Abstract
The canonical Wnt pathway and β-catenin have been implicated in the pathophysiology of mood disorders. We generated forebrain-specific CRE-mediated conditional β-catenin knockout mice to begin exploring the behavioral implications of decreased Wnt pathway signaling in the central nervous system. In situ hybridization revealed a progressive knockout of β-catenin that began between 2 and 4 weeks of age, and by 12 weeks resulted in considerably decreased β-catenin expression in regions of the forebrain, including the frontal cortex, hippocampus, and striatum. A significant decrease in protein levels of β-catenin in these brain regions was observed by western blot. Behavioral characterization of these mice in several tests (including the forced swim test, tail suspension test (TST), learned helplessness, response and sensitization to stimulants, and light/dark box among other tests) revealed relatively circumscribed alterations. In the TST, knockout mice spent significantly less time struggling (a depression-like phenotype). However, knockout mice did not differ from their wild-type littermates in the other behavioral tests of mood-related or anxiety-related behaviors. These results suggest that a considerable β-catenin reserve exists, and that a 50-70% β-catenin reduction in circumscribed brain regions is only capable of inducing subtle behavioral changes. Alternatively, regulating β-catenin may modulate drug effects rather than being a model of mood disorder pathophysiology per se.
Keywords: depression, bipolar disorder, mood, Wnt pathway, glycogen synthase kinase, behavior, mice
Major challenges for neuropsychiatric research have been the characterization of biochemical pathways involved in the pathophysiology of mood disorders and the identification of truly novel therapeutic targets for their treatment [30]. The canonical Wnt pathway is a signaling pathway that has been implicated in a number of different aspects of mood disorder pathophysiology and treatment. Activation of the Wnt signaling pathway leads to inhibition of the enzyme glycogen synthase kinase-3 (GSK-3), and thereafter to an increase in β-catenin, which acts as a nuclear transcription factor. Specifically, the constitutively active enzyme GSK-3 is a critical negative regulator of this pathway, in which it phosphorylates β-catenin, resulting in its ubiqitin-dependent degradation [1, 32]. When GSK-3 is inhibited within a protein complex in the Wnt signaling pathway, GSK-3 does not phosphorylate β-catenin. The unphosphorylated β-catenin is therefore not degraded, leading to increased cellular levels of β-catenin [4, 33, 41]. The importance of the Wnt signaling pathway to mood disorder pathophysiology and treatment is suggested by the results of research on a number of levels. GSK-3 and β-catenin are regulated either directly or indirectly by a number of psychotrophic medications including, lithium, valproate, antidepressants, and antipsychotics [2, 7, 12, 21, 22, 24]. Decreasing the activity of GSK-3 either pharmacologically or through hemizygous deletion in mice results in an increase in brain levels of β-catenin, as well as antidepressant-like effects in the forced swim test (FST) and a decreased sensitivity to the hyperlocomotive effects of amphetamine [3, 14, 20, 31]. Further, our group has recently reported that over-expression of β-catenin in the mouse brain results in a decrease in amphetamine-induced hyperlocomotion and a decrease in immobility time in the FST [15], linking increased levels of β-catenin with alterations in behaviors related to mood stabilization in humans. The regulatory effect of β-catenin in the differentiation and proliferation of neural stem cells and differentiated neurons [17-19, 35] may also be related to mood disorder pathophysiology, given the possible roles of neurogenesis, neuroplasticity, and cellular resilience in these disorders. For example, antidepressants may, at least in part, exert their effects on proliferation of neurons in the subgranular zone of the hippocampus by increasing levels of β-catenin [27].
In order to understand further the role of β-catenin in behavioral models of mood disorders, we generated Cre/LoxP β-catenin conditional KO mice, under the control of the Ca2+/calmodulin-dependent protein kinase II alpha (CaMK2α) promoter. We then tested these mice in a number of behavioral models that are utilized to evaluate mood-and anxiety-related behavior.
MATERIALS AND METHODS
Mice
Floxed β-catenin mice were obtained from The Jackson Laboratory and had been backcrossed to C57BL/6 for at least ten generations (stock number 004152; Bar Harbor, Maine) [6]. Mice carrying the R26R LacZ reporter allele, obtained originally from Dr. Phillipe Soriano (University of Washington), were maintained on a C57BL/6 background and provided to us by Kazutoshi Nakazawa (NIMH, Bethesda, Maryland) [37]. The T29-1 Cre recombinase transgenic mice, in which Cre expression is restricted in the forebrain region by the CaMK2α promoter, were obtained originally from Dr. Susumu Tonegawa (Massachusetts Institute of Technology), and are maintained as heterozygotes on the C57BL/6 background [39]. The T29-1 Cre mice were also provided to us by Kazutoshi Nakazawa (NIMH, Bethesda, Maryland). T29-1 Cre recombinase mice were backcrossed to floxed β-catenin mice and thereafter Cre positive mice were mated with homozygous floxed β-catenin mice that had been maintained as a separate colony.
