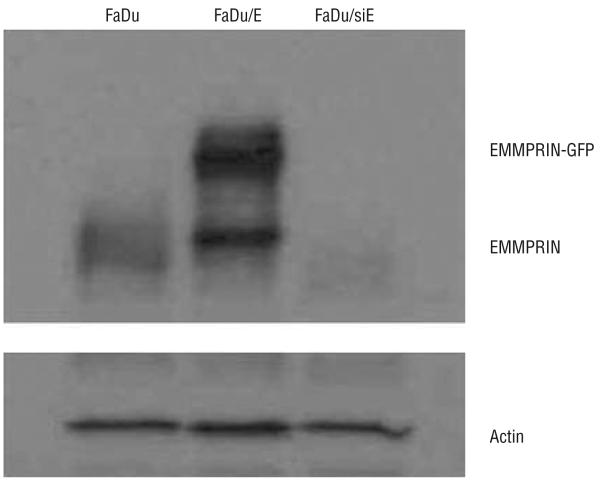
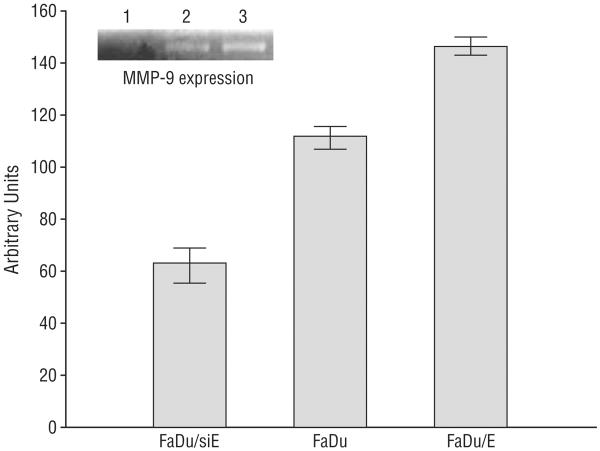
Western blot analysis was performed to verify varying EMMPRIN expression in the transfected FaDu cell lines (Figure 1). Results verified silencing of EMMPRIN expression in the FaDu/siE cell line and successful transfection of EMMPRIN–green fluorescent protein expression vector to generate the FaDu/E cell line. Intermediate levels of expression were seen in the control vector–transfected line (FaDu). To ensure that the EMMPRIN in each cell line was functional, NDFs were exposed to membrane preparations from each of the 3 cell lines. Ly-sates were then collected and MMP-9 levels were measured by zymogram (Figure 2). Results demonstrated graded expression of MMP-9 with increasing expression of EMMPRIN (P≤.003).
Figure 1.
Extracellular matrix metalloprotease inducer (EMMPRIN) expression in transfected FaDu cell lines (FaDu/E). Control vector-transfected cells (FaDu) expressed intermediate basal levels of EMMPRIN. Western blot analysis confirms overexpression of the EMMPRIN-green fluorescent protein (GFP) fusion protein. Expression of EMMPRIN was reduced in the FaDu transfected with small-interfering RNA against EMMPRIN (FaDu/siE) cell line. Equal protein loading was confirmed by actin loading.
Figure 2.
Matrix metalloprotease-9 (MMP-9) expression by FaDu human cancer cell lines. After successfully transfecting cells to overexpress extracellular matrix metalloprotease inducer (EMMPRIN) (FaDu/E), cell lysates prepared from tumor xenografts were analyzed by zymogram to ensure that the EMMPRIN produced was functional (P≤.003). Error bars indicate SEM; FaDu/siE, FaDu transfected with small-interfering RNA against EMMPRIN.
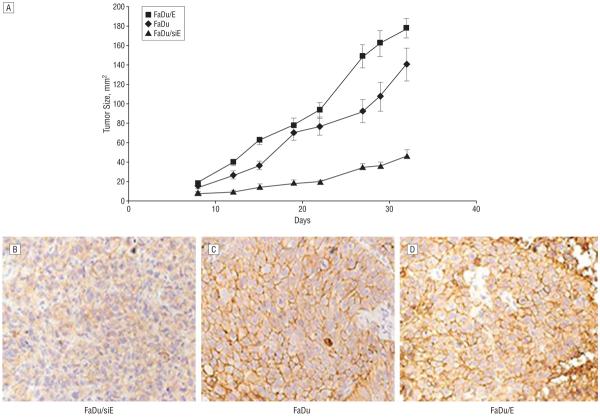
Tumor growth and tumor formation rate positively correlate with increasing EMMPRIN expression. To determine the effect of EMMPRIN expression on tumor growth rate, tumor size was measured for all 3 groups (n=5 per group), beginning at day 8 postinoculation. The EMMPRIN-transfected group (FaDu/E) had a larger tumor area (18.1 mm2) than did the control vector-transfected group (FaDu, 14.9 mm2), which was larger than the knockdown group (FaDu/siE, 8.8 mm2). FaDu/E tumor growth (Figure 3) was significantly larger at day 29 (161.9 mm2) compared with FaDu tumors (107.1 mm2; P=.006). Similarly, the FaDu tumors were significantly larger at day 29 (107.1 mm2) than FaDu/siE (36.84 mm2; P<.001). After the final measurement, the animals were killed, and immunohisto-chemical analysis for EMMPRIN was performed on xenografts from each tumor cell line (Figure 3B-D). Staining demonstrated that the relative amounts of EMMPRIN expression between the cell lines persisted through the tumor growth period, which was verified by Western blot analysis (data not shown).
Figure 3.
Tumor size measurement began at day 8 postinoculation of tumor cells with normal dermal fibroblasts. As shown in the graph (A), overexpressing extracellular matrix metalloprotease inducer (EMMPRIN)-transfected cells (FaDu/E) had the largest amount of growth and a corresponding greater rate of tumor formation than the control FaDu (P=.006, 2-tailed), which was significantly larger than the small-interfering RNA against EMMPRIN-transfected FaDu (FaDu/siE) group (P<.001). The FaDu/siE group showed the least amount of growth as well as the slowest tumor formation rate. Error bars indicate SEM. Levels of EMMPRIN expression in xenografts (FaDu/siE [B], FaDu [C], and FaDu/E [D]) obtained at day 32 were confirmed by both immunohistochemical staining (original magnification ×400) and Western blot analysis (data not shown).
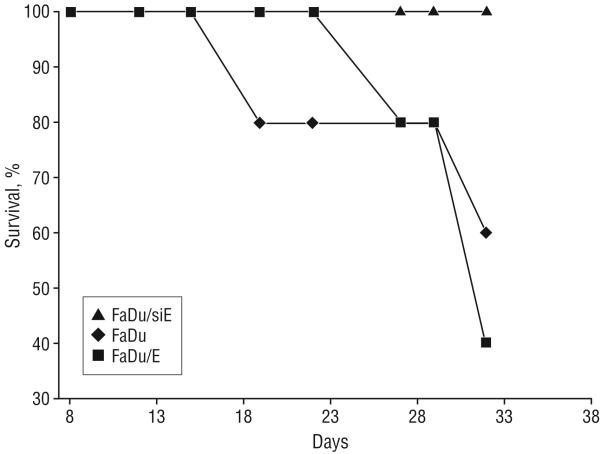
INVERSE RELATION BETWEEN MOUSE SURVIVAL AND EMMPRIN EXPRESSION
All mice transfected with the siRNA against EMMPRIN (FaDu/siE) survived throughout the course of the experiment, while 60% of those receiving the EMMPRIN-transfected FaDu (FaDu/E) cells died by the termination of the experiment (Figure 4). The FaDu control group had an intermediate percent survival, with 40% dying during the course of the experiment. This survival reached significance when comparing FaDu/siE and FaDu/E (P=.05), but not when comparing the other groups (P≥.13).
Figure 4.