LacZ Staining
Mice were perfused transcardially with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB). The brains were removed and post-fixed in the same fixative at 4°C for 30 min. Saggital sections (50-um thick) were cut on a Vibratome and collected in PB. Sections were then incubated in 0.1 M PB containing 0.01% SDS, 0.02% NP-40, and 2 mM MgCl2 at 4°C for 15 min, followed by β-galactosidase reaction in 1 X PBS pH 8.0 containing 0.5 mg/ml X-Gal, 5 mM K4Fe(CN)6/3H2O, 5 mM K3Fe(CN)6, 2 mM MgCl2 at 37°C for 24 hours. Section were post fixed in 10% formalin for at least 2 hours, and counterstained with Nuclear Fast Red (Poly Scientific).
Genotyping
DNA was extracted from tail clips by proteinase K digestion followed by ethanol precipitation. The presence of the CRE-expressing transgene was detected by PCR amplification of a 444 bp product using the sense primer, 5' CCGGGCTGCCACGACCAA 3' and the anti-sense primer, 5' GGCGCGGCAACACCATTTTT 3' [36].
In situ hybridization
In situ hybridization procedures were performed as described for ribonucleotide (cRNA) probes [40]. Tissue sections were fixed with 4% formaldehyde acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine-HCl, pH 8.0, dehydrated, and delipidated with chloroform. A plasmid containing cDNAs encoding a 414 bp fragment of murine β-catenin mRNA (nucleotides 135-549) was amplified using primers 5'CATGGAGCCGGACAGAAAAGCTGCTG3' and 5'CGTGTGGAAGTTCCGCGTCATCCTG3', and cloned into pCRII-TOPO (the complete plasmid was a generous gift of Dr. Johanna Laurikkala, Institute of Biotechnology, Helsinki, Finland; [23]). Antisense ribonucleotide probes were transcribed from the previously mentioned plasmid linearized with HindIII using the Riboprobe System (Promega Biotech, Madison, WI) with 35SUTP (specific activity >1000 Ci/mmol; Perkin Elmer, Boston, MA) and T7 polymerase. Radiolabeled probes were diluted in a hybridization buffer, pH 7.5, and applied to brain sections (approximately 500,000 CPM/section). Slides were incubated overnight at 55°C in a humidified chamber. To reduce non-specific binding of the probe, slides were washed in 20 g/ml RNase solution for 30 min at room temperature, followed by 1 h each in 2X SSC at 50°C and 0.2X SSC at 55°C and 60°C. Slides were dehydrated and air-dried for autoradiography.
Audioradiography
The hybridized sections were exposed to Kodak BioMax MR film for 72 h to estimate approximate exposure time. Based on these results, films were again exposed to Kodak BioMax film for 24-96 hours. Films were developed under safelight conditions on a Kodak X-OMAT 2000A processor (Eastman Kodak Company, Rochester, NY). Audioradiographic images were digitized using a CoolSnap cf Color camera (Photometrics, Tucson, AZ) and IPLabs 3.6.1 software.
Western blots
At 16 weeks of age, WT (n = 8) and KO (n = 8) mice were sacrificed by cervical dislocation. Frontal cortex, striatum, hippocampus, and cerebellum were dissected immediately following decapitation. Brain specimens were then rapidly frozen in liquid nitrogen and stored at -80°C until further analysis. Western blots were performed essentially as described [12]. Briefly, samples were subjected to SDS-PAGE on 10% gels. Following homogenization, protein concentrations were determined using the Bio-Rad protein assay kit [5]. The linearity of the protein concentration for immunoblotting was ascertained by resolution of selected concentrations of protein. Due to the high levels of β-catenin in the brain the amount of protein loaded was 0.25 ug per lane. Proteins thus resolved were then electrophoretically transferred to nitrocellulose membranes. Nonspecific binding was blocked with TBST with 5% nonfat dry milk, and the membranes were then incubated with primary antibodies. Mouse monoclonal antibodies were from BD Bioscience (anti-β-catenin 610154; San Jose, CA); and Chemicon international (anti-actin MAB1501; Temulca, CA). After blotting with anti-mouse secondary antibody, the protein was detected with the ECL plus kit (Amersham Biosciences, Piscataway, NJ) and BioMax MR scientific imaging film (Kodak; New Haven, CT). Reported densitometry values are a ratio of β-catenin to actin, and normalized between gels using a common standard.
Behavioral Phenotyping
All behavioral experiments were performed with 16- to 20- week-old randomly selected male littermates derived from multiple litters. Mice were housed, 2-4 per cage in an animal room with constant temperature (22 ± 1 °C) and a 12 hour light/dark cycle (lights on/off at 6:00 A.M/P.M.), with free access to food and water. The majority of tests were conducted using naïve cohorts of animals; however, in circumstances where the same cohort was used for multiple tests, tests were performed in order from least to most stressful. Experiments were performed during the light phase of the light/dark cycle. All experimental procedures were approved by the National Institute of Mental Health Animal Care and Use Committee and were conducted in full accordance with the Guide for the Care and Use of Laboratory Animals.
Rotarod
An accelerating rotarod (Accuscan Instruments, Inc.) was used for testing motor coordination. The rotarod was set to accelerate from 4 to 40 RPM over 5 minutes [8]. On the first day, mice were placed on the rotating drum until they did not fall for a period of 30 seconds. Mice were then tested in 3 trials. A minimum of 2 minutes elapsed between trials.
Black-white box
The black-white box used (43 cm × 25 cm × 30 cm) has an open door between the light and dark compartments. The light compartment is 2/3 of the total size, and the overhead lighting had previously been optimized to be especially sensitive to anxiety-like behavior. Mice were placed in the light compartment and videotaped for 10 min. The number of transitions between the two chambers and time spent in each was later measured by an observer blind to genotype.
Tail suspension test
The tail suspension test was performed using a protocol that minimizes tail-climbing in C57/BL6 mice [16]. A 15 cm length of tape (TimeMed Labeling Systems, Inc, Burr Ridge, Illinois) was wrapped around the end of the tail, with approximately 2 mm of the tip protruding. Mice were suspended by their tails for six minutes. Sessions were videotaped and later scored by an observer blind to genotype. Any mice that did climb their tails were removed from the experimental analysis. Mobility was defined as movement of the hind legs.
Forced swim test
Because a depressive-like phenotype was expected, a two-day procedure was used for the forced swim test [11]. Mice were placed for 10 min in a cylinder of water 23-25°C. This session was videotaped for later analysis. The mice were tested in a six-minute session the following day. This session was also videotaped. For each session, mobility, defined as any movement beyond what is necessary to maintain the head above water, and was analyzed during the time period between 2 to 6 minutes.
Wire hanging
Mice were placed on a wire cage grate, which was subsequently turned upside down. The length of time before they fell into the cushioned cage below was measured. Time spent hanging was limited to one minute [8].
Hot plate
Mice were placed on a plate that had been heated to 55°C. Latency (in seconds) to jump or to lick the paws was measured. After the first incidence of biting/licking, mice were immediately removed from the hot plate and returned to the home cage.
Learned helplessness
Mice were exposed to 120 inescapable shocks, each 15 sec and 0.3 mA, in one compartment of the two-compartment GEMINI Avoidance System (San Diego Instruments; San Diego, CA). Average interval between shocks was 10 sec. The following day, mice were placed in the same chamber as that used for training. Testing consisted of 30 trials, with 15 sec shocks, 0.3 mA. The average intertrial interval was 20 sec. A gate opened 1 sec before the shock began, and stayed open for the duration of the shock. Shock administration stopped if mice passed through the gate to the other compartment.
Stimulant-induced sensitization
d-Amphetamine sulfate (2 mg/kg) was administered i.p. to mice immediately before each was placed in a 35 × 35 × 35 cm Plexiglas arena. Behavior was recorded for 90 minutes by the Ethovision videotracking system. Data analysis was performed by Ethovision software, and total distance moved was calculated. To ensure that sensitization would not be affected by exploratory behavior, mice were habituated to the arenas for five consecutive days. They received saline injections each day, and were immediately placed in the Plexiglas arenas for 60 min each day. On days 7-9, amphetamine (2 mg/kg) was administered i.p. before the mice were placed in the center of the same arenas. Ten days later, amphetamine was injected again and the mice were placed in the arenas. Ethovision videotracking recorded the behavior of the mice on both the habituation and the sensitization days. A modified version of the sensitization procedure was repeated with cocaine. Mice received 20 mg/kg of cocaine (Sigma, St. Louis, MO) i.p. and were immediately placed in the arenas. Movement was recorded by the Ethovision tracking system for 90 minutes. This procedure was repeated over five consecutive days, and again nine days later.
Statistics
Statistical analysis was performed by Graphpad Prism Version 4. Statistics were 2-tailed t-test, or a repeated measure two-way ANOVA. Data are reported as mean ± SE. p < 0.05 was considered significant.
RESULTS
Reporter Mouse
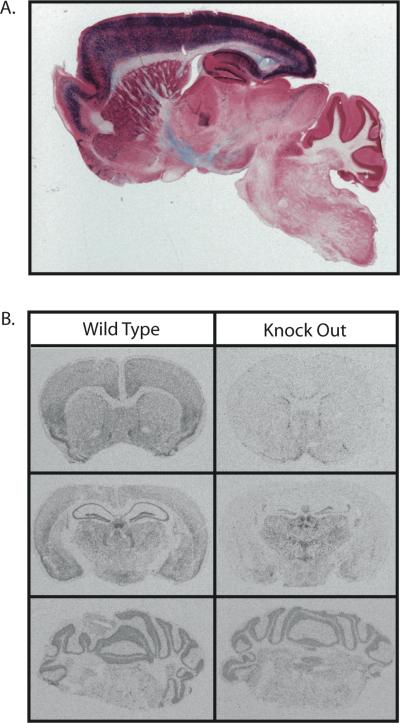
The T29-1 Cre expressing line has been previously reported to have Cre expression restricted to the CA1 pyramidal cell layer of the hippocampus [39]. Because transgene expression can change over time and vary due to the mouse background strain utilized, we first characterized the expected spatial pattern of recombination for the background of the T29-1 Cre expressing line we were utilizing, by mating this strain of mice with the R26R-LacZ reporter strain [37]. Analysis of the progeny of this mating at 8 weeks of age revealed a high degree of recombination in the forebrain but minimal, if any, recombination in mid and hind brain regions (Figure 1A). Recombination was highest in all areas of the cortex, as well as the CA1 region of the hippocampus. Recombination was also observed in CA3 and dentate gyrus regions of the hippocampus, as well as diffusely throughout the striatum (Figure 1A).