Inverse relationship between extracellular matrix metalloprotease inducer (EMMPRIN) expression and survival. All mice bearing FaDu transfected with small-interfering RNA against EMMPRIN (FaDu/siE) tumors survived the course of the experiment, while only 40% of those bearing FaDu transfected with EMMPRIN (FaDu/E) tumors survived. Animals bearing FaDu (control group) had an overall survival of 60%. This survival reached significance when comparing FaDu/siE and FaDu/E (P=.05), but not when comparing the other groups (P≥.13).
INCREASED TUMOR PROLIFERATION IN THE PRESENCE OF EMMPRIN-TRANSFECTED CELLS
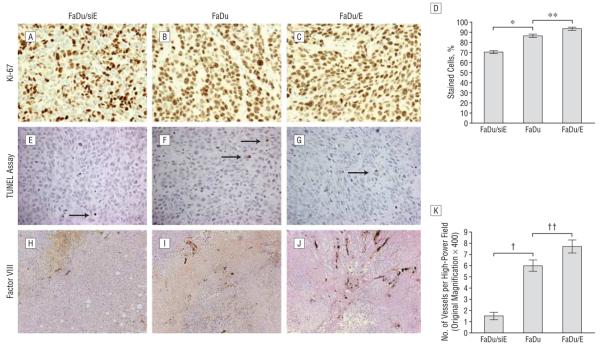
To assess tumor proliferation relative to EMMPRIN expression, the tumor xenografts underwent immunohistochemical analysis for Ki-67, which identifies proliferating cells (Figure 5A-C). The percentage of cells stained with Ki-67 (Figure 5D) was significantly higher for the FaDu/E group (93.31%) compared with FaDu/siE group (70.28%; P.001), indicating a greater proportion of proliferating cells in the FaDu/E group. The percentage of stained cells in the FaDu control group (86.57%) was significantly higher than that of the FaDu/siE group (70.28%; P>.001). Differences in proliferation between the FaDu and FaDu/E groups was also found to be statistically significant (P<.001). The presence of apoptosis among the tumor samples was determined by TUNEL assay. The number of apoptotic cells per high-power field (original magnification ×400) ranged from 0 to 6 in nonnecrotic areas of tissue samples (Figure 5E-G). The number of apoptotic cells in FaDu xenograft specimens was not significantly altered by modification of EMMPRIN expression. This finding was consistent among the 3 groups without significant variation; pairwise comparisons demonstrated P values all ≥.37.
Figure 5.
Immunohistochemical staining (original magnification ×400) for Ki-67 (A-C), TUNEL (terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end labeling) assay (E-G), and factor VIII (H-J). Comparison of the effects of increasing extracellular matrix metalloprotease inducer (EMMPRIN) expression on proliferation of tumor cells assessed by Ki-67 immunohistochemical analysis (D) demonstrated higher levels of proliferation as EMMPRIN levels increased (*FaDu [control group]>FaDu/siE [FaDu transfected with small-interfering RNA against EMMPRIN], P<.001; **FaDu/E [FaDu transfected with EMMPRIN]>FaDu, P<.001). The TUNEL assay (E-G) was used to determine degree of apoptosis, which did not vary with EMMPRIN expression (P≥.37) (error bars indicate SEM; arrows, apoptotic cells). The degree of vascularization, measured using factor VIII staining (K), showed greater blood vessel density as EMMPRIN levels increased (†FaDu>FaDu/siE, P<.001; ††FaDu/E>FaDu, P=.03).
HIGHER DENSITY OF VASCULARIZATION IN EMMPRIN-EXPRESSING TUMORS
To determine if EMMPRIN expression increases angio-genesis, tumor samples were stained with factor VIII (Figure 5H-J). Analysis of the specimens demonstrated progression in vascularity as EMMPRIN expression increased across the 3 tumor lines. Differences in vessel density between FaDu and FaDu/siE (Figure 5K) were statistically significant (P<.001), as were the differences between FaDu and FaDu/E (P=.03).