Figure 1.
Brain region specific nature of β-catenin KO. Sagittal section of an 8 week old reporter mouse brain showing LacZ expression (blue) following Cre recombination in ROSA26 mice mated with Cre expressing mice. Note the high expression in cortex and hippocampus (A). Audioradiographs visualizing the in situ hybridization of β-catenin in 12 week old wild type and knock out animals at the level of (from top to bottom) the striatum, dorsal hippocampus, and cerebellum (B).
In situ hybridization
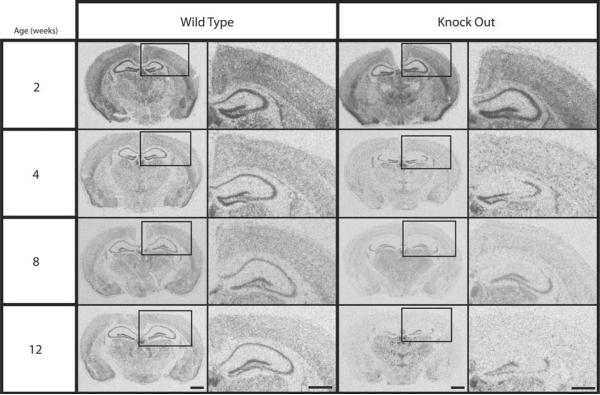
The R26R-LacZ reporter strain predicts a “best-case” scenario for Cre/LoxP mediated recombination [37]. In situ hybridization was used to characterize the degree of recombination in conditional β-catenin KO mice. The conditional β-catenin KO mouse line was generated by mating homozygous floxed β-catenin mice mated with T29-1 Cre recombinase transgenic mice and then backcrossing to a homozygous floxed β-catenin background. In situ hybridization revealed a post-natal down-regulation of β-catenin in selected brain regions. Specifically, at 12 weeks, β-catenin mRNA expression was reduced relative to WT controls in the frontal cortex, striatum, and dorsal hippocampus, while expression in the thalamus and cerebellum was equivalent in the WT and KO animals (Figure 1B). Figure 1B also illustrates the selective down-regulation in the hippocampus, where the knock-out is virtually complete in the CA1 and CA3 regions, but where some mRNA expression remains in the CA2 region and the dentate gyrus. Temporally, no decrease in β-catenin mRNA was observed at 2 weeks, but a decrease was evident by 4 weeks and continued to increase through 12 weeks (Figure 2). Because of these in situ hybridization results, we decided to conduct the behavioral experiments on mice between 16 and 20 weeks of age, to ensure that the expression of β-catenin had been reduced as much as possible.
Figure 2.
β-catenin mRNA expression throughout development. Audioradiographs visualizing the in situ hybridization of β-catenin in wild type and knock out animals. As age increases, β-catenin expression decreases in the cortex and hippocampus of knock-out animals. Although the reduction in mRNA expression is high in the CA1 and CA3 regions of the hippocampus, the reduction is considerably lower in the CA2 region and the dentate gyrus. Columns 2 and 4 are higher magnification of boxed areas in adjacent images (columns 1 and 3). Scale bars: Columns 1 and 3, 1.0mm; Columns 2 and 4, 0.5mm.
β-catenin protein levels
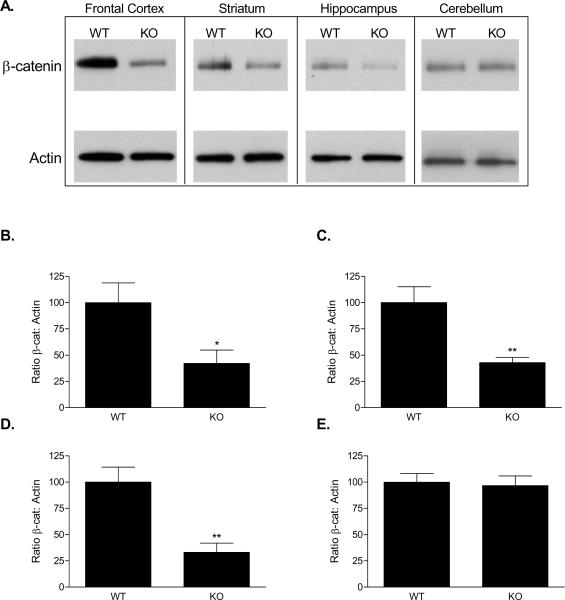
We confirmed the in situ findings by measuring protein levels of β-catenin. As predicted from the in situ hybridization results, 16 week-old KO mice had significantly reduced β-catenin protein in regions of the forebrain relative to their WT littermates, with no reduction in the cerebellum (Figure 3). Specifically, western blot analysis showed a 58% decrease in β-catenin levels in the frontal cortex (57.85 ± 22.78%; t = 2.54, df =14, p = 0.024); a 57% decrease in the striatum (57.12 ± 15.96%; t = 3.58, df =14, p = 0.003); a 67% decrease in the hippocampus (66.88 ± 16.76%; t = 3.99, df =14, p = 0.0013); and no significant change in the cerebellum (3.41 ± 12.43%; t = 0.27, df =14, p = 0.79).
Figure 3.
β-catenin protein expression in knock-out mice at 16-20 weeks. Western blot revealed decreased expression in the frontal cortex, striatum, and hippocampus, but no difference in the cerebellum (A). Densitometry revealed that β-catenin protein levels were reduced by 58% in the frontal cortex (B), 57% in the striatum (C), and 67% in the hippocampus (D). No difference was observed in the cerebellum (E).
Rotarod
WT and KO mice performed similarly in the rotarod test of motor coordination. Two-way ANOVA revealed a significant effect of trial number [F(2, 54) = 8.75, p < 0.001], but no significant effect of genotype [F(1, 54) = 0.031, p = 0.86] and no significant interaction [F(2, 54) = 1.32, p = 0.28].
Black-white box
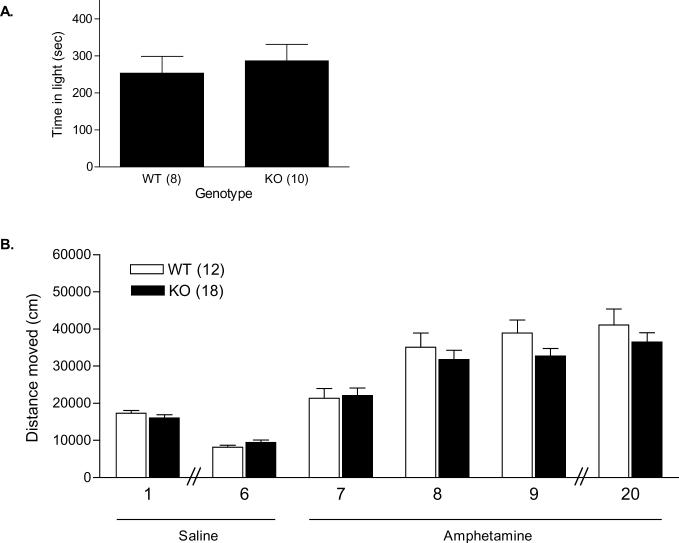
The amount of time spent in the light compartment of a black-white box, as well as the numbers of entries into the light compartment were evaluated (Figure 4B). There were no significant differences in the percentage of time spent in the light compartment between WT (42.25 ± 7.55%) and KO mice (47.75 ± 7.38%; t = 0.52, df =16, p = 0.61). There was also no significant difference in the number of entries into the light compartments between WT (16.88 ± 2.69) and KO mice (20.00 ± 3.91; t = 0.62, df = 16, p = 0.54).
Figure 4.
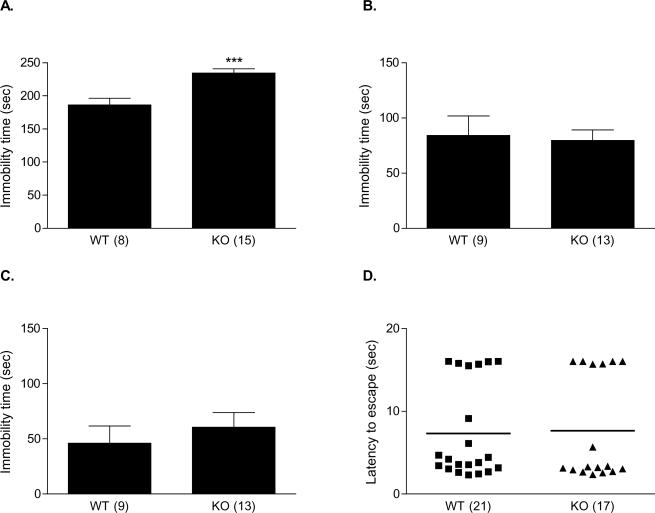
Behavioral phenotyping of WT and β-catenin KO mice. There was no difference in WT and KO mice in the amount of time spent in the light compartment of a light-dark box (A); or habituation to an environment and sensitization to amphetamine within the open field. Mice were administered saline on days 1 through 6, and amphetamine on days 7, 8, 9, and 20. Days are indicated on the X axis (B).