EMMPRIN STIMULATES VEGF FROM NDFs
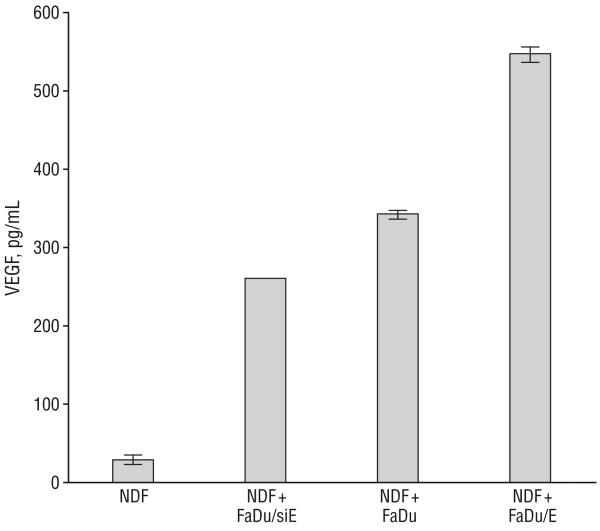
To test the hypothesis that EMMPRIN stimulates VEGF in our system, membrane preparations were prepared from FaDu/siE, FaDu, and FaDu/E tumor cell lines and used to stimulate NDFs in vitro. After stimulation, levels of VEGF were measured by ELISA and plotted in relation to basal levels expressed by NDFs (Figure 6). Exposure of NDFs to increasing levels of EMMPRIN (FaDu vs FaDu/siE) caused a 3-fold increase in VEGF, which showed a graded response to further increases in EMMPRIN expression (exposure to FaDu/E), increasing another 702% (P=.001).
Figure 6.
Effects of extracellular matrix metalloprotease inducer (EMMPRIN) expression on vascular endothelial growth factor (VEGF) expression in normal dermal fibroblasts (NDFs). After stimulation of membrane fractions prepared from FaDu transfected with small-interfering RNA against EMMPRIN (FaDu/siE), FaDu transfected with control vector (FaDu), or FaDu transfected with EMMPRIN (FaDu/E), VEGF was harvested from NDFs, which showed a graded response between all 3 tumor lines (P=.001). Error bars indicate SEM.
COMMENT
Increased EMMPRIN expression has been widely demonstrated in primary HNSCC tumor samples, but to our knowledge, its potential as a therapeutic target has not been explored. To that end, we demonstrate for the first time in HNSCC that silencing EMMPRIN results in statistically significant suppression of tumor growth (P < .001); however, increased survival advantage in EMMPRIN knockdown tumor xenografts were not statistically significant (P≥.13). Furthermore, we show that suppression of growth occurs through down-regulation of vessel ingrowth and proliferation, not through an increase in apoptosis.
It has recently been shown that tumor growth and vascularization can be inhibited by suppressing EMMPRIN expression, in both breast cancer6 and malignant melanoma.11 In the present study, we demonstrate that head and neck cancer tumor growth correlates with levels of EMMPRIN expression. The tumors that constitutively express lower levels of EMMPRIN (FaDu/siE) proved to have lower overall growth, to be less vascularized, and to have lower levels of cell proliferation than did those tumors with higher EMMPRIN levels (FaDu and FaDu/E). The differences among the 3 groups did not progress in a stepwise fashion for all parameters studied. We anticipated a greater difference in growth between control vector–transfected and EMMPRIN overexpression cells. One explanation for the moderate differences seen in tumor growth between these groups is that EMMPRIN is so highly expressed in HNSCC tumor cells and that tumor growth potential is already near maximal with these constitutive levels. This could also explain why the difference in vascularity between the control vector–transfected and EMMPRIN overexpression groups was not as large as the difference between EMMPRIN knockdown and control vector groups.