Habituation and stimulant-induced sensitization
We first determined whether there was an effect of genotype on habituation to a novel environment. Following i.p. injection of saline, mice were placed in the 35 by 35 cm arenas for 60 min. This process was repeated over 6 consecutive days. Mice were videotracked on the first and the sixth day of habituation. Both WT and KO mice moved similarly in this open field, and habituated to the environment at a similar rate (Figure 4B). Two-way ANOVA revealed a significant effect of day on distance moved [F(1, 28) = 97.39; p < 0.0001], but no significant effect of genotype [F (1, 28) = 0.000051; p = 0.99], and no significant interaction [F(1, 28) = 2.33; p = 0.14]. Subsequently, mice were treated with 2 mg/kg i.p. d-AMP for 3 consecutive days (Figure 4B). Over the course of the three days, locomotor response increased. Two-way ANOVA revealed a significant effect of day on total distance moved [F(2, 56) = 55.59; p < 0.0001], but no significant effect of genotype [F(1, 56) = 0.71; p = 0.41], and no significant interaction [F (2, 56) = 2.85; p = 0.066]. Ten days later, d-AMP was administered again. There was no difference between the two genotypes in total distance moved in response to this administration of d-AMP (WT: 41118 ± 4319 cm; KO: 36503 ± 2539 cm; t = 0.98, df = 28, p = 0.33; Figure 4C). In another cohort of mice, a similar procedure was used with cocaine administration over five days, without a habituation phase. A two-way ANOVA for total distance moved over each of the 5 days showed a significant effect of day [F(4, 80) = 5.14; p < 0.001], indicating that both WT and KO mice sensitized to cocaine. However, there was no significant effect of genotype [F(1, 80) = 0.0008; p = 0.77], and no significant interaction [F(4, 80) = 0.45; p = 0.78]. Reexposure to cocaine on day 14 days also resulted in no significant difference in total distance moved between genotypes (WT: 48530 ± 3050 cm; KO: 47590 ± 2208 cm; t = 0.23, df = 20, p = 0.82).
Tail Suspension Test
The 6-minute TST was scored for immobility time (Figure 5A). There was a significant difference between the immobility times of WT (186.3 ± 10.10) and KO mice (234.5 ± 6.30 s; t = 4.26, df = 21, p < 0.001). In order to ensure that this effect was not due to a chance occurrence among our mutiple tests, we repeated the test with a naïve cohort of mice. Again, the KO mice exhibited significantly more immobility than the WT mice (WT: 205.6 ± 9.89 s; KO: 237.7 ± 7.74 s; t = 2.56 df = 20, p < 0.05). In the second cohort we considered that tail length could influence TST performance. The difference was not attributable to a difference in tail length, as the tail lengths of WT and KO mice did not differ significantly (WT: 7.96 ± 0.08 cm; KO: 7.91 ± 0.09 cm; t = 0.45, df = 20, p = 0.66). In order to determine whether the difference in immobility seen in the TST was due to a difference in strength between the two genotypes, we evaluated their performance in the wire-hanging test. There was no significant difference in hanging time between the WT and KO mice, as measured by this test (WT: 53.75 ± 2.63 s; KO: 50.31 ± 4.19 s; t = 0.70, df = 30, p = 0.49).
Figure 5.
Behavioral phenotyping of WT and β-catenin KO mice in models relevant to depression. Tail suspension test immobility in WT and KO mice; relative to their WT littermates, KO mice showed significantly increased immobility in the TST (A). Forced swim test immobility time did not differ significantly between WT and KO mice on either the first (B) or the second (C) day of a two-day testing paradigm. In the learned helplessness test, WT and KO mice exhibited similar escape latencies following one day of inescapable shock training (D).
Forced Swim Test
We also tested a cohort of mice in the forced swim test, another model of antidepressant efficacy (Figure 5B, C). A two-day testing paradigm, which may be more sensitive to depressive-like behavior, was used [11]. The first day consisted of a ten-minute training session; of the first 6 minutes, the last 4 were scored for immobility time, as they would be in a standard test. We observed that in their first exposure to the test, there was no difference in immobility time between the WT and KO mice (WT: 84.00 ± 17.78 s; KO: 79.54 ± 9.54 s; t = 0.24, df = 20, p = 0.81). Because we were predicting a depressive-like phenotype based upon the results of the TST experiment, we repeated the test the following day. The last 4 minutes of this 6-minute test were scored for immobility time. There was no significant difference between the immobility times of WT (46.11 ± 15.39 s) and KO mice (60.62 ± 13.16 s; t = 0.71, df = 20, p = 0.48). In the control group there was one outlier (greater that 2 standard deviations from the group mean) with high immobility. However, with this outlier removed the difference remained non-significant (34.0 ± 10.77 vs 60.62 ± 13.16 s; t = 1.41, df = 19, p = 0.17; data not shown).