Investigation into the possible mechanism(s) by which EMMPRIN mediates tumor proliferation and angiogenesis have led others to conclude that the up-regulation of VEGF by EMMPRIN, through interaction with fibro-blasts, is responsible.6,11 We demonstrate a similar correlation between EMMPRIN and VEGF expression, since VEGF and vessel density (factor VIII staining) showed a graded progression in expression levels with exposure to increasing levels of EMMPRIN. Our results indicate that inhibition of apoptosis is not a prominent means by which EMMPRIN promotes tumor growth. Because our laboratory10 and others6 have shown the importance of the interaction of EMMPRIN with surrounding fibro-blasts, we coinjected NDFs with tumor cells to generate xenografts in the present study. Although multiple mechanisms may be responsible for our findings, our evidence aligns with the findings of others in highlighting a significant relationship between EMMPRIN, NDFs, and VEGF production. We demonstrate a correlation between EMMPRIN expression and angiogenesis, but direct evidence of this relationship is still required. Whether this is the end pathway or one of several in the modulation of tumor cells by EMMPRIN and the tumor micro-environment has yet to be determined.
In conclusion, EMMPRIN appears to have multiple roles in cancer progression, including cell proliferation and vascularization. The precise understanding of how EMMPRIN activity is involved in angiogenesis requires further study and has significant implications for cancer treatment. By down-regulating EMMPRIN and demonstrating a suppression of tumor growth in vivo, this study suggests that anti-EMMPRIN-targeted therapy may prove to be an effective treatment option in HNSCC.
Acknowledgments
Funding/Support: This work was supported by grant NCI K08CA102154 from the American Head and Neck Society, the National Cancer Institute and grant 2T32 CA 091078-06 from the National Institutes of Health.
Footnotes
Financial Disclosure: None reported.
Previous Presentation: This study was presented at the Seventh International Conference on Head & Neck Cancer; July 22, 2008; San Francisco, California.
REFERENCES
- 1.Yan L, Zucker S, Toole BP. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost. 2005;93(2):199–204. doi: 10.1160/TH04-08-0536. [DOI] [PubMed] [Google Scholar]
- 2.Vigneswaran N, Beckers S, Waigel S, et al. Increased EMMPRIN (CD 147) expression during oral carcinogenesis. Exp Mol Pathol. 2006;80(2):147–159. doi: 10.1016/j.yexmp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Caudroy S, Polette M, Nawrocki-Raby B, et al. EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin Exp Metastasis. 2002;19(8):697–702. doi: 10.1023/a:1021350718226. [DOI] [PubMed] [Google Scholar]
- 4.Bordador LC, Li X, Toole B, et al. Expression of emmprin by oral squamous cell carcinoma. Int J Cancer. 2000;85(3):347–352. [PubMed] [Google Scholar]
- 5.Rosenthal EL, Shreenivas S, Peters GE, Grizzle WE, Desmond R, Gladson CL. Expression of extracellular matrix metalloprotease inducer in laryngeal squamous cell carcinoma. Laryngoscope. 2003;113(8):1406–1410. doi: 10.1097/00005537-200308000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Tang Y, Nakada MT, Kesavan P, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65(8):3193–3199. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- 7.Jones CLA. Cancer biology. In: Longe JL, editor. Gale Encyclopedia of Cancer. 2nd ed. Vol. 1. Gale Cengage; Detroit, MI: 2006. pp. 192–198. [Google Scholar]
- 8.Moses MA. The regulation of neovascularization of matrix metalloproteinases and their inhibitors. Stem Cells. 1997;15(3):180–189. doi: 10.1002/stem.150180. [DOI] [PubMed] [Google Scholar]
- 9.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18(5):1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal EL, Vidrine DM, Zhang W. Extracellular matrix metalloprotease inducer stimulates fibroblast-mediated tumor growth in vivo. Laryngoscope. 2006;116(7):1086–1092. doi: 10.1097/01.mlg.0000224368.58870.3c. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Lin J, Kanekura T, et al. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res. 2006;66(23):11323–11330. doi: 10.1158/0008-5472.CAN-06-1536. [DOI] [PubMed] [Google Scholar]