Learned Helplessness
We first used the hot plate test of pain sensitivity to ascertain that WT and KO mice responded comparably to pain. In the hot plate test, there was no significant difference in the length of time prior to jumping or licking the paws (WT: 9.70 ± 0.79 s; KO: 10.37 ± 0.53 s; t = 0.73, df = 29, p = 0.47). With a separate cohort of mice we then determined whether there was a difference between WT and KO mice in their tendency to develop and maintain helplessness following a single day of inescapable shock training (Figure 5D). Both the latency to escape and the number of escape failures were recorded over 30 trials, 24 h following inescapable shock training. KO mice did not differ from their WT littermates in either escape latency (WT: 7.33 ± 1.24 s; KO: 7.66 ± 1.53 s; t = 0.17, df = 36, p = 0.87; Figure 5D) or number of escape failures (WT: 9.43 ± 2.77; KO: 10.82 ± 3.49 t = 0.32, df = 36, p = 0.75).
DISCUSSION
Using Cre/loxP technology, we generated a conditional, forebrain-restricted knock-out of β-catenin in the mouse brain. These mice had reduced levels of β-catenin mRNA and β-catenin protein in the cortex, striatum, and hippocampus. The knockout was not present at 2 weeks of age, but increased in extent up to at least 12 weeks of age. For this reason we tested mice between 16 and 20 weeks of age. In multiple behavioral paradigms conditional β-catenin knock-out mice performed similarly to their wild-type littermates. However, in the TST, a test for antidepressant efficacy, β-catenin KO mice showed increased immobility, a depressive-like phenotype.
Antidepressant-like behavior was not observed in two other models of antidepressant efficacy, namely the FST or learned helplessness models. The lack of differences in these additional models, especially between the FST and TST, could be viewed as a major concern regarding how generalizable the data might be. It is possible that modifications to the FST procedure such as varying water temperature or perhaps changing the age of testing would identify differences between genotypes in the FST. However, while the neurobiological and genetic underpinnings of the FST and TST measures of “behavioral despair” are commonly viewed as being related, the literature contains a number of examples of performance differences in the two tests, among both outbred and inbred strains, in response to medications within these strains, as well as in genetically manipulated mice [9, 10, 25, 26, 28, 29, 34, 38]. Understanding the fundamental differences between the FST and TST will require additional research, but our results, and the results of others, suggest that there may be differences in the neurobiology that underlies performance on the two tests [28, 38].
Lithium, commonly used in the treatment of bipolar disorder and as an adjunct treatment for unipolar depression, directly inhibits GSK-3, which is upstream of β-catenin in the canonical Wnt pathway. Whereas the constitutive activity of GSK-3 marks intracellular β-catenin for ubiquitin-mediated degradation, the inhibtion of the enzyme leads to increased β-catenin levels in the cell. This increase allows for the importin-mediated translocation of β-catenin to the nucleus, where it dimerizes and acts as a transcription factor for Wnt-regulated genes. Lithium has antimanic- and antidepressant-like effects in various mouse behavioral models, and both the decreased activity of GSK-3 and the over-expression of β-catenin result in similar behavioral effects [14, 15, 20, 31]. Indeed, the results of a number of studies suggest the importance of the Wnt signaling pathway for the molecular and behavioral effects of diverse classes of psychotropic medications [13]. It will therefore be critical to study the mice we have generated in their response to lithium and other psychotropic medications.
The reduced expression of β-catenin might be expected to have the opposite effect as lithium or antidepressant administration, or β-catenin over-expression, in behavioral models. As such, we would expect to see a depressive- and/or a manic-like phenotype in the β-catenin knock-out mice. Indeed, the increased immobility of the knock-out mice in the TST is in keeping with this hypothesis. Reasons for the restricted nature of our findings may be multifold. As discussed above, the actions of β-catenin in these models may be more relevant to the behavioral effects of the medication than to baseline pathophysiology. However, the fact that the observed behavioral effects are limited may also be related to the specific characteristics of the mouse we generated, rather than to a limited role of β-catenin generally. In β-catenin KO mice, β-catenin protein levels were reduced by 58% in the frontal cortex, 57% in the striatum, and 67% in the hippocampus. It is possible that decrease we found in β-catenin protein and mRNA levels may not necessarily be associated with a change in the function of β-catenin targets such as transcription factors. This residual expression of β-catenin may be due to specific cell types or neuronal population that do not activate the CaMK2α promoter. It is possible that a more substantial reduction in β-catenin protein levels would lead to more extensive behavioral effects. Another possibility is that the KO mice develop mechanisms to compensate for the reduction of β-catenin. For example, alterations in the activity of GSK-3 could lead to less phosphorylation of β-catenin, making β-catenin more stable. Although the down-regulation does not begin until at least 2 weeks of age, the mice were 16 to 20 weeks old when they were tested in behavioral paradigms; thus, enough time may have elapsed in the post-natal period of development for compensatory mechanisms to have been established.
ACKNOWLEDGMENTS
We thank Dr. Miles Herkenham (NIMH) for assistance with the in situ hybridization experiments, Dr. Kazutoshi Nakazawa (NIMH) for the R26R-LacZ reporter and the T29-1 Cre recombinase transgenic mice, Dr. Juan Belforte (NIMH) for help with the LacZ staining, and Dr. Johanna Laurikkala (Institute of Biotechnology, Helsinki, Finland) for the α-catenin probe. The Intramural Research Program of the National Institute of Mental Heath supported our research. The authors have no conflicts of interest, financial or otherwise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. Embo J. 1997;16:3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Antipsychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry. 2005;57:533–42. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- [3].Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–9. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- [5].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- [6].Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- [7].Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999;72:1327–30. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- [8].Crawley JN. What's wrong with my mouse? : behavioral phenotyping of transgenic and knockout mice. Wiley-Liss; New York: 2000. p. xiii.p. 329. [Google Scholar]
- [9].Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- [10].David DJ, Renard CE, Jolliet P, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the forced swimming test. Psychopharmacology (Berl) 2003;166:373–82. doi: 10.1007/s00213-002-1335-4. [DOI] [PubMed] [Google Scholar]
- [11].Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–70. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- [12].Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004;29:32–8. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- [13].Gould TD, Dow ER, O'Donnell KC, Chen G, Manji HK. Targeting signal transduction pathways in the treatment of mood disorders: recent insights into the relevance of the wnt pathway. CNS Neurol Disord Drug Targets. 2007;6:193–204. doi: 10.2174/187152707780619308. [DOI] [PubMed] [Google Scholar]
- [14].Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–90. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- [15].Gould TD, Einat H, O'Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–83. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- [16].Gould TD, O'Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. doi: 10.1016/j.neuropharm.2007.11.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hall AC, Brennan A, Goold RG, Cleverley K, Lucas FR, Gordon-Weeks PR, Salinas PC. Valproate Regulates GSK-3-Mediated Axonal Remodeling and Synapsin I Clustering in Developing Neurons. Mol Cell Neurosci. 2002;20:257–70. doi: 10.1006/mcne.2002.1117. [DOI] [PubMed] [Google Scholar]
- [18].Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:269–79. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Israsena N, Hu M, Fu W, Kan L, Kessler JA. The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol. 2004;268:220–31. doi: 10.1016/j.ydbio.2003.12.024. [DOI] [PubMed] [Google Scholar]
- [20].Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry. 2004;55:781–4. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- [21].Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–9. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kozlovsky N, Amar S, Belmaker RH, Agam G. Psychotropic drugs affect Ser9-phosphorylated GSK-3beta protein levels in rodent frontal cortex. Int J Neuropsychopharmacol. 2005;1-6 doi: 10.1017/S1461145705006097. [DOI] [PubMed] [Google Scholar]
- [23].Laurikkala J, Pispa J, Jung HS, Nieminen P, Mikkola M, Wang X, Saarialho-Kere U, Galceran J, Grosschedl R, Thesleff I. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development. 2002;129:2541–53. doi: 10.1242/dev.129.10.2541. [DOI] [PubMed] [Google Scholar]
- [24].Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–31. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu X, Gershenfeld HK. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol Psychiatry. 2001;49:575–81. doi: 10.1016/s0006-3223(00)01028-3. [DOI] [PubMed] [Google Scholar]
- [26].Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–22. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- [27].Madsen TM, Newton SS, Eaton ME, Russell DS, Duman RS. Chronic electroconvulsive seizure up-regulates beta-catenin expression in rat hippocampus: role in adult neurogenesis. Biol Psychiatry. 2003;54:1006–14. doi: 10.1016/s0006-3223(03)00700-5. [DOI] [PubMed] [Google Scholar]
- [28].Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- [29].Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29:1050–62. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- [30].Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- [31].O'Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–8. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735–8. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- [33].Peifer M, Pai LM, Casey M. Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Dev Biol. 1994;166:543–56. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- [34].Ripoll N, David DJ, Dailly E, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the tail suspension test. Behav Brain Res. 2003;143:193–200. doi: 10.1016/s0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- [35].Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Sudhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–93. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- [36].Scheel JR, Garrett LJ, Allen DM, Carter TA, Randolph-Moore L, Gambello MJ, Gage FH, Wynshaw-Boris A, Barlow C. An inbred 129SvEv GFPCre transgenic mouse that deletes loxP-flanked genes in all tissues. Nucleic Acids Res. 2003;31:e57. doi: 10.1093/nar/gng057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- [38].Swiergiel AH, Leskov IL, Dunn AJ. Effects of chronic and acute stressors and CRF on depression-like behavior in mice. Behav Brain Res. 2008;186:32–40. doi: 10.1016/j.bbr.2007.07.018. [DOI] [PubMed] [Google Scholar]
- [39].Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–26. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- [40].Whitfield HJ, Jr., Brady LS, Smith MA, Mamalaki E, Fox RJ, Herkenham M. Optimization of cRNA probe in situ hybridization methodology for localization of glucocorticoid receptor mRNA in rat brain: a detailed protocol. Cell Mol Neurobiol. 1990;10:145–57. doi: 10.1007/BF00733641. [DOI] [PubMed] [Google Scholar]
- [41].Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